Abstract
A hallmark of heart failure (HF) is adverse extracellular matrix (ECM) remodeling, which is regulated by the collagen cross-linking enzyme, lysyl oxidase (LOX). In this study, we evaluate the efficacy of LOX inhibition to prevent adverse left ventricular (LV) remodeling and dysfunction using an experimental model of HF. Sprague–Dawley rats were subjected to surgically induced volume overload (VO) by creation of aortocaval fistula (ACF). A LOX inhibitor, beta-aminopropionitrile (BAPN; 100 mg/kg/day), was administered to rats with ACF or sham surgery at eight weeks postsurgery. Echocardiography was used to assess progressive alterations in cardiac ventricular structure and function. Left ventricular (LV) catheterization was used to assess alterations in contractility, stiffness, LV pressure and volume, and other indices of cardiac function. The LV ECM alterations were assessed by: (a) histological staining of collagen, (b) protein expression of collagen types I and III, (c) hydroxyproline assay, and (d) cross-linking assay. LOX inhibition attenuated VO-induced increases in cardiac stress, and attenuated increases in interstitial myocardial collagen, total collagen, and protein levels of collagens I and III. Both echocardiography and catheterization measurements indicated improved cardiac function post-VO in BAPN treated rats vs. untreated. Inhibition of LOX attenuated VO-induced decreases in LV stiffness and cardiac function. Overall, our data indicate that LOX inhibition was cardioprotective in the volume overloaded heart.
Keywords: Fibrosis, collagen, heart failure, cardiac function, cardioprotection
Introduction
Cardiovascular disease (CVD) represents the leading cause of morbidity and mortality in the United States in both men and women.1,2 One of the major causes of heart failure (HF) in humans is dilated cardiomyopathy (DCM). In this study, we use the aortocaval fistula (ACF) model of biventricular volume overload (VO) to induce DCM. The ACF model is a widely used model of VO-induced HF. Many studies have demonstrated that remodeling in response to VO is characterized by an increase in ECM turnover.3–5 The ventricles progressively dilate in response to VO, resulting in a disproportionate ratio between LV end diastolic diameter and wall thickness, which elevates myocardial wall stress and promotes adverse ECM remodeling and HF.6,7
Various factors have been attributed to ECM remodeling and collagen turnover during stress, including increased expression of collagen and lysyl oxidase (LOX). The ECM is primarily composed of fibrillar collagens, type I and III. Collagens are produced by cardiac fibroblasts, which work in conjunction with cardiac myocytes during states of cardiac stress.7 Collagens play a crucial role in the maintenance of ventricular shape, size, and function.8,9 Due to its high tensile strength, small changes in collagen expression have been shown to exert marked effects on the passive mechanical properties of the heart.10 The relative expression of collagen types I and III, and the degree of collagen cross-linking, dictate the passive mechanical properties of the heart.11 For collagen to be deposited into the ECM, it must first be cross-linked.
LOX is a copper-dependent amine oxidase that plays a critical role in covalently cross-linking fibrillar collagens.12 The degree of collagen cross-linking among collagen fibrils determines the insolubility, stiffness, and resistance to degradation of the resulting fibers.11 Accordingly, increased cardiac LOX expression is found in patients with HF, and is associated with diastolic dysfunction and myocardial fibrosis.13,14 Aminonitriles, such as beta-aminopropionitrile (BAPN), have been shown to irreversibly inhibit LOX by forming a covalent adduct with the enzyme.15 The purpose of this study was to assess whether the LOX inhibiting agent, BAPN, could attenuate or reverse VO-induced alterations in LV ECM, structure and function.
Materials and methods
Studies were performed using eight-week-old male Sprague–Dawley rats (Harlan Hsd:SD) weighing ∼250 g at surgery. Rats were housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the principles of the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011), and were approved by our Institution’s Animal Care and Use Committee.
Surgical model of VO
The infrarenal abdominal ACF model was used to create VO.16 All instruments were autoclaved prior to performing surgeries. Anesthesia was induced with isoflurane (4% induction/3% maintenance, balance oxygen). A ventral abdominal laparotomy was performed to expose the aorta and caudal vena cava. Both vessels were occluded and an 18-gauge needle inserted into the exposed ventral abdominal aorta and advanced through the medial wall into the vena cava to create the fistula. Creation of a successful fistula was visually evident by the pulsatile flow of oxygenated blood into the vena cava. Sham surgeries were similarly performed, but without the creation of a fistula.
Experimental protocol
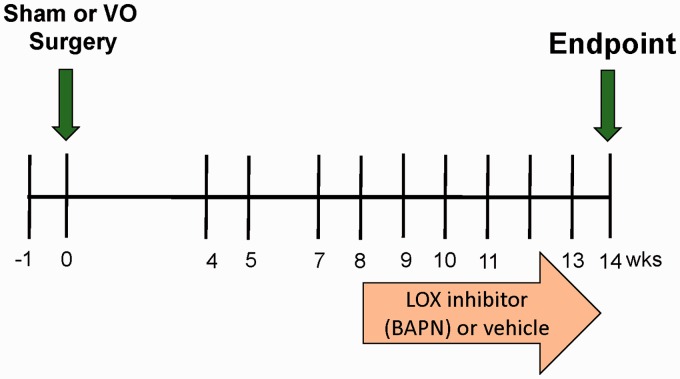
The effects of LOX inhibition on remodeling induced by VO were studied in four age-matched groups: Sham-operated controls (SHAM), Sham-operated controls treated with BAPN (SHAM + B), fistula-induced VO, and VO treated with BAPN (VO + B). Eight weeks after SHAM or ACF surgery, the LOX inhibitor, BAPN, was administered at 100 mg/kg/day via osmotic minipump (Alzet; Cupertino, CA, USA) inserted into the abdominal cavity. Eight weeks of VO causes extensive cardiac structural and ECM remodeling with associated dysfunction.11,17,18 We chose this eight-week time point to initiate LOX inhibition in rats with established LV remodeling and dysfunction (Figure 1).
Figure 1.
Experimental timeline. Echocardiography was used to assess cardiac function at the time points indicated. A baseline echo was performed a week before surgery (−1 week). At 14 weeks, left ventricular pressure–volume catheterization was performed and tissue was collected. (A color version of this figure is available in the online journal)
Echocardiography
LV dimension and function were assessed in sedated rats by echocardiography (VEVO 770; Visual Sonics, Toronto, Ontario, Canada) 1 week prior to surgery and weekly, starting at four weeks postsurgery, for 14 weeks. The rats were sedated with 1% isoflurane. LV short-axis view was used to obtain B-mode 2D images and M-mode tracings of ventral and dorsal LV wall using a two-dimensional reference sector. Echocardiography was used to assess LV internal diameter (LVID) and posterior wall thickness (LVPW) at both diastole (d) and systole (s). Fractional Shortening was calculated as (LVIDd – LVIDs)/LVIDd. Eccentric index was evaluated by (2 × LVPWd)/LVIDd. All measurements were performed on multiple cardiac cycles and averages were calculated for each time point.
