Abstract
This study assessed the effects of thyroid hormones on the enzymes involved in l-arginine metabolism and the metabolites generated by the different metabolic pathways. Compounds of l-arginine metabolism were measured in the kidney, heart, aorta, and liver of euthyroid, hyperthyroid, and hypothyroid rats after 6 weeks of treatment. Enzymes studied were NOS isoforms (neuronal [nNOS], inducible [iNOS], and endothelial [eNOS]), arginases I and II, ornithine decarboxylase (ODC), ornithine aminotransferase (OAT), and l-arginine decarboxylase (ADC). Metabolites studied were l-arginine, l-citrulline, spermidine, spermine, and l-proline. Kidney heart and aorta levels of eNOS and iNOS were augmented and reduced (P < 0.05, for each tissue and enzyme) in hyper- and hypothyroid rats, respectively. Arginase I abundance in aorta, heart, and kidney was increased (P < 0.05, for each tissue) in hyperthyroid rats and was decreased in kidney and aorta of hypothyroid rats (P < 0.05, for each tissue). Arginase II was augmented in aorta and kidney (P < 0.05, for each tissue) of hyperthyroid rats and remained unchanged in all organs of hypothyroid rats. The substrate for these enzymes, l-arginine, was reduced (P < 0.05, for all tissues) in hyperthyroid rats. Levels of ODC and spermidine, its product, were increased and decreased (P < 0.05) in hyper- and hypothyroid rats, respectively, in all organs studied. OAT and proline levels were positively modulated by thyroid hormones in liver but not in the other tissues. ADC protein levels were positively modulated by thyroid hormones in all tissues. According to these findings, thyroid hormone treatment positively modulates different l-arginine metabolic pathways. The changes recorded in the abundance of eNOS, arginases I and II, and ADC protein in renal and cardiovascular tissues may play a role in the hemodynamic and renal manifestations observed in thyroid disorders. Furthermore, the changes in ODC and spermidine might contribute to the changes in cardiac and renal mass observed in thyroid disorders.
Keywords: Hyperthyroidism, hypothyroidism, rat, l-arginine metabolism, aorta, heart kidney
Introduction
l-Arginine and its metabolites are at the center of various metabolic pathways. l-Arginine is the main source of nitric oxide (NO) generation via NO synthase (NOS).1 All three NOS isoforms (neuronal [nNOS], inducible [iNOS], and endothelial [eNOS]) are present in tissues related to cardiovascular regulation and in renal tissue, and all play a role in cardiovascular and renal physiology.2,3
Arginases are responsible for the hydrolysis of arginine into ornithine and urea.4 There are two distinct isoforms of mammalian arginase, arginases I and II. Arginase I (AI, cytosolic enzyme) is highly expressed in the liver and to a much lesser extent in a few other cell types, whereas the expression of arginase II (AII, mitochondrial enzyme) is found in the kidney where it is more widely distributed.5,6 Both arginase isoforms are expressed in endothelial and smooth muscle cells of the vascular wall.7,8
Arginases compete with arginine for NO synthesis. Although the Km for arginine is in the µM range for NOS isozymes and in the mM range for arginases, the Vmax of arginases is more than 1000-fold higher than that of NOS isozymes. Hence, high levels of arginases can limit the availability of arginine for NO synthesis by intact cells.9
l-Ornithine is precursor in the synthesis in mammalian cells of the polyamines putrescine, spermidine, and spermine via the enzyme ornithine decarboxylase (ODC).10 Increased activity of this enzyme is essential for cell proliferation and tissue repair.11 l-Ornithine is also the substrate for ornithine aminotransferase (OAT), which generates l-proline, required for collagen production.12
Another l-arginine metabolizing enzyme, l-arginine decarboxylase (ADC), is responsible for the generation of agmatine, which is elevated in the normal kidney13 and might contribute to the biological effects of l-arginine supplementation.
In hyperthyroid rats, plasma nitrite/nitrate levels are augmented14 and NOS activity is upregulated in tissues primarily related to blood pressure control.15 Our group previously reported that blood pressure was increased in thyroxine-treated rats by oral administration of the non-specific NO inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) and of the iNOS inhibitor aminoguanidine at doses without pressor activity in normal rats.14,16 These reports all evidence an association between the hyperdynamic circulation of hyperthyroidism and an increase in NO production.
With this background, we performed the first investigation in the metabolism of l-arginine in kidney, heart, and aorta in thyroid disorders. The aim is to analyze the effects of excesses and deficits in thyroid hormone levels on the protein abundance of the enzymes involved in l-arginine metabolism and on the amount of metabolites generated by different metabolic pathways.
