Abstract
Advances in depression research have targeted inflammation and oxidative stress to develop novel types of treatment. The JAK/STAT signaling pathway plays pivotal roles in immune and inflammatory responses. The present study was designed to investigate the effects of N-acetylcysteine, a putative precursor of the antioxidant glutathione, in an animal model of depression, with an emphasis on the JAK/STAT signaling pathway. Fluoxetine, a classical antidepressant drug was also under investigation. Male Wistar rats were subjected to forced swimming test and given N-acetylcysteine and fluoxetine immediately after the pre-test session, 5 h later and 1 h before the test session of the forced swimming test. N-acetylcysteine decreased immobility time (P < 0.05), serum corticosterone (P < 0.001), and hydrogen peroxide (P < 0.001), while restored glutathione concentration. Treatment of the rats with N-acetylcysteine produced significant (P < 0.001) down-regulation of STAT3 mRNA expression and protein phosphorylation. On the other hand, N-acetylcysteine significantly (P < 0.001) increased SOCS3 gene expression; however, SOCS3 protein was not changed. In conclusion, our study suggests that modulation of the JAK/STAT pathway might mediate the antidepressant-like effects of N-acetylcysteine. Therefore, depression research may target the JAK/STAT signaling pathway to provide a novel effective therapy.
Keywords: Depression, oxidative stress, inflammation, forced swimming test, glutathione, SOC3
Introduction
Major depressive disorder (MDD) is a common mental disease and one of the leading causes of disability worldwide.1 The pathogenesis of depression is thought to occur according to the monoamine hypothesis, which suggests that there is a deficiency or imbalance in the monoamine neurotransmitters, including serotonin, dopamine, and norepinephrine.2 In addition, alterations in neurogenesis and neuroendocrine functions could play roles in the pathogenesis of MDD.3,4 Recent developments in research investigating major depression have led to the hypothesis that oxidative stress and inflammatory processes are also involved in the pathogenesis of major depression and may contribute to dysfunction of the serotonergic and noradrenergic systems.5,6
Oxidative stress has been reported to play key pathologic roles in diverse disease states and may be a common mechanism underlying several major psychiatric disorders.7 The brain is more vulnerable to the detrimental effects of reactive oxygen species (ROS) due to its high metabolic rate and low antioxidant levels, which could explain why oxidative stress is a central feature in most neurodegenerative diseases, including major depression.6,8 Multiple studies have demonstrated that depression is associated with increased levels of the redox products malondialdehyde, 8-iso-prostaglandin F2 9–12 and 8-hydroxy-2′-deoxyguanosine.9 Overproduction of ROS generates an inflammatory response and increases the release of proinflammatory cytokines. In addition, a large body of evidence has shown that major depression is characterized by disturbances in inflammatory pathways, including the expression of cytokines such as interleukin-1β (IL-1β), IL-6, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α).13 Therefore, antioxidants might represent efficient therapies with beneficial effects in treating depression.
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway is associated with inflammation, and its components are widely expressed in several areas of the brain such as the cerebral cortex and hippocampus.14,15 This pathway plays important roles in cell growth, survival, development, and differentiation and in the regulation of gene expression.16 Dysregulation of the JAK/STAT pathway has been found to be a key factor in a variety of neurodegenerative diseases, which highlights the importance of determining how this pathway influences the fate and functions of brain cells.17 The JAK/STAT signaling pathway acts downstream of the binding of various ligands, such as cytokines, growth factors, and ROS18 and is inhibited by the action of suppressor of cytokine signaling (SOCS) proteins.19 The observation that ROS such as hydrogen peroxide (H2O2) activate certain components of the JAK/STAT pathway (STAT1, STAT3 and JAK2) indicates that increases in ROS associated with depression could result in activation of the JAK/STAT pathway and that this effect might be inhibited by antioxidants such as glutathione (GSH), which is the most important low molecular weight antioxidant that is synthesized in cells.20–22
Accordingly, several clinical studies have targeted inflammation and oxidative stress to develop novel types of treatments for MDD to overcome the treatment resistance associated with most antidepressants. It has been demonstrated that patients who suffer from depression have significantly decreased concentrations of GSH in their blood.23 Therefore, patients with major depression could benefit from GSH treatment. However, oral administration of GSH alone does not adequately restore GSH levels because this compound is rapidly hydrolyzed in the liver and intestines and because its ability to cross the blood–brain barrier is poor.24 In contrast, the administration of N-acetylcysteine (NAC) results in a significant increase in the plasma level of cysteine, leading to an increase in the plasma level of GSH, which can protect against ROS.25,26 NAC has been examined in the context of diverse neuropsychiatric disorders, and add-on treatment with NAC was shown to exert significant effect on depressive symptoms by up-regulating the GSH pathway in a placebo-controlled randomized trial.27 In addition, NAC showed antidepressant-like activity in several preclinical models, including the rat forced swimming test (FST),28 mouse tail suspension test (TST),29 and the FST in bulbectomized rats.30 Unlike GSH, NAC has been shown to successfully penetrate the blood–brain barrier.31,32
NAC acts through different mechanisms to mediate its antidepressant-like effects, and it is likely that NAC exerts these effects through mechanisms beyond those of being a precursor of the antioxidant GSH, which modulates the glutamatergic,33 dopaminergic,34 and inflammatory pathways.35 Therefore, the current study was designed to investigate the potential involvement of the JAK/STAT signaling pathway in the pathogenesis of depression and the modulatory effect of NAC.