Pressure–volume analysis
At the experimental end point of 14 weeks, LV pressure–volume loop analysis was used to assess cardiac function. The rats were intubated and attached to a ventilator. The chest was opened to expose the apex of the heart. A small needle was used to puncture the heart at the apex, and then a Scisense pressure–volume catheter (product #: FTS-1912B-9018, fixed segment for SHAM; FTE-1918B-E218, multisegment for VO) was inserted into the LV. After establishing stable baseline function for ∼5 min, pressure–volume loop data were collected for analysis of cardiac functional parameters. Data were acquired using an iWorx 308 T data acquisition system and Labscribe software. After establishing steady-state pressure–volume loops, load independent parameters of cardiac function were established by occluding the vena cava for ∼3 s using a cotton tip swab while continuously recording pressure–volume loops. After loop recording, the chest cavity was further opened to expose the base of the heart and aorta. Cardiac output was measured using a Doppler flow probe (model 3SB; Transonic Systems; Ithaca, NY, USA) on the aortic arch. Data analysis was performed using Labscribe software with built-in pressure–volume loop analysis functions. To calibrate volume data, the catheter was inserted into blood-filled cuvettes of known volume, the output recorded, and a calibration curve generated. The data were also corrected using cardiac output measures from the Transonic flow probe (alpha correction). All measures of cardiac function were evaluated from a minimum of 10 consecutive pressure–volume loops. After functional assessment, the heart was removed, placed in ice-cold phosphate-buffered saline (PBS), and the left and right ventricles were separated and weighed. A portion of the mid-LV region was fixed with 4% paraformaldehyde, and the remainder was snap frozen in liquid nitrogen for further assay. Lung wet weight was also recorded.
Analysis of the collagen matrix
Interstitial collagen volume fraction (CVF) was measured in mid-LV sections. LV sections were fixed in 4% paraformaldehyde for 24 h, and then embedded in paraffin. Five-micron sections were cut, attached to slides, and stained with collagen specific Picrosirius Red (PSR). The CVF of the section was determined by analyzing 15 interstitial regions from two sections of each heart. Perivascular collagen was excluded from the measurements. Images were captured (20×) and processed using a Nikon Eclipse model #TE2000-U fluorescence microscope and NIS Elements Software. The mean interstitial CVF was expressed as a percentage of the total area for each LV section, and then group averaged.
Hydroxyproline assay
Total collagen content was quantitatively estimated by measuring hydroxyproline content of myocardial free wall samples. A total of 30–40 mg of wet LV tissue were dried at 65℃ overnight and hydrolyzed in 6N HCl for 24 h at 110℃. The hydrosylate solution was charcoal filtered, evaporated and resuspended in 1 ml of citrate buffer. The solution was then reacted with isopropanol, antioxidant solution (Chloramine T), and Ehrlich Reagent (p-dimethylamino benzaldehyde). Absorbance was measured at 540 nM and compared to curve of known hydroxyproline standards. Hydroxyproline levels were expressed as ug of hydroxyproline per mg of dry tissue.
LOX-dependent collagen cross-linking
Pyridinoline was quantified in mid-LV heart tissue using commercial assay (Quidel PYD kit, 8019; SD, CA, USA). PYD assay was performed on diluted hydrosylates of LV.
Western blot analysis
Tissue was homogenized with RIPA buffer and HALT Protease Inhibitor Cocktail with EDTA (Pierce; Rockford, IL, USA). Western blots were performed as previously described.19 Enhanced chemiluminescence was used to visualize the immunostaining. Antibodies used for this study include: collagen I (Abcam; Ab34710), collagen III (Abcam; Ab7778), elastin (Santa Cruz; SC-17581), and GAPDH (Abcam; Ab9485). Data were collected and analyzed using a Carestream Gel Logic 2200 Pro imaging system.
Data and statistical analysis
Statistical analyses were performed using Graphpad software (San Diego, CA, USA). Grouped data comparisons were made by one-way analysis of variance. When a significant F ratio (P < 0.05) was obtained, inter-group comparisons were made using Dunnett’s post-test.
Results
Measurements of heart, lung and body weight per group
There were no significant differences in body weight (BW) between the four analyzed groups: SHAM, SHAM + B, VO, and VO + B. VO induced significant increases in the LV, RV, and lung to BW ratios, (92% increase, 121% increase, and 36% increase vs. SHAM, respectively; Table 1). VO also induced significant increases in lung wet weight to dry weight ratios (16% increase vs. SHAM; Table 1). There was a significant decrease in LV and lung to BW ratios between the VO-treated group and VO (12 and 19% decrease, respectively vs. VO; Table 1). LOX inhibition in VO also induced a decrease in wet weight to dry weight ratio when compared to untreated VO (6% decrease vs. VO; Table 1). However, the BAPN-treated VO group still had significantly increased wet weight to dry weight ratios when compared with SHAM (8% increase; Table 1).
Table 1.
Group averaged weights
| SHAM | SHAM + B | VO | VO + B | |
|---|---|---|---|---|
| LV/BW (mg/kg) | 1.96 ± 0.06 | 2.01 ± 0.16 | 3.77 ± 0.26* | 3.31 ± 0.14*† |
| RV/BW (mg/kg) | 0.52 ± 0.04 | 0.52 ± 0.06 | 1.19 ± 0.11* | 1.06 ± 0.08* |
| Lung/BW (mg/kg) | 4.02 ± 0.29 | 3.94 ± 0.47 | 5.45 ± 0.29* | 4.43 ± 0.37*† |
| Lung Wet Weight/ Dry Weight (mg/mg) | 1.22 ± 0.09 | 1.22 ± 0.08 | 1.41 ± 0.04* | 1.32 ± 0.01*† |
| BW (g) | 446 ± 6 | 428 ± 37 | 468 ± 34 | 464 ± 21 |
Reported values are expressed as mean ± SD (BW, body weight; LV, left ventricle; RV, right ventricle; SHAM, sham operated; VO, volume overload; B, lysyl oxidase inhibitor. Statistical significance denoted as P < 0.05 vs. SHAM (*) and vs. VO (†)).