Materials and methods
Animals
Male Wistar rats born and raised in the experimental animal service of the University of Granada were used. Experiments were performed according to European Union guidelines for the ethical care of animals. Rats initially weighing 200–250 g with 6 weeks of age were maintained on standard chow and tap water ad libitum except where stated. The animals were divided into three groups: euthyroid control, hyperthyroid, and hypothyroid (n = 8 each group). Hyperthyroidism was induced by injecting s.c. thyroxine 75 µg/rat/d dissolved in isotonic saline (100 mL) plus 1 mL of 0.5 N NaOH and hypothyroidism was induced by the continuous administration of 0.03% methimazole via drinking water, as previously reported.17,18 Control rats were injected with the same solution as the hyperthyroid rats but without thyroxine. These treatments were administered for 6 weeks.
Experimental protocol
Tail systolic BP and heart rate were recorded using tail-cuff plethysmography in unanesthetized rats (LE 5001-Pressure Meter, Letica SA, Barcelona, Spain) twice at the end of the experimental period on alternate days. Blood samples were drawn from the femoral artery to determine plasma thyroid hormone levels (T3 and T4). After anesthetizing the rats with ethyl ether and killing them by exsanguination, the heart, kidneys, aorta, and liver were removed.
Analytical procedures
Free circulating T3 and T4 were determined in plasma using rat radioimmunoassay kits according to the instructions of the manufacturer (Diagnostic Products Corporation, Los Angeles, CA, USA). Rat plasma TSH was measured by a solid phase competitive chemiluminiscent enzyme immunoassay using the IMMULITE 2000 Analyzer (EURO/DPC, Llanberis, Gwynedd, UK).
Compounds of the l-arginine metabolism
All dissected tissues were weighed and homogenized in ice-cold 10% perchloric acid (50 mg wet weight/mL) and then centrifuged at 10,000 rpm for 10 min to precipitate protein. Supernatants (perchloric acid extracts) were immediately frozen and stored at −80℃ until analysis.
HPLC conditions
l-Citrulline, spermine, spermidine, praline, and arginine were determined by high-performance liquid chromatography (HPLC). Sample extracts were reconstituted in the positive ion mode. The chromatographic separation was carried out using an HSS T3 2.1 × 100 mm column. The mobile phase was acetonitrile:water (80:20 v/v) containing 0.1% PFBA (perfluorobutanoic acid), and the total flow rate was 0.25 mL/min; 10 µL of each standard plus sample solution was injected, with a total run time of 4.5 min.
Mass spectrometer settings
Detection of derivatized metabolites and internal standards was analyzed by liquid chromatography/tandem mass spectrometry (LC/MS/MS) in electrospray positive ionization mode (Waters XEVO-TQS, Scientific Instrument Center of University of Granada, Granada, Spain). Capillary voltage was set at 2.3 kV, source temperature at 150℃, nebulizer gas temperature at 200℃, nebulizer at 800 L/h, collision gas (argon) pressure in second quadrupole at 0.16 (mL/min), and dwell time and interchannel delay were each set at 0.025 s.
Method validation
Calibration standards were analyzed in triplicate for each unlabeled compound to determine retention time, limit of detection (LOD), limit of quantitation (LOQ), coefficient of regression (R2), and dynamic range. Calibration curves for each analyte were fitted using linear regression. Assay reproducibility was tested by analyzing calibration standards in triplicate on three different days. The coefficient of variation (%CV) was calculated at each concentration within the linear range of the assay.
Protein abundance of the enzymes related to l-arginine metabolism
The tissues were homogenized in 50 mm HCl-Tris (pH 7.4) containing 1% Triton X-100 and centrifuged for 15 min at 1000g. Protein abundance of eNOS, iNOS, nNOS), Arginases I and II, ODC, ADC, and OAT were measured in heart, kidney, aorta, and liver. The enzymes were analyzed by indirect ELISA using commercial kits from Bethyl Laboratorios Inc. (Montgomery, TX, USA). Briefly, homogenized tissues containing 10 µg/mL of total protein were fixed in a 96-well plate overnight at 4℃. After blocking, the plate was probed with 1:1000 rabbit anti-eNOS, anti-iNOS, anti-ADC, anti-OAT, anti-ODC (Abcam, Cambridge, UK), 1:1000 rabbit anti-Arg-1 or anti-nNOS (Epitomic, Burlingame, CA, USA), or 1:1000 mouse anti-Arg-2 antibody (Abcam, Cambridge, UK) as primary antibodies, and with 0.2 µg/mL of mouse anti-rabbit HRP-linked IgG antibody (KPL Inc., Gaithersburg, MD, USA) or 1 µg/mL of horse anti-mouse HRP-linked IgG antibody (Vector Laboratories, Burlingame, CA, USA), as secondary antibodies. All samples were analyzed in duplicate. Results are expressed as percentage of the mean absorbance of the control group. Tissue protein was determined with the DC Protein Assay kit (Bio-Rad, Madrid, Spain).