Materials and methods
Experimental animals
Adult male Wistar rats weighing 150–175 g were supplied by the Animal Care Centre at the College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The rats were housed in standard polypropylene cages in controlled environment (25℃ ± 1℃) on a 12 h light/dark cycle and were provided free access to food and water. Rats were kept under observation for 1 week to acclimatize to the laboratory conditions prior to the experiment. All animal procedures were undertaken with the approval of Institutional Research Ethics Committee of the College of Pharmacy at King Saud University (Riyadh, Saudi Arabia).
Drugs and chemicals
NAC and fluoxetine were purchased from Sigma-Aldrich (St. Louis, MO, USA). H2O2 Amplex® Red assay kit was supplied by Invitrogen (USA) and corticosterone assay kit was purchased from MyBioSource (San Diego, CA, USA). All other chemicals were of analytical grade and obtained from either Sigma-Aldrich (St. Louis, MO, USA) or Cell Signaling Technology (Beverly, MA, USA).
Treatments and behavioral tests
The FST was performed as described by Porsolt et al.36,37 with slight modification. Rats were individually forced to swim in an open tank (25 × 30 cm, height 40 cm) filled with water at 24 ± 1℃ (the animals could not touch the bottom). On the first experimental day, the animals were gently placed in water for 15 min (pre-test session). The next day, they were once more placed gently in the water and observed for 5 min (test session). Immobility time of the rats was recorded in each 5 s during the test session. A rat was considered immobile when no additional activity (floating with only occasional alternate movements of paws and tail necessary to keep its head above the water) is observed. After each swim session, the rat was removed from the cylinder, dried with paper towels, placed in a resting cage for 20 min, and returned to its home cage. Water in the cylinder was changed between subjects.38,39
Forty-eight rats were included in the present study and were randomly allocated into four groups having 12 in each as follows:
-
Group I (Control): Rats received 0.9% NaCl intraperitoneally (i.p.).
Group II (Depressed): Rats received i.p. 0.9% NaCl.
Group III (NAC): Rats treated with NAC (50 mg/kg, i.p.).28
Group IV (Fluoxetine): Rats treated with fluoxetine (10 mg/kg, i.p.).40
NAC and fluoxetine were dissolved in sterile 0.9% NaCl. Drugs and vehicles were administered immediately after the pre-test session, 5 h later and 1 h before the test session of the FST.38,39
Open field test
To rule out any interference of locomotor activity in interpretation of the results obtained for the FST, ambulatory behavior was assessed in an open field test (OFT).41 Briefly, the apparatus consists of a large open field (70 cm × 70 cm × 40 cm) divided into 16 equal squares drawn on the floor of the arena. The test room was dimly illuminated with a red bulb (25 W) located 130 cm above the center of the arena. A single rat was placed in the center of the floor and the number of squares crossed (with the four paws), the number of rears (posture sustained with hind-paws on the floor), and the time spent grooming (including washing or mouthing of forelimbs, hind paws, face, body and genitals) were counted for 5 min. The open field was cleaned with 90% ethanol after each rat was tested to prevent the possible cueing effects of odors left by the previous subject.42 This test was performed 1 h after the last dose of drug was administered and before the test session of the FST (approximately 1 h between tests).