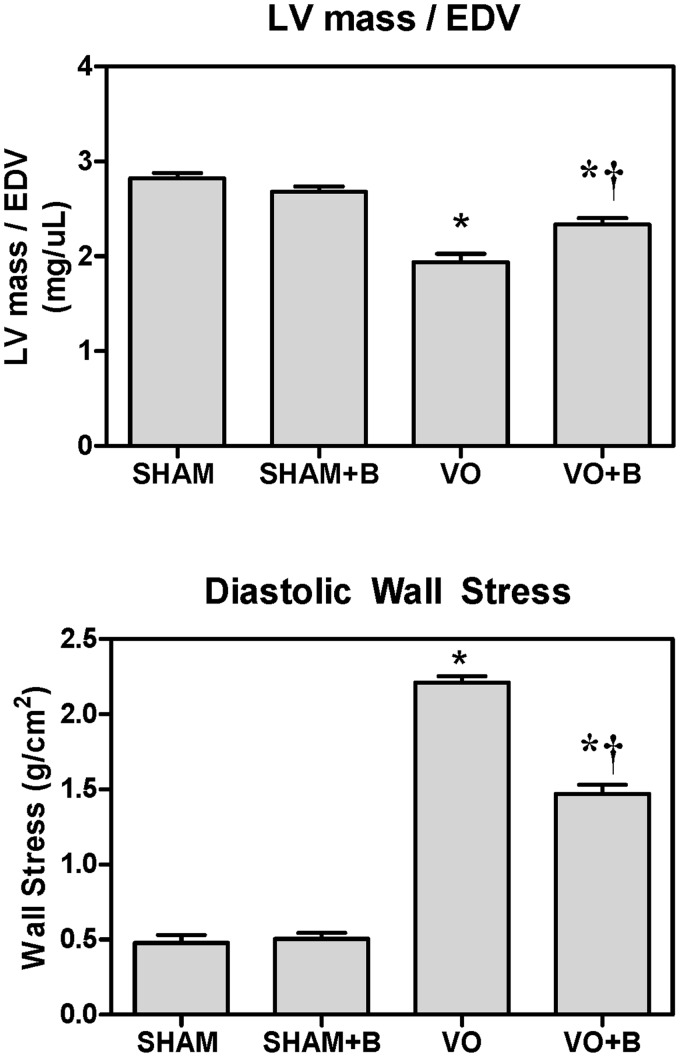
LOX inhibition attenuated VO-induced cardiac wall stress
To assess LV wall stress, we utilized two different calculations. The first establishes a ratio between LV mass and EDV. Based on Laplace’s law, this ratio is inversely proportional to cardiac wall stress. VO significantly decreased LV mass-to-volume ratio (32% decrease in VO vs. SHAM, Figure 2). LOX inhibition attenuated this VO-induced reduction in mass-to-volume ratio (21.5% decrease in VO + B vs. VO). LV mass was obtained by weighing the tissue using a laboratory scale. EDV was measured using a pressure–volume catheter. LV mass and EDV were also calculated via echocardiography (data not shown), and similar results for LV mass-to-volume ratio were obtained using both methods. The second means of estimating cardiac wall stress used the following equation:
| (1) |
where, P is LV diastolic pressure (mmHg), V is LVEDV (ml), and M is LV mass (g). Using equation (1) to calculate wall stress, VO caused a significant increase (293% increase in VO vs. SHAM, Figure 2). LOX inhibition attenuated the VO-induced increase, but did not completely restore wall stress to SHAM levels (62% decrease in VO + B vs. VO, Figure 2).
Figure 2.
Lysyl oxidase (LOX) inhibition attenuated volume overload (VO)-induced increases in cardiac wall stress. TOP: A ratio between left ventricular (LV) mass and end diastolic volume (EDV) was calculated to assess cardiac stress. LV mass was determined from wet weight and LV EDV was determined by pressure–volume catheterization. The ratio decreased in VO, indicative of increased cardiac stress. LOX inhibition with beta-aminoproprionitrile (BAPN;B) attenuated these decreases. Similar results and statistical significance were obtained using LV mass and volume estimated by echocardiography (data not shown). BOTTOM: A calculation of diastolic stress using Equation 1 confirms that LOX inhibition attenuated VO-induced increases in cardiac stress. Statistical significance denoted as P < 0.05 vs. Sham (*) and VO (†); n = 4–8 per group
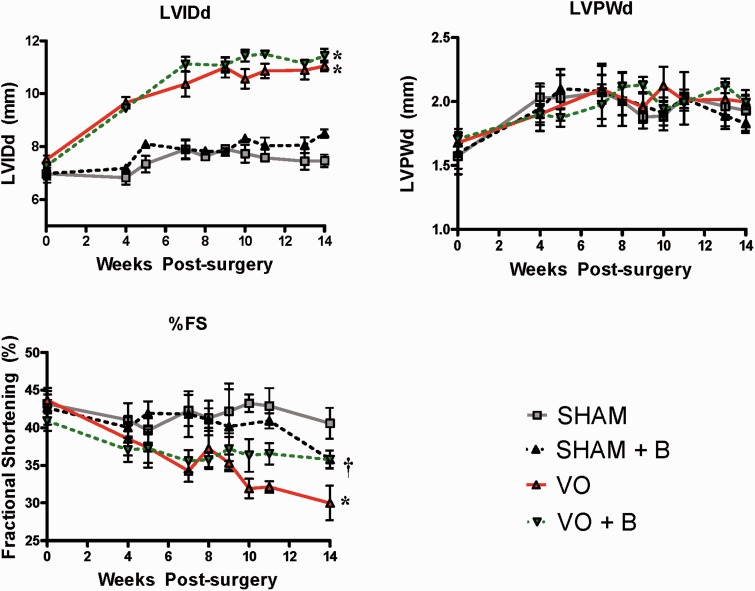
LOX inhibition attenuated VO-induced cardiac dysfunction
By ultrasound echocardiography, there were no significant changes in wall thickness (i.e. LVPWd), chamber dilation (i.e. LVIDd) or fractional shortening (FS%) in the SHAM treated group when compared to SHAM (Figure 3). However, VO significantly increased LVIDd (67% increase vs. SHAM). VO caused a significant decrease in FS compared to SHAM (26% decrease vs. SHAM; P < 0.05). There were no significant changes in LVIDd or LVPWd in the VO + B group when compared with VO. However, the VO + B group had significantly higher FS when compared with VO (19% increase vs. VO; P < 0.05). Although LOX inhibition did not fully restore FS to untreated SHAM levels, the difference was not statistically significant (12% decrease in VO + B vs. SHAM).
Figure 3.