Statistical analysis
A one-way ANOVA was used for the comparisons of each variable. When the overall ANOVA result was significant, pairwise comparisons were performed with Bonferroni's method. P < 0.05 was considered significant. SPSS for Windows 15.0 (IBM Inc, Chicago, IL) was used for the analyses. P < 0.05 was considered significant in all tests.
Results
Biological variables
Table 1 exhibits the effects of hyper-and hypothyroiodism on biological variables. Animals receiving thyroxine or methimazole for 6 weeks gained significantly less weight than their age-matched controls over this period. Systolic blood pressure, heart rate, renal weight, heart weight, and plasma FT3 and FT4 levels were increased in hyperthyroid rats and decreased in hypothyroid rats, whereas plasma TSH were decreased and increased in hyper-and hypothyroid rats, respectively. Therefore, rats receiving thyroxine for 6 weeks developed the characteristic manifestations of hyperthyroidism, whereas those receiving methimazole for the same period developed hypothyroidism.
Table 1.
Biological variables in control, hypothyroid (methimazole treated, 0.03% in drinking water), and hyperthyroid (T4-treated, 75µg/rat/d s.c.) rats (n = 8 each group)
| Hypothyroid | Control | Hyperthyroid | |
|---|---|---|---|
| Body, heart and kidney weight | |||
| Body weight (g) | 263 ± 2.7*** | 358 ± 4.2 | 314 ± 3.2* |
| Heart weight (mg) | 600 ± 4.1 | 930 ± 4.2 | 1120 ± 3.5 |
| Kidney weight (mg) | 770 ± 25*** | 1100 ± 32 | 1450 ± 43*** |
| Hemodynamic variables | |||
| Systolic blood pressure (mmHg) | 110 ± 4.0*** | 125 ± 4.1 | 154 ± 2.5*** |
| Heart rate (bpm) | 348 ± 5.4** | 390 ± 8.9 | 477 ± 10.2*** |
| Thyroid hormone levels | |||
| FT3 (pg/mL) | 1.60 ± 0.10*** | 3.35 ± 0.07 | 8.20 ± 0.33*** |
| FT4 (ng/dL) | 0.07 ± 0.002*** | 2.44 ± 0.20 | 8.56 ± 0.32*** |
| TSH (mIU/l) | 6.03 ± 0.10*** | 2.40 ± 0.05 | 0.16 ± 0.02*** |
Data are mean ± SEM. P < 0.05; P < 0.01; P < 0.001 vs. controls.
Role of thyroid hormone levels on the compounds of l-arginine metabolism
Figure 1 shows the effects of hypo-and hyperthyroidism on different compounds of l-arginine metabolism measured in the study. The highest levels of l-arginine were found in the kidney, since the kidney is the major organ involved in endogenous arginine synthesis.19 The hyperthyroid group showed reduced l-arginine levels in kidney, heart, and aorta, whereas in the hypothyroid group, they were unchanged. The liver showed the lowest levels of l-arginine, which were positively modulated by the thyroid hormone levels.
Figure 1.
Compounds of l-arginine metabolism in tissues from hyperthyroid and hypothyroid rats. Data are means ± SEM. *P < 0.05; vs. controls; + P<0.05 vs. hypothyroid rats (n = 8 in each group)
Renal levels of l-citrulline, a product of NOS activity, were negatively modulated by thyroid hormone levels, as were renal levels of the substrate l-arginine, being higher in kidneys from hypothyroid rats and lower in those from hyperthyroid rats. The highest levels of l-citrulline were found in the heart. Cardiac l-citrulline levels were reduced by both hypo- and hyper-thyroidism. Aorta l-citrulline levels were increased in the hypothyroid group and normal in the hyperthyroid group. l-Citrulline levels were lowest in the liver, as in the case of the substrate l-arginine, and were positively modulated by the thyroid hormone levels.
Spermidine is generated by the activity of spermidine synthase on putrescine, which is the product of the activity of ornithine decarboxylase on l-ornithine. Spermidine was reduced in all organs from hypothyroid rats and was significantly increased in hyperthyroid rats.