Blood and brain samples collection
Animals were sacrificed by decapitation following exposure to behavioral tests. Since anesthetic drugs affect the levels of corticosterone,43 decapitation was done without anesthesia. Trunk blood was collected from the site, where the animal was decapitated in anticoagulant-free tubes. Blood samples were centrifuged at 1000 × g for 15 min to separate serum. Sera were then collected and kept at −20℃ for further analysis.
The whole brain was quickly removed, rinsed with cold saline, and dissected on an ice-cold plate. The prefrontal cortex and hippocampus were harvested, washed, weighed, frozen in liquid nitrogen and stored at −80℃. Frozen samples (10% w/v) were homogenized in chilled phosphate-buffered saline (PBS) using tissue homogenizer (Omni International Inc., Kennesaw, GA, USA), and the homogenates were centrifuged at 1500 × g for 10 min. The clear homogenates were collected and stored at −20℃ for subsequent assays. Other samples were kept frozen at −80℃ and used for western blotting and gene expression assays.
Biochemical assays
Measurement of H2O2 content
The H2O2 content in the tissue homogenates of the hippocampus and prefrontal cortex was determined using the Amplex Red® assay kit according to the manufacturer’s instructions. This assay is based on the reaction between H2O2 and N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red), which is a colorless and non-fluorescent derivative of dihydroresorufin, in a 1:1 stoichiometry to produce the red-fluorescent oxidation product, resorufin.44 Resorufin has excitation and emission maxima of approximately 571 nm and 585 nm, respectively. This reaction can be used to detect as little as 10 picomoles of H2O2.
Measurement of GSH content
GSH content was determined based on Ellman’s method.45 Briefly, tissue homogenates were deprotonated with trichloroacetic acid (TCA) and incubated at room temperature for 5 min followed by centrifugation at 10,000 × g for 10 min at 4℃. The clear supernatant was then allowed to react with 6 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent dissolved in absolute methanol. The volume was completed to 3 ml with 0.2 M Na2HPO4. The absorbance was measured at 412 nm against a blank reagent using Ultrospec 2100 Pro (Amersham, GE Healthcare Life Science, Piscataway, NJ, USA). Different concentrations of standard GSH were diluted for standard curve preparation. The concentration of GSH was expressed in nmol/100 mg tissue.
Measurement of serum corticosterone level
Serum levels of corticosterone were assayed using specific enzyme-linked immunosorbent assay kit (MyBioSource, San Diego, CA, USA) following the manufacturer’s instructions. The assay sample and buffer were incubated together with corticosterone-horseradish peroxidase (HRP) conjugate in pre-coated plate for 1 h. The wells were decanted, washed five times, and incubated with a substrate for HRP enzyme. A stop solution was added and intensity of the formed yellow color was measured spectrophotometrically at 450 nm in a microplate reader. A standard curve was plotted and coricosterone concentration in each sample was interpolated from this standard curve.
RNA isolation and quantitative polymerase chain reaction
Total RNA was isolated from approximately 50 mg of frozen hippocampus and prefrontal cortex using RiboPure™ RNA purification kit (Life Technologies, Eugene, Oregon, USA), and concentrations were quantified at 260 nm using NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA samples with A260/A280 ratios ≥ 1.7 were selected. The RNA integrity was assessed using 1% agarose gel electrophoresis. Reverse transcription of RNA to complementary DNA (cDNA) was performed with 2 µg RNA using GeneAmp® RNA PCR core kit (Life Technologies, Eugene, Oregon, USA), in Applied Biosystems® 2720 thermal cycler.
cDNA samples were mixed with TaqMan® universal PCR master mix, TaqMan® MGB probes, and RNase-free water in a final volume of 20 μl. STAT3 and SOCS3 fluorescent probes were designed and synthesized by Applied Biosystems. Samples were loaded in a 96-well plate and run in triplicate using the primer set described in Table 1. The experiments were performed twice and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Amplification was performed using the Applied Biosystems 7900 HT sequence detection system, and the analysis was conducted using sequence detection software, version 2.4 according to the supplier’s recommendations (Applied Biosystems, Foster City, CA, USA). The qRT-PCR was set up as follows: 50℃ for 2 min, 95℃ for 10 min, then 40 cycles: 95℃ for 15 s, 60℃ for 6 s. The amplification products were confirmed on 1% agarose gel stained with ethidium bromide. The obtained amplification data were analyzed by the 2−ΔΔCt method,46 and the values were normalized to GAPDH.