Echocardiography assessment determined that lysyl oxidase (LOX) inhibition attenuated cardiac dysfunction following volume overload (VO). VO induced significant increases in left ventricle chamber size (LVIDd), but had no significant effect on wall thickness (LVPWd). VO caused a significant decrease in fractional shortening (FS) when compared to SHAM. LOX inhibition (B) did not significantly alter LVPWd or LVIDd in either SHAM or VO rats. However, LOX inhibition did significantly attenuate the decrease in %FS caused by VO. Statistical significance denoted as P < 0.05 vs. Sham (*) and VO (†). (A color version of this figure is available in the online journal)
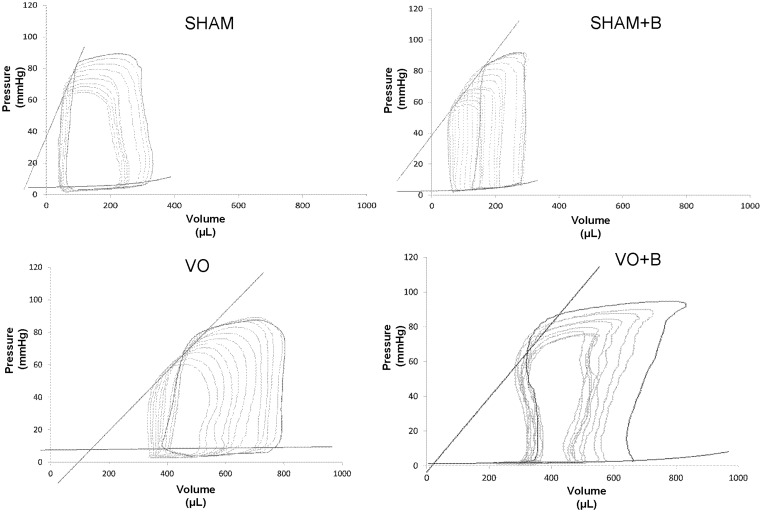
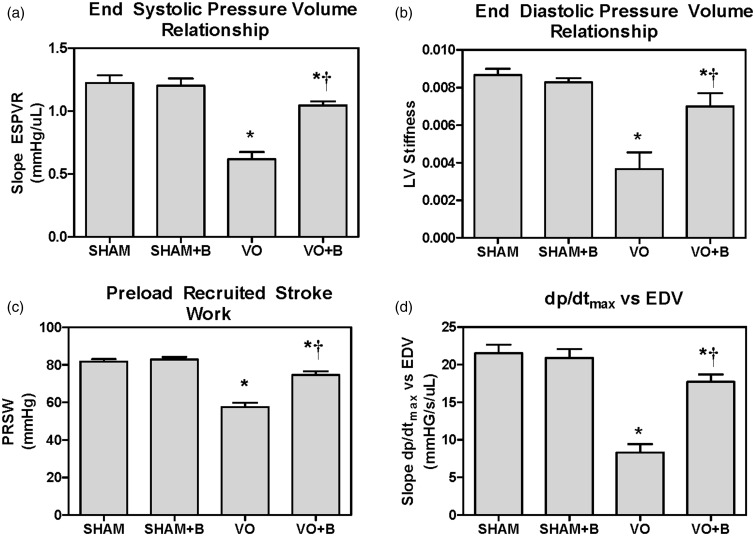
LV dimension and function were also assessed using pressure–volume catheterization at the 14-week postsurgical endpoint. Representative pressure–volume loops for each group are shown in Figure 4. Both VO groups developed significant LV dilatation, indicated by a rightward shift of the pressure–volume loop. Steady state data were collected for each of the groups and compiled in Table 2. There were no significant differences in HR, ESP, or Tau (LV relaxation time constant) between any of the groups. LOX inhibition in SHAM had no significant effect on any of the measured parameters. VO caused significant increases in CO, ESV, EDV, EDP, and SV, which were associated with a significant decrease in EF (18% decrease vs. SHAM). LOX inhibition did not prevent the VO-induced increases in CO, ESV, EDV, EDP, and SV. However the reduction in EF in treated VO + B rats was less than that found in untreated VO, suggesting that LOX inhibition improved cardiac function. Load independent parameters of cardiac function and contractility were also calculated for each group using the pressure–volume catheterization data. ESPVR, EDPVR, PRSW, and dP/dt vs. EDV slope were significantly decreased in VO (50, 59, 30, and 61.5% decrease, respectively vs. SHAM; Figure 5), indicative of reduced cardiac function. LOX inhibition attenuated these VO-induced changes, but did not fully restore cardiac function to SHAM levels.
Figure 4.
Representative pressure–volume loops for each group collected by left ventricular (LV) pressure–volume catheterization. Sham-operated (SHAM) controls were not significantly altered by the lysyl oxidase inhibitor (B). Volume overload (VO) caused significant LV dilatation (rightward shift), which was not prevented by lysyl oxidase inhibition (B)
Table 2.
Steady State parameters of cardiac function as measured by pressure–volume catheterization
| SHAM | SHAM + B | VO | VO + B | |
|---|---|---|---|---|
| HR (bpm) | 318 ± 33 | 319 ± 43 | 357 ± 21 | 321 ± 44 |
| CO (ml/min) | 62 ± 8 | 64 ± 11 | 147 ± 9.1* | 118 ± 9.2*,† |
| ESV (ul) | 131 ± 36 | 145 ± 51 | 381 ± 21* | 310 ± 58* |
| EDV (ul) | 310 ± 48 | 321 ± 110 | 801 ± 38* | 680 ± 118* |
| ESP (mmHg) | 86.0 ± 17.2 | 82.9 ± 5.9 | 81.6 ± 12.6 | 89.4 ± 12.9 |
| EDP (mmHg) | 4.4 ± 0.2 | 4.2 ± 1.4 | 12.6 ± 5.0* | 8.2 ± 3.0* |
| SV (ul/beat) | 195 ± 19 | 199 ± 24 | 411 ± 12* | 385 ± 13* |
| EF (%) | 62.9 ± 1.6 | 62.1 ± 2.3 | 51.9 ± 1.4* | 56.7 ± 1.9*,† |
| Tau (ms) | 16.0 ± 2.2 | 15.3 ± 1.9 | 16.1 ± 0.7 | 17.3 ± 1.2 |
SHAM = sham operated; VO = volume overload; B = LOX inhibitor; HR = heart rate; CO = cardiac output; ESV = end systolic volume; EDV = end diastolic volume; ESP = end systolic pressure; EDP = end diastolic pressure; SV = stroke volume; EF = ejection fraction; Tau = left ventricular relaxation time constant. Statistical significance denoted as P < 0.05 vs. SHAM (*) and vs. VO (†).
Figure 5.