Spermine is generated by the activity of spermine synthase on spermidine; its levels did not significantly change in any organ by thyroid hormone levels except for an increase in the liver of the hyperthyroid group.
Proline is the product of the activity of OAT with l-ornithine as a substrate, and the highest levels were found in the kidney. Kidney and heart proline levels did not differ significantly between the hypothyroid and hyperthyroid groups, while aorta levels were reduced in both groups. Liver proline levels were positively modulated by the thyroid hormone levels, reaching significance in the hypothyroid group.
Protein abundance of the enzymes involved in l-arginine metabolism
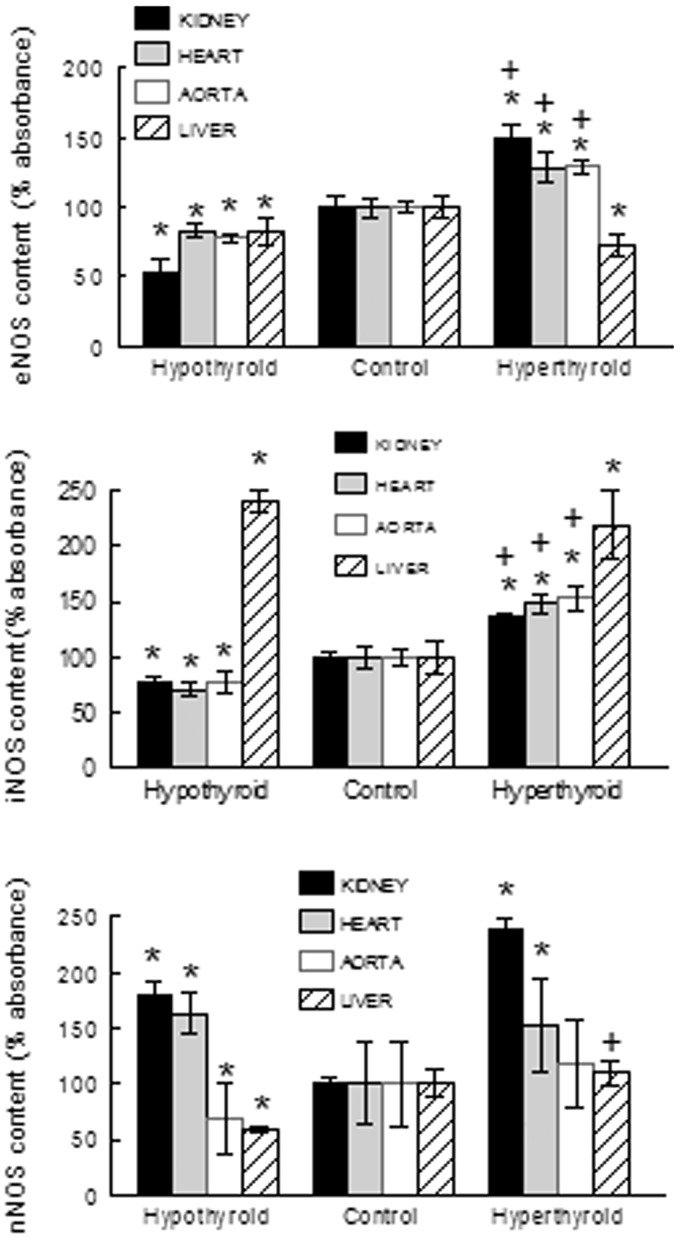
The values of NOS isoforms are shown in Figure 2. In general, the organs directly related to BP control (heart, kidney, and aorta) showed increased levels of the enzymes involved in l-arginine metabolism in the hyperthyroid state, especially in comparison with the methimazole-treated group. Levels of eNOS and iNOS in heart, kidney, and aorta were increased in hyperthyroid rats and decreased in hypothyroid rats. In the liver, the levels of eNOS were decreased and of iNOS increased in both hyper-and hypothyroid rats. Levels of nNOS in heart and kidney were increased in both hypo-and hyperthyroid rats; but in the aorta and in the liver, they were reduced in hypothyroid rats.
Figure 2.