Table 1.
Primer pairs used for qPCR
| Gene | GenBank accession number | Sequence 5′-3′ |
|---|---|---|
| STAT3 | NM-012747.2 | F:GCAGTTTAGACAGGGAGGGG |
| R:CACTGTCTCTGGGGCTGAAG | ||
| SOCS3 | NM-053565.1 | F:CCCCGCTTTGACTGTGTACT |
| R:AAAGGAAGGTTCCGTCGGTG | ||
| GAPDH | NM_017008.1 | F:CAACAGGGTGGTGGACCTCA |
| R:GAGTTGGGATGGGGACTCTCAG |
PCR: quantitative polymerase chain reaction.
Western blotting
Protein expression of SOCS3, STAT3, and GAPDH was determined in hippocampus and prefrontal cortex tissue homogenates using Western blotting analysis. Briefly, 100 mg of tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer supplemented with proteinase inhibitors. The homogenate sample was then centrifuged at 10,000 × g for 15 min at 4℃. Protein concentration in the supernatant was determined using Bradford protein assay.47 Samples were mixed with Laemmli loading buffer, denatured at 99℃ for 5 min and separated by 12% SDS-PAGE. The separated proteins were transferred overnight at 4℃ to polyvinylidene difluoride (PVDF) membranes using wet transfer system (Bio Rad Trans-Blot® Cell, Hercules, CA, USA). Membranes were blocked using 5% (w/v) non-fat dry milk in tris buffered saline – tween 20 (TBST) for 1 h at room temperature. Membranes were incubated overnight at 4℃ with rabbit polyclonal anti-p-STAT3 (Tyr-705) diluted 1:1000, anti-STAT3 (1:1000 dilution), anti-SOCS3 (1:2000 dilution), and anti-GAPDH (1:1000 dilution). Membranes were then washed three times with TBST and incubated with HRP-conjugated anti-rabbit secondary antibody (1:2000 dilution) for 1 h at room temperature. All antibodies were supplied by Cell Signaling Technology (Beverly, MA, USA) and diluted in TBST. After washing, protein bands were visualized by Super Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) using the ImageQuant™ LAS 4000 mini biomolecular imager (GE Healthcare Life Science, Piscataway, NJ, USA) and quantified using ImageJ software (NIH, USA). The pSTAT3 was normalized to STAT3 and GAPDH, while the SOCS3 protein level was determined after normalization to the GAPDH and presented as % of control.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA), and all statistical comparisons were made by means of the one-way analysis of variance test followed by Tukey’s test post hoc analysis. Results were expressed as mean ± standard error of the mean (SEM) and a P-value < 0.05 was considered significant.
Results
Effect of NAC and fluoxetine on immobility time and locomotor activity
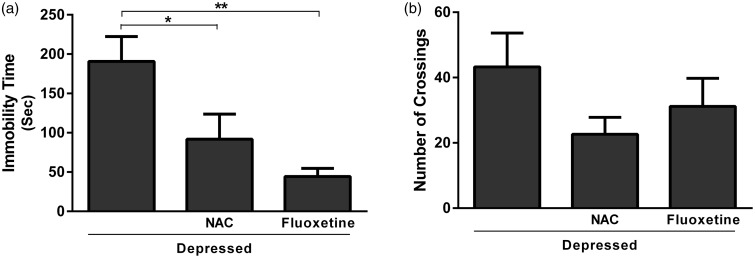
Data represented in Figure 1(a) show the effect of NAC and fluoxetine on immobility time in rats subjected to FST. Treatment with NAC significantly (P < 0.05) decreased immobility time when compared with the depressed control rats; the recorded data were 91.67 ± 23.01 and 190.8 ± 31.61 s for NAC and depressed group, respectively. Rats treated with fluoxetine showed a significant (P < 0.01) decrease in immobility time (44.17 ± 10.60 s) when compared with the corresponding control group. On the other hand, the effect of either NAC or fluoxetine on the locomotor activity measured in the OFT was non-significant (P > 0.05) when compared with the control rats (Figure 1(b)).