Left ventricular (LV) pressure–volume catheterization indicates that lysyl oxidase (LOX) inhibition using beta-aminoproprionitrile (BAPN; B) improved contractility and cardiac function in the volume overloaded heart. Volume overload (VO) induced significant decreases in (A) end systolic pressure–volume relationship (ESPVR), (B) end diastolic pressure–volume relationship (EDPVR), (C) preload recruited stroke work (PRSW), and (D) the rate of LV pressure change per unit time (dP/dt max) vs. end diastolic volume (EDV). LOX inhibition attenuated the VO-induced decreases in each of these parameters. Statistical significance denoted as P < 0.05 vs. Sham (*) and VO (†)
LOX inhibition attenuated interstitial fibrosis
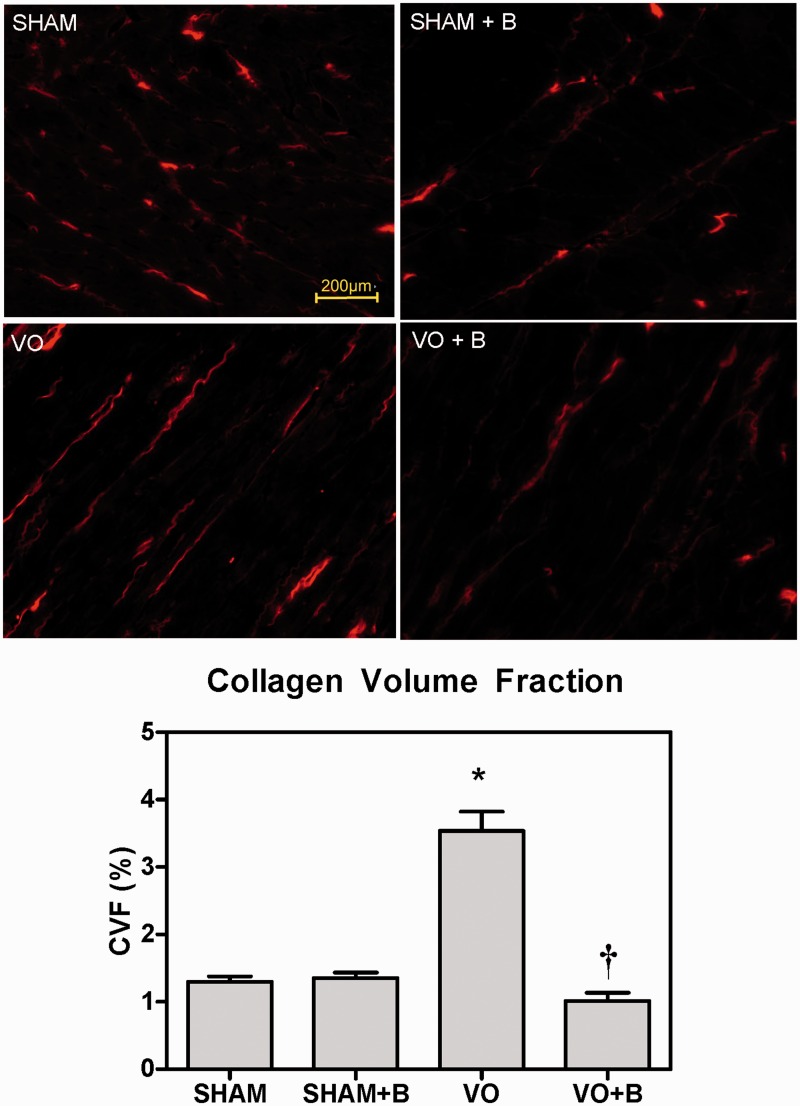
Interstitial CVF was measured and assessed from PSR stained mid-LV sections at 14 weeks postsurgery. LOX inhibition initiated at eight weeks did not alter CVF in SHAM animals. When compared to SHAM, hearts of VO animals had significantly increased interstitial CVF (172% increase vs. SHAM; Figure 6). VO animals treated with LOX inhibitor had CVF levels similar to SHAM (71% decrease in VO + B vs. VO; Figure 6).
Figure 6.
Lysyl oxidase (LOX) inhibition attenuated volume overload (VO)-induced increases in interstitial collagen. Representative picrosirius red (PSR)-stained left ventricular sections at 14 weeks postsurgery and calculated collagen volume fraction (CVF) are shown for each group. CVF was increased in the VO group compared to SHAM. LOX inhibition (B) attenuated the VO-induced increases in collagen. Statistical significance denoted as P < 0.05 vs. Sham (*) and VO(†)
LOX inhibition attenuated increases in total collagen content
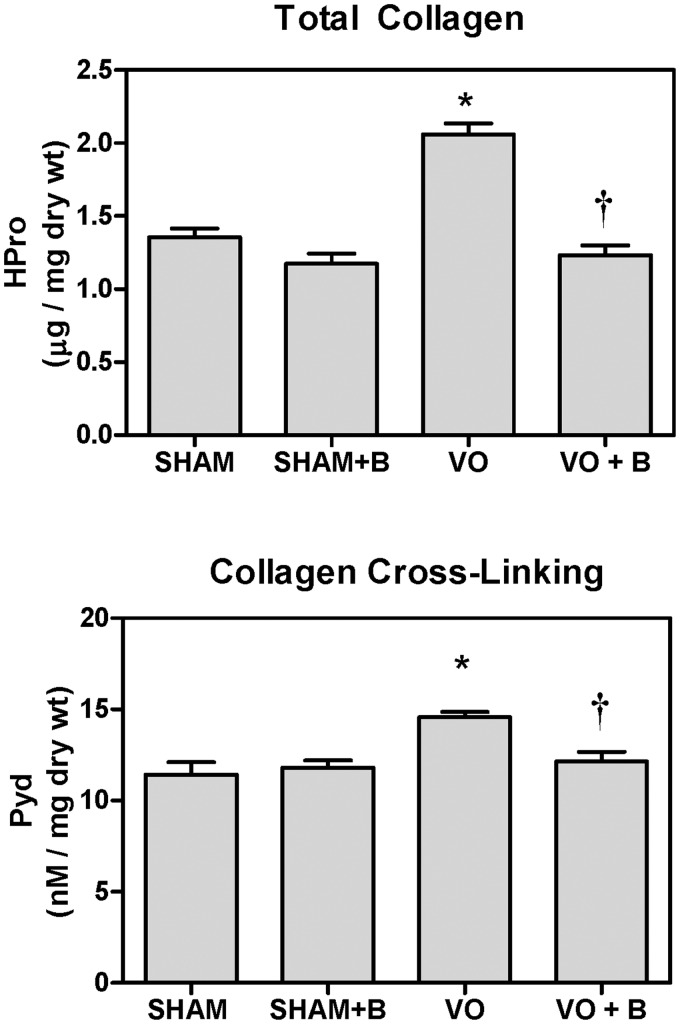
To further assess alterations in collagen, a hydroxyproline assay was performed on LV free wall tissue. Hydroxyproline is a reliable marker of total collagen content.20 There were no significant differences in total collagen content between the SHAM and SHAM + B treated groups. When compared to SHAM, the VO group exhibited a significant increase in total collagen content (33% increase vs. SHAM; Figure 7). LOX inhibition blocked this VO-induced increase in total collagen (NS vs. SHAM).
Figure 7.
Lysyl oxidase (LOX) inhibition (B) attenuated volume overload (VO)-induced increases in left ventricular (LV) total collagen and collagen cross-linking. Hydroxyproline (Hpro) concentration in mid-LV free wall was significantly increased in the VO group, but not different from SHAM in VO + B group. Pyridinoline (PYD) levels are a measure of LOX-dependent collagen cross-links. Fourteen weeks of VO caused a significant increase in LV PYD levels. Treatment with the LOX inhibitor attenuated the increase in PYD (P < 0.05 vs. Sham (*) and VO(†))
LOX inhibition attenuated VO-induced increases in collagen cross-linking
Pyridinoline (PYD) assay was used to assess collagen cross-links formed by LOX. There were no significant changes in PYD levels between the SHAM and SHAM + B groups (Figure 7). There was a modest, but significant increase in LV PYD in the VO group compared to the SHAM (26% increase; P < 0.05). PYD levels in the VO + B treated group were not different from SHAM.