NOS isoforms content in tissues from hyperthyroid and hypothyroid rats. Data are means ± SEM. *P < 0.05; vs. controls; + P < 0.05 vs. hypothyroid rats (n = 8 in each group)
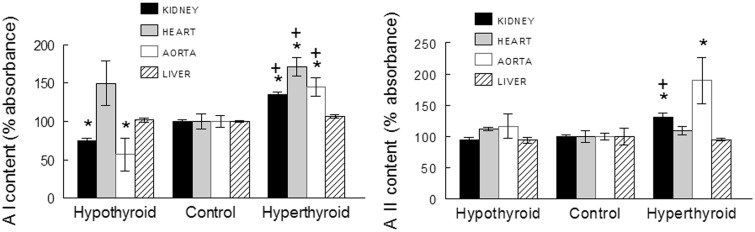
Arginase I abundance in aorta, heart, and kidney was significantly increased in T4-treated rats in comparison with controls and was decreased in kidney and aorta of hypothyroid rats (Figure 3). Arginase II was augmented in aorta and kidney of hyperthyroid rats and remained unchanged in the heart and liver from this group and in all organs from the hypothyroid rats (Figure 3).
Figure 3.
Arginase I (AI) and II (AII) content in tissues from hyperthyroid and hypothyroid rats. Data are means ± SEM. * P < 0.05; vs. controls; + P < 0.05 vs. hypothyroid rats (n = 8 in each group)
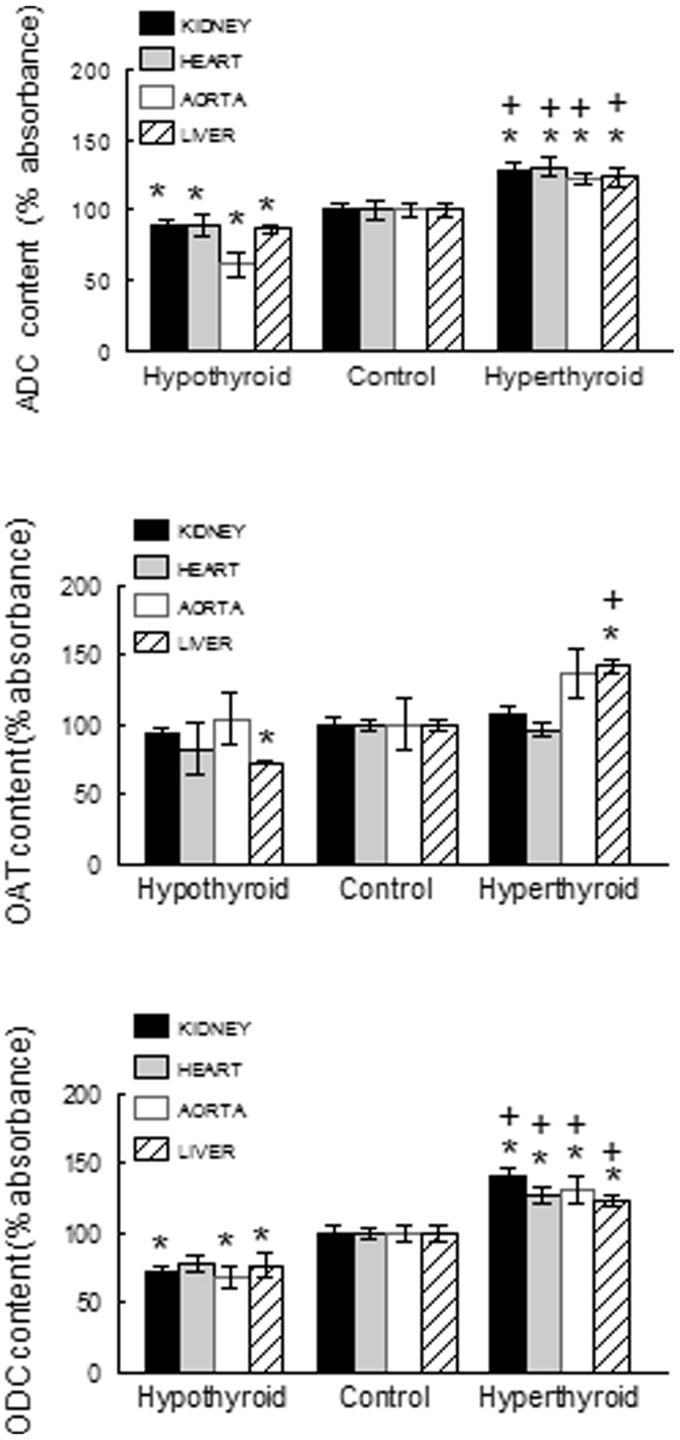
ODC was increased in all studied organs of hyperthyroid rats and reduced in all those of hypothyroid rats (Figure 4). The protein abundance of OAT (Figure 4) was positively modulated by thyroid hormone levels in the liver but was not significantly changed in any other tissue in either group (hyper- or hypothyroid). The protein abundance of ADC in heart, kidney, aorta, and liver was increased in hyperthyroid rats and reduced in hypothyroid rats (Figure 4).
Figure 4.
Arginine decarboxylase (ADC), ornithine aminotransferase (OAT), and ornithine decarboxylase (ODC) content in tissues from hyperthyroid and hypothyroid rats. Data are means ± SEM. *P < 0.05; vs. controls; P < 0.05 vs. hypothyroid rats (n = 8 in each group)
The data that are reported is summarized in Figure 5.
Figure 5.
Summary of l-arginine metabolism in mammalian cells and summary of the results. K, kidney; H, heart; A, aorta, L, liver. Symbols in red: effect (left) of the hyperthyroid state. Simbols in blue (right): effects of the hypothyroid state. ↑ increase, ↓ decrease, ↔ not change. The data concerning iNOS and nNOS have not been included for clarity. (A color version of this figure is available in the online journal.)
Discussion
The results of this study provide evidence that thyroid hormones positively modulate different l-arginine metabolic pathways, especially in regard to tissues and enzymes related to blood pressure control (Figure 5). Thus, levels of eNOS, iNOS and both arginases in the kidney, aorta, and heart are elevated in hyperthyroid rats and reduced in hypothyroid rats. l-Arginine, the substrate for these enzymes, is consequently reduced in hyperthyroid rats.
These observations are in line with a previous report by our group that NOS activity is upregulated in tissues primarily related to blood pressure control in hyperthyroid rats.15 The augmented expression of eNOS in aorta and kidney in hyperthyroid rats and its reduction in hypothyroid rats are consistent with their increase and decrease, respectively, in total peripheral vascular resistance,20 renal blood flow,21 and endothelium-dependent vasodilation in conductance and resistance vessels.22
The present study did not address the potential mechanisms responsible for the changes in NOS isoform expression in rats with thyroid disorders, but various factors may play a role, either alone or in combination. These include a direct effect of thyroid hormone on NOS activity,23 blood pressure changes,24 abnormal levels of vasoactive agents,25,26 and/or changes in shear stress due to the hyperdynamic circulation of hyperthyroid rats and hypodynamic circulation of hypothyroid rats.20
There is growing evidence that vascular arginase participates in the pathophysiology of vascular diseases. Thus, increased arginase activity and expression can be observed in the heart and aorta of animals with genetic and secondary forms of hypertension, diabetes, and aging.27 In the present study, the abundance of arginases I and II was augmented in aorta and kidney from hypertensive hyperthyroid rats.
Several factors may underlie the higher arginase activity observed,27 including angiotensin II or cytokine levels, oxidative stress, or hemodynamic forces such as blood pressure elevation, which are all increased in hyperthyroid rats.22,25,26 The augmented abundance of arginases I and II found in cardiovascular and renal tissues may contribute to the hemodynamic and renal manifestations of thyroid disorders. In fact, we recently reported that the blood pressure and proteinuria of hyperthyroid rats was reduced by the administration of nor-NOHA, an arginase inhibitor.28
ODC, the enzyme involved in the production of putrescine, spermidine, and spermine, in this order, was augmented in all studied tissues from hyperthyroid rats and reduced in all those from hypothyroid rats. Spermidine, which is the product of the activity of ornithine decarboxylase over l-ornithine, was, therefore, reduced in all organs from hypothyroid rats and augmented significantly in hyperthyroid rats. However, spermine, generated by the activity of spermine synthase on spermidine, was not modulated by thyroid hormone levels.
It is well established that all inducers of cardiac hypertrophy increase ODC and polyamine concentrations.29 Thus, administration of an ODC inactivator reduced polyamine content and attenuated isoproterenol30 and clenbuterol-induced cardiac hypertrophy.31 Moreover, polyamines are also involved in vascular smooth muscle cell proliferation and migration.32 Therefore, the changes in ODC and polyamines reported in the present paper might contribute to the elevation and reduction, respectively, of cardiac and renal mass in hyper and-hypothyroid rats and to the abnormal vascular function20 reported in thyroid disorders.
The protein abundance of OAT and proline levels were positively modulated by thyroid hormones in the liver but not in the organs related to blood pressure control.
The protein content of ADC, an enzyme that generates agmatine, was positively modulated by thyroid hormone in the tissues studied. Agmatine modulates heart and vascular function by acting on calcium homeostasis,33 and its infusion into the renal interstitium increases glomerular filtration and tubular resorption.13 Hence, the changes in the protein content of ADC and in eNOS expression reported here may participate in the peripheral resistance alterations observed in thyroid disorders.