LOX inhibition blocks VO-induced increases in collagen I and III, but had no effect on elastin protein expression
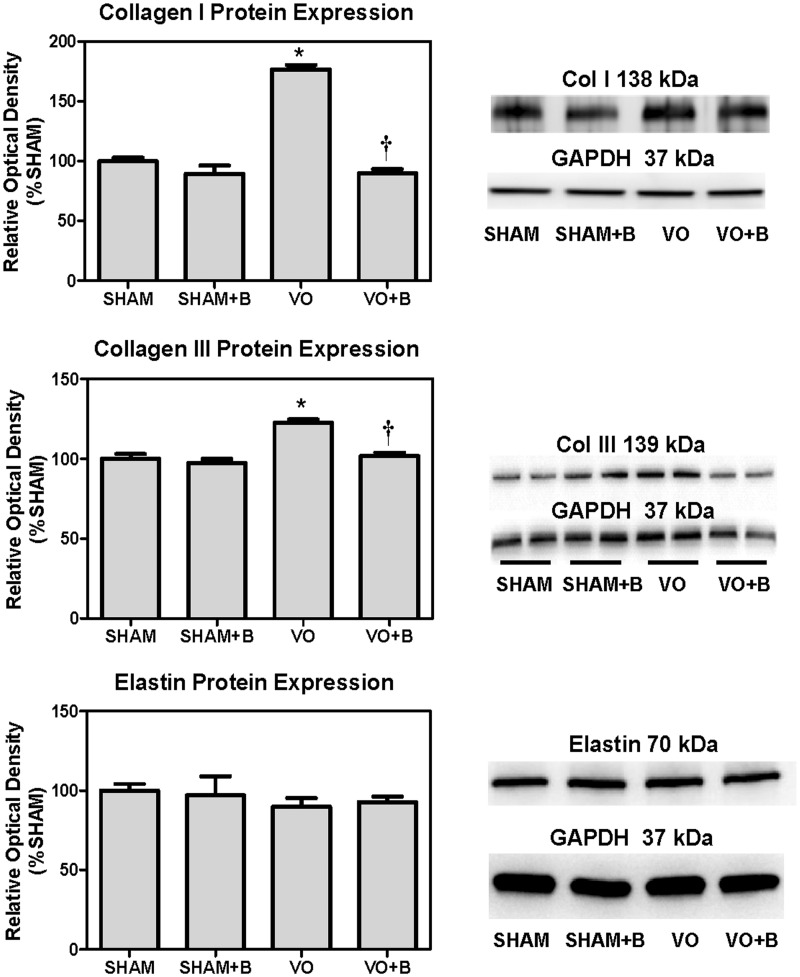
There were no significant changes in the cardiac expression of collagen I or collagen III in the SHAM + B treated group as compared to SHAM (Figure 8). Fourteen weeks of VO caused a significant increase in the LV expression of both collagen I and III (120 and 23% increase vs. SHAM, respectively; P < 0.05). When compared to VO, the VO + B treated group had a significant decrease in collagen I and III, resulting in levels that were not different from SHAM. There were no significant differences in the protein expression of elastin between the groups (SHAM, SHAM + B, VO, and VO + B).
Figure 8.
Lysyl oxidase (LOX) inhibition (B) attenuated volume overload (VO)-induced increases in collagen I and III, but did not alter elastin expression. Protein expression of collagen I and collagen III in left ventricular (LV) extracts was determined by western blot analysis (n = 4 to 6 per group). VO significantly increased LV expression of both collagen types I and III, when compared to SHAM. Rats with volume overload that were treated with LOX inhibitor had collagen I and III levels similar to SHAM. LV elastin expression was similar among all groups (P < 0.05 vs. Sham (*) and VO(†))
LOX inhibition reverses rather than attenuates VO-induced increases in interstitial collagen
To determine if LOX inhibition reversed rather than prevented the volume-overload induced changes in LV interstitial collagen, we assessed interstitial CVF at six weeks postsurgery, which was two weeks before initiation of treatment with the LOX inhibitor. There were no significant differences in interstitial CVF between the SHAM groups (six-week SHAM vs. 14-week SHAM). However, six weeks of VO caused a significant increase in CVF (six-week VO = 2.69 ± 0.23%, an 82% increase vs. SHAM). These findings indicate that CVF was significantly increased due to VO prior to the initiation of treatment with LOX inhibitor.
Discussion
VO causes compensatory remodeling of the ventricles driven by increased ventricular wall stress. Our findings confirm previous studies which found that rats with ACF-induced VO exhibit ventricular hypertrophy, chamber dilatation, and collagenous ECM alterations.11,21–23 These studies and ours demonstrated a progressive decline in cardiac function caused by chronic VO. Here, we investigated the efficacy of a LOX inhibitor to attenuate the adverse ventricular remodeling and cardiac dysfunction associated with chronic VO. Eight weeks after surgical induction of VO, BAPN treatment was initiated, which covalently and irreversibly binds to the active site of LOX inhibiting its activity and ability to cross-link collagen.24–27
Several factors dictate the degree of stress imposed on the ventricular wall, including: pressure in the chamber, radius of the chamber, and wall thickness. According to Laplace’s Law, the stress imposed on the walls of the chamber is directly proportional to the radius and the pressure of the chamber, but inversely proportional to the thickness of its walls.28,29 We found that VO increased wall stress, which was attenuated by LOX inhibition. An increase in mass-to-volume ratio is an indicator that compensatory remodeling mechanisms are normalizing wall stress.21 Rats with VO developed a decrease in LV mass-to-volume ratio, indicating an unsuccessful remodeling response to VO and an inability to normalize wall stress. This result is confirmed by studies that show that the inability to fully compensate for wall stress leads to progressive ventricular remodeling and eventual HF.21,30 LOX inhibition attenuated VO-induced decreases in mass-to-volume ratio, indicating a more successful myocardial remodeling response to the VO insult, and thus, reduced wall stress relative to untreated rats.
Data obtained via pressure–volume catheterization found that chronic VO stress caused a decrease in contractility. Clinical studies on patients with LV hypertrophy demonstrate that increased wall stress eventually leads to decreased contractility and impaired cardiac pump function.31 In our study, VO led to a significant decrease in contractility, as confirmed by previous studies.23,32–36 A possible explanation for decreased contractility could be depressed sarcomere cross-bridging.37 The prolonged decrease in contractility eventually leads to myocardial failure.38 We found that LOX inhibition attenuated the VO-induced depression in contractility demonstrated by significant increases in ESPVR, PRSW and dP/dt vs. EDV indices, when compared to untreated VO. This is the first study to demonstrate a positive systolic effect of LOX inhibition on VO-induced dysfunction. This improvement in contractility is surprising for two reasons: (a) levels of ventricular collagen and degree of cross-linking are thought to primarily impact diastolic function, and (b) reducing cross-linking in the volume overloaded heart could potentially reduce ventricular wall integrity and promote dilatation. However, our data clearly indicate an improvement in systolic function in VO-stressed rats treated with LOX inhibitor.
Stiffness of the LV was also assessed by measuring EDPVR using pressure–volume catheterization. Consistent with previous findings, VO led to decreased stiffness of the ventricle, which is associated with increased compliance.10,21,39,40 LOX inhibition attenuated the VO-induced effects on LV wall stiffness. Diastolic chamber properties are largely determined by the relative expression and distribution of collagen in the heart. The protective effects of LOX inhibition on diastolic function are likely mediated by its impact on collagen (discussed below).
Cardiac structure and function are directly affected by the composition of the ECM, and alterations of the ECM promote the progression of HF.11,41,42 To better understand what causes VO-induced cardiac dysfunction and improvement of function following LOX inhibition, we analyzed LV levels of collagen types I and III, which are the primary components of the cardiac ECM. The relative amounts of collagen types I and III in the heart largely determine the physical properties of the tissue, which impacts function, including ventricular compliance and diastolic stiffness.43 In our study, rats with VO developed increased levels of myocardial collagen, as demonstrated by both histological and biochemical assessments. This increase was likely achieved through fibroblast activation, which initiates compensatory mechanisms to counteract increased preload and wall stress by increasing wall strength (i.e., through collagen deposition). Protein expression of both collagen types I and III were upregulated in response to VO. Each of these alterations in collagen was attenuated by LOX inhibition, and was associated with an improvement in both systolic in diastolic cardiac function. The increase in collagen found in the untreated VO group appears discordant with the diastolic findings of reduced stiffness. Because of these unexpected results, we also evaluated LV elastin expression, due to its impact on cardiac tissue mechanical properties. However, we found no changes in elastin between any of our groups. Although our data do not provide definitive proof, a potential explanation of the mismatch between LV collagen and stiffness is that the spatial distribution of collagen in the VO heart is such that it does not alter mechanical properties as expected. Another explanation could be that the elastin or collagen is fragmented in the tissue, or the impact of enlarged LV chamber size on tissue stiffness.44 Overall, these findings strongly indicate that LOX is a key regulator of adverse collagenous ECM alterations in the volume overloaded heart.
Pulmonary edema, a condition in which fluid builds up in the lungs, has been shown to result from myocardial failure and, specifically, VO.45 The untreated VO rats developed pulmonary edema, which was attenuated following LOX inhibition. These data suggest that LOX inhibition slowed or prevented the progression of HF by VO.
In our studies, we initiated treatment with the LOX inhibitor after eight weeks of VO, allowing us to analyze whether the inhibition would prevent the progression of HF or reverse established disease. We found increased collagen staining in the LV of rats after six weeks of VO, which further increased by 14 weeks. LOX inhibition initiated at eight weeks returned collagen staining to levels similar to Sham-operated controls. However, while LOX inhibition prevented further depression of cardiac function beyond eight weeks, inhibition did not fully restore cardiac function. These data indicate that LOX inhibition prevents the progression of HF, and can reverse many of the collagenous ECM alterations associated with VO. One question that remains unanswered is “How does LOX inhibition improve systolic function?”. One potential mechanism is that elevated or excessive LOX activity could produce increased oxidative load and injury to the cardiac tissue. The enzymatic action of LOX on collagen produces hydrogen peroxide. The significance of this hydrogen peroxide production, and how it may negatively impact cardiomyocyte survival and function, is not known. Also, studies have found that LOX can bind to, and inhibit the signaling of, growth factors, including TGF-β1 and Fibroblast Growth Factor (FGF-2), albeit in non-cardiac cells.46,47 The detrimental effects of TGF inhibition on the stressed heart were demonstrated using a dominant negative TGF-receptor mouse model.48 In response to pressure overload, mice with reduced TGF signaling rapidly developed ventricular dilation and HF. The adverse effects of excess LOX activity on the stressed heart warrant further study.
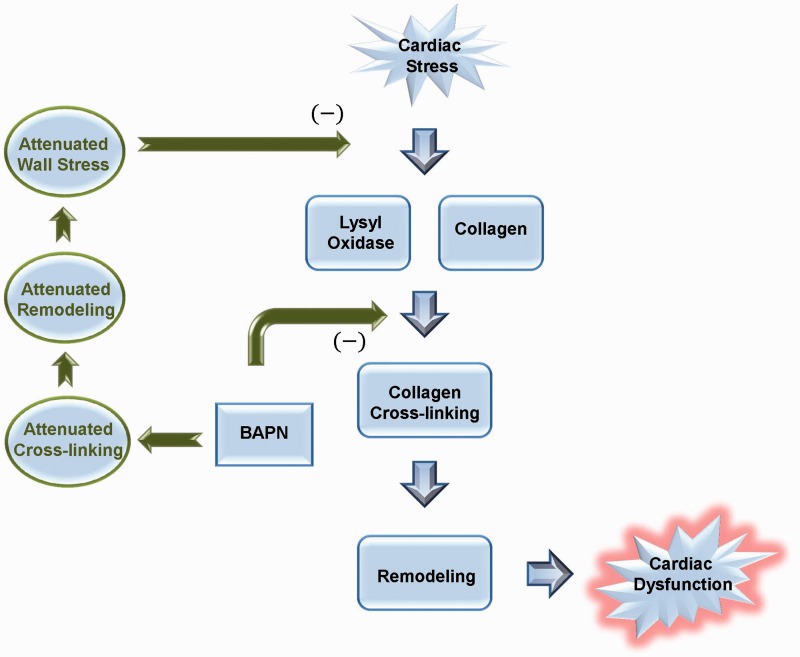
The major findings of this study are outlined in Figure 9. We found that LOX inhibition improved cardiac function and reversed many of the adverse ECM collagen changes caused by chronic VO. LOX inhibition reduced ventricular wall stress and increased mass-to-volume ratio, indicating a more successful compensatory adaptation to VO following treatment. LOX inhibition prevented progression of HF in our model, as demonstrated by maintenance of cardiac function and reduction of pulmonary edema. Our findings identify the importance of LOX as a key modulator of ECM remodeling in heart disease. Further studies are needed to determine the mechanisms responsible for the cardioprotective effects of LOX inhibition and to evaluate LOX as a potential therapeutic target for the treatment or prevention of HF.
Figure 9.
The beneficial effects of lysyl oxidase (LOX) inhibition in the rat model of volume overload. Volume overload (VO)-induced cardiac stress increases both LOX and collagen expression. The increased deposition of collagen results in extracellular matrix (ECM) remodeling and progressive cardiac dysfunction. Inhibiting LOX with beta-aminoproprionitrile (BAPN), attenuates cross-linking and ECM remodeling, leading to reduced ventricular wall stress, which halts further progression of cardiac dysfunction. (A color version of this figure is available in the online journal)
Author contributions and Acknowledgments
All authors participated in the collection and analysis of data, and review of the manuscript; E.C.E.H. drafted the manuscript, performed and analyzed collagen III blots, analyzed PYD assay, and contributed to the experimental design. M.C.E.H. performed and analyzed CVF on 14 weeks animals and hydroxyproline assay. V.K.N. performed and analyzed collagen I western blots and CVF on six-week LV tissue. J.D.G. contributed to experimental design, collection and analysis of data, and writing and editing of the manuscript. We would also like to acknowledge Ms. Conni Corll for her expertise and assistance in performing the PYD assay. Funding for these studies was provided by the American Heart Association Greater Southeast Affiliate #11GRNT7700002 (PI: Gardner), NIH/NIAAA R21 # 1R21AA022690-01 (PI: Gardner), and NIH/NCRR COBRE #2P20RR018766-09 (PI: Kapusta).