Finally, food intake, and, therefore, l-arginine intake, is known to be increased and decreased in hyper- and hypothyroid rats, respectively.33.These changes in l-arginine intake might contribute to differences in l-arginine metabolism observed here in thyroid disorders. However, the amount of oral l-arginine supplement required to produce biological effects34,35 is much larger than the difference in l-arginine intake between hyper- and hypothyroid rats.
Study limitations include its largely descriptive nature, being designed to record changes induced by thyroid disorders in the main compounds of l-arginine metabolism. However, these observations open up new perspectives for research into the mechanisms underlying these alterations and their pathophysiological consequences.
In summary, the results of the present paper show that thyroid hormone positively modulates different l-arginine metabolic pathways. The tissue content of eNOS, arginases I and II, ADC, ODC, and spermidine in kidney, heart, and aorta is increased in hyperthyroid rats and reduced in hypothyroid rats. These alterations may participate in the hemodynamic and renal manifestations and cardiac and renal mass abnormalities of thyroid disorders.
Acknowledgements
The authors are grateful to R. Davies for help with the English version. This study was supported by a grant (CTS-6704) from the Junta de Andalucía and from the Carlos III Health Institute of the Spanish Ministry of Health and Consumer Affairs (Red de Investigación Renal, REDinREN 012/0021). “FEDER una manera de hacer Europa.”
Author Contributions
Conceived and designed the experiments: FV and RW. Performed the experiments: IRG, JNM, AQ, SMM, and PVT. Analyzed the data: FV and IRG. Wrote the paper: AO and RW.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Moncada S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann NY Acad Sci 1997; 811: 60–7. [DOI] [PubMed] [Google Scholar]
- 2.Thorup C, Persson AE. Nitric oxide and renal blood pressure regulation. Curr Opin Nephrol Hypertens 1998; 7: 197–202. [DOI] [PubMed] [Google Scholar]
- 3.Raij L, Bayli C. Glomerular actions of nitric oxide. Kidney Int 1995; 48: 20–32. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol 1996; 114: 107–32. [DOI] [PubMed] [Google Scholar]
- 5.Morris SM, Jr, Bhamidipati D, Kepka-Lenhart D. Human type II arginase: sequence analysis and tissue-specific expression. Gene 1997; 193: 157–61. [DOI] [PubMed] [Google Scholar]
- 6.Shi O, Kepka-Lenhart D, Morris SM, Jr, O’Brien WE. Structure of the murine arginase II gene. Mamm Genome 1998; 9: 822–4. [DOI] [PubMed] [Google Scholar]
- 7.Buga GM, Singh R, Pervin S, Rogers NE, Schmitz DA, Jenkinson CP, Cederbaum SD, Ignarro LJ. Arginase activity in endothelial cells: inhibition by N[omega]-hydroxy-l-arginine during high-output NO production. Am J Physiol 1996; 271: H1988–98. [DOI] [PubMed] [Google Scholar]
- 8.Ignarro LJ, Buga G, Wie LH, Bauer PM, Wu G, del Soldato P. Role of arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA 2001; 98: 4202–42089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J 1998; 336: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih VE. Regulation of ornithine metabolism. Enzyme 1981; 26: 254–8. [DOI] [PubMed] [Google Scholar]
- 11.Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. Am J Physiol 1994; 267: F331–46. [DOI] [PubMed] [Google Scholar]
- 12.Berry CL, Henrichs KJ. Morphometric investigation of hypertrophy in the arteries of DOCA-hypertensive rats. J Physiol 1982; 136: 85–94. [DOI] [PubMed] [Google Scholar]
- 13.Morrissey J, McCracken R, Ishidoya S, Klahr S. Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int 1995; 47: 1458–61. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Gómez I, Sainz J, Wangensteen R, Moreno JM, Duarte J, Osuna A, Vargas F. Increased pressor sensitivity to chronic nitric oxide deficiency in hyperthyroid rats. Hypertension 2003; 42: 220–5. [DOI] [PubMed] [Google Scholar]
- 15.Quesada A, Sainz J, Wangensteen R, Rodriguez-Gomez I, Vargas F, Osuna A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur J Endocrinol 2002; 147: 117–22. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Gómez I, Wangensteen R, Moreno JM, Chamorro V, Osuna A, Vargas F. Effects of chronic inhibition of inducible nitric oxide synthase in hyperthyroid rats. Am J Physiol Endocrinol Metab 2005; 288: E1252–7. [DOI] [PubMed] [Google Scholar]