Declaration of Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007; 13: 1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal 2006; 8: 1737–44. [DOI] [PubMed] [Google Scholar]
- 3.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 2010; 122: 2727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci 2010; 87: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 2007; 117: 568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet 2006; 367: 356–67. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson KR, Stewart JA, Jr., Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol 2010; 48: 564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janicki JS, Matsubara BB, Kabour A. Myocardial collagen and its functional role. Adv Exp Med Biol 1993; 346: 291–8. [DOI] [PubMed] [Google Scholar]
- 9.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol 2003; 284: H122–32. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi RR, Herron T, Simmons R, Shore D, Kumar P, Sethia B, Chua F, Vassiliadis E, Kentish JC. Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation 2010; 121: 979–88. [DOI] [PubMed] [Google Scholar]
- 11.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 2006; 30: 604–10. [DOI] [PubMed] [Google Scholar]
- 12.Kagan HM, Vaccaro CA, Bronson RE, Tang SS, Brody JS. Ultrastructural immunolocalization of lysyl oxidase in vascular connective tissue. J Cell Biol 1986; 103: 1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem 2008; 307: 159–67. [DOI] [PubMed] [Google Scholar]
- 14.Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension 2009; 53: 236–42. [DOI] [PubMed] [Google Scholar]
- 15.Tang SS, Trackman PC, Kagan HM. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem 1983; 258: 4331–8. [PubMed] [Google Scholar]
- 16.Voloshenyuk TG, Gardner JD. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am J Physiol Regul Integr Comp Physiol 2010; 299: R683–93. [DOI] [PubMed] [Google Scholar]
- 17.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 2010; 298: H497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail 2002; 8: 101–7. [DOI] [PubMed] [Google Scholar]
- 19.El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res 2014; 38: 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 1991; 88: 1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner JD, Brower GL, Janicki JS. Effects of dietary phytoestrogens on cardiac remodeling secondary to chronic volume overload in female rats. J Appl Physiol (1985) 2005; 99: 1378–83. [DOI] [PubMed] [Google Scholar]
- 22.Carabello BA, Nolan SP, McGuire LB. Assessment of preoperative left ventricular function in patients with mitral regurgitation: value of the end-systolic wall stress-end-systolic volume ratio. Circulation 1981; 64: 1212–7. [DOI] [PubMed] [Google Scholar]
- 23.Guggilam A, Hutchinson KR, West TA, Kelly AP, Galantowicz ML, Davidoff AJ, Sadayappan S, Lucchesi PA. In vivo and in vitro cardiac responses to beta-adrenergic stimulation in volume-overload heart failure. J Mol Cell Cardiol 2013; 57: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol 2010; 299: H1–9. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 1998; 16: 387–98. [DOI] [PubMed] [Google Scholar]
- 26.Kato S, Spinale FG, Tanaka R, Johnson W, Cooper Gt, Zile MR. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol 1995; 269(3 Pt 2): H863–8. [DOI] [PubMed] [Google Scholar]
- 27.Roy R, Polgar P, Wang Y, Goldstein RH, Taylor L, Kagan HM. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: interactions among TGF-beta, IL-1 beta, and prostaglandin. Eur J Cell Biochem 1996; 62: 411–7. [DOI] [PubMed] [Google Scholar]
- 28.Drazner MH. The progression of hypertensive heart disease. Circulation 2011; 123: 327–34. [DOI] [PubMed] [Google Scholar]
- 29.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102: 470–9. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein S, Ali AS, Sabbah H. Ventricular remodeling. Mechanisms and prevention. Cardiol Clin 1998; 16: 623–32, vii–viii. [DOI] [PubMed] [Google Scholar]
- 31.Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, Dahlof B, Devereux RB. Losartan Intervention For Endpoint Reduction in Hypertension S. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2002; 39: 739–43. [DOI] [PubMed] [Google Scholar]
- 32.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: implications for management. Circulation 2008; 118: 2298–303. [DOI] [PubMed] [Google Scholar]
- 33.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation 2006; 113: 296–304. [DOI] [PubMed] [Google Scholar]
- 34.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol 1996; 271(5 Pt 2): H2071–8. [DOI] [PubMed] [Google Scholar]
- 35.Brower GL, Janicki JS. Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol Heart Circ Physiol 2001; 280: H674–83. [DOI] [PubMed] [Google Scholar]
- 36.Carabello BA, Zile MR, Tanaka R, Cooper Gt. Left ventricular hypertrophy due to volume overload versus pressure overload. Am J Physiol 1992; 263(4 Pt 2): H1137–44. [DOI] [PubMed] [Google Scholar]
- 37.Wessely R, Henke A, Zell R, Kandolf R, Knowlton KU. Low-level expression of a mutant coxsackieviral cDNA induces a myocytopathic effect in culture: an approach to the study of enteroviral persistence in cardiac myocytes. Circulation 1998; 98: 450–7. [DOI] [PubMed] [Google Scholar]
- 38.de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res 1998; 37: 367–80. [DOI] [PubMed] [Google Scholar]
- 39.Heller LJ, Mohrman DE, Prohaska JR. Decreased passive stiffness of cardiac myocytes and cardiac tissue from copper-deficient rat hearts. Am J Physiol Heart Circ Physiol 2000; 278: H1840–7. [DOI] [PubMed] [Google Scholar]
- 40.Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA, Jr., Murray DB, Chancey AL. Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res 2006; 69: 657–65. [DOI] [PubMed] [Google Scholar]
- 41.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007; 87: 1285–342. [DOI] [PubMed] [Google Scholar]
- 42.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 1989; 13: 1637–52. [DOI] [PubMed] [Google Scholar]
- 43.Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res 2003; 57: 632–41. [DOI] [PubMed] [Google Scholar]
- 44.Hutchinson KR, Saripalli C, Chung CS, Granzier H. Increased myocardial stiffness due to cardiac titin isoform switching in a mouse model of volume overload limits eccentric remodeling. J Mol Cell Cardiol 2015;79:104--14. [DOI] [PMC free article] [PubMed]
- 45.Carlson RW, Schaeffer RC, Jr., Michaels SG, Weil MH. Pulmonary edema fluid. Spectrum of features in 37 patients. Circulation 1979; 60: 1161–9. [DOI] [PubMed] [Google Scholar]
- 46.Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J Biol Chem 2010; 285: 7384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem 2008; 283: 34229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas JA, Zhang Y, Li P, Gong K, Miller AP, Hassan E, Hage F, Xing D, Wells B, Oparil S, Chen YF. Inhibition of transforming growth factor-beta signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am J Physiol Heart Circ Physiol 2010; 298: H424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]