- 17.Vargas F, Baz MJ, Luna JD, Andrade J, Jodar E, Haro JM. Urinary excretion of digoxin-like immunoreactive factor and arginine vasopressin in hyper-and hypothyroid rats. Clin Sci 1991; 81: 471–6. [DOI] [PubMed] [Google Scholar]
- 18.Vargas F, Atucha N, Sabio JM, Quesada T, García-Estañ J. Pressure–diuresis–natriuresis response in hyper- and hypothyroid rats. Clin Sci 1994; 87: 323–8. [DOI] [PubMed] [Google Scholar]
- 19.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol 1990; 259: E437–42. [DOI] [PubMed] [Google Scholar]
- 20.Vargas F, Moreno JM, Rodríguez-Gómez I, Wangensteen R, Alvarez-Guerra M, Osuna A, García-Estañ J. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol 2006; 154: 1–17. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Gómez I, Banegas I, Wangensteen R, Quesada A, Jimenez R, Gómez-Morales M, O’Valle F, Duarte J, Vargas F. Influence of thyroid state on cardiac and renal capillary density and glomerular morphology in rats. J Endocrinol 2012; 216: 1–10. [DOI] [PubMed] [Google Scholar]
- 22.Vargas F, Fernández-Rivas A, García Estañ J, García del Río C. Endothelium-dependent and endothelium-independent vasodilation in hyperthyroid and hypothyroid rats. Pharmacology 1995; 51: 308–14. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti N, Ray AK. Rise of intrasynaptosomal Ca2+ level and activation of nitric oxide synthase in adult rat cerebral cortex pretreated with 3-5-30-L-triiodothyronine. Neuropsychopharmacology 2000; 22: 36–41. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND, Zhenmin N, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension 1998; 31: 1248–54. [DOI] [PubMed] [Google Scholar]
- 25.Vargas F, Rodriguez-Gómez I, Vargas-Tendero P, Jiménez E, Montiel M. The renin–angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J Endocrinol 2012; 213: 25–36. [DOI] [PubMed] [Google Scholar]
- 26.Singh G, Thompson EB, Gulati A. Altered endothelin 1 concentration in brain and peripheral regions during thyroid dysfunction. Pharmacology 1994; 49: 184–91. [DOI] [PubMed] [Google Scholar]
- 27.Popolo A, Adesso S, Pinto A, Autore G, Marzocco S. l-Arginine and its metabolites in kidney and cardiovascular disease. Amino Acids 2014; 46: 2271–86. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Gómez I, Moreno JM, Jimenez R, Quesada A, Montoro-Molina S, Vargas-Tendero P, Wangensteen R, Vargas F. Effects of arginase inhibition in hypertensive hyperthyroid rats. Am J Hypertens (doi:10.1093/ajh/hpv049). [DOI] [PubMed]
- 29.Pegg AE, Hibasami H. Polyamine metabolism during cardiac hypertrophy. Am J Physiol 1980; 239: E372–8. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Zhang X, Wang L, Zhao Y, Li H, Xiao W, Xu C, Liu J. Polyamine depletion attenuates isoproterenol-induced hypertrophy and endoplasmic reticulum stress in cardiomyocytes. Cell Physiol Biochem 2014; 34: 1455–65. [DOI] [PubMed] [Google Scholar]
- 31.Cubria JC, Reguera R, Balana-Fouce R, Ordoñez C, Ordoñez D. Polyamine-mediated heart hypertrophy induced by clenbuterol in the mouse. J Pharm Pharmacol 1998; 50: 91–6. [DOI] [PubMed] [Google Scholar]
- 32.Kucharzewska P, Welch JE, Svensson KJ, Belting M. Ornithine decarboxylase and extracellular polyamines regulate microvascular sprouting and actin cytoskeleton dynamics in endothelial cells. Exp Cell Res 2010; 316: 2683–91. [DOI] [PubMed] [Google Scholar]
- 33.Raghavan SAV, Dikshit M. Vascular regulation by the l-arginine metabolites, nitric oxide and agmatine. Pharmacol Res 2004; 49: 397–414. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa H, Hirata Y, Suzuki E, Kimura K, Kikuchi K, Nagano T, Hirobe M, Omata M. Long-term administration of l-arginine improves nitric oxide release from kidney in deoxycorticosterone acetate-salt hypertensive rats. Hypertension 1994; 23: 752–6. [DOI] [PubMed] [Google Scholar]
- 35.Abreu GR, Futuro-Neto HA, Cabral AM, Vasquez EC. L-arginine restores the effect of ouabain on baroreceptor activity and prevents hypertension. Hypertension 1999; 34: 729–32. [DOI] [PubMed] [Google Scholar]