Figure 1.
Effect of NAC and fluoxetine on (a) the immobility time in FST and (b) the number of crossings in OFT. Data are mean ± SEM (N = 12).*P < 0.05 and **P < 0.01
Effect of NAC and fluoxetine on H2O2 and GSH content
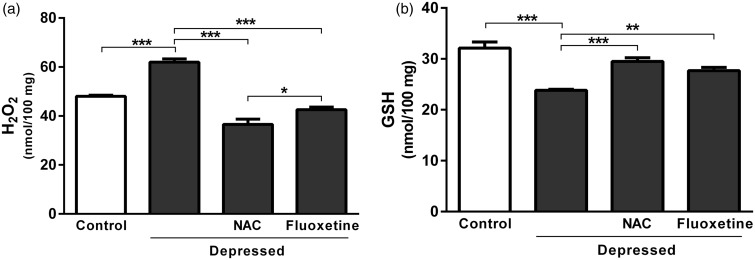
Rats exposed to FST showed a significant (P < 0.001) increase in hippocampus and prefrontal cortex H2O2 concentration (61.99 ± 1.30 nmol/100 mg) when compared with the normal control rats (48.01 ± 0.52 nmol/100 mg), as represented in Figure 2(a). The observed increase was significantly (P < 0.001) reversed after treatment with either NAC or fluoxetine; the recorded results were 36.58 ± 2.11 and 42.60 ± 1.02 nmol/100 mg, respectively.
Figure 2.
Effect of NAC and fluoxetine on (a) hydrogen peroxide and (b) reduced glutathione in rats subjected to the FST. Data are mean ± SEM (N = 12).*P < 0.05, **P < 0.01 and ***P < 0.001.
NAC: N-acetylcysteine; H2O2: hydrogen peroxide; GSH: reduced glutathione
On the contrary, GSH content of FST-exposed rats was significantly (P < 0.001) declined when compared with the corresponding control rats. Treatment with NAC significantly (P < 0.001) restored GSH levels, as represented in Figure 2(b). Similarly, FST-induced decrease in GSH was markedly (P < 0.01) alleviated following treatment with fluoxetine. NAC was more potent than fluoxetine in ameliorating GSH levels in the brain of rats exposed to FST (Figure 2(b)).
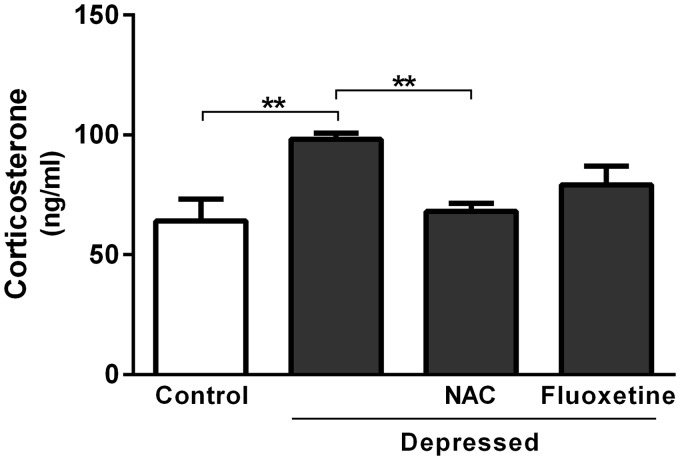
Effect of NAC and fluoxetine on serum corticosterone levels
Data concerning the effect of acute treatment with either NAC or fluoxetine on serum levels of corticosterone are represented in Figure 3. The results revealed significant (P < 0.01) increase in serum corticosterone after exposure to FST. The recorded results were 98.17 ± 2.65 and 64.20 ± 8.95 ng/ml for the depressed and control rats, respectively. On the other hand, NAC-treated rats showed significantly (P < 0.001) decreased serum corticosterone level (68.07 ± 3.35 ng/ml) when compared with the non-treated depressed rats. Treatment of the FST-exposed rats with fluoxetine produced non-significant (P > 0.05) change in serum corticosterone level (Figure 3).
Figure 3.
Effect of NAC and fluoxetine on serum corticosterone levels in rats subjected to the FST. Data are mean ± SEM (N = 12). **P < 0.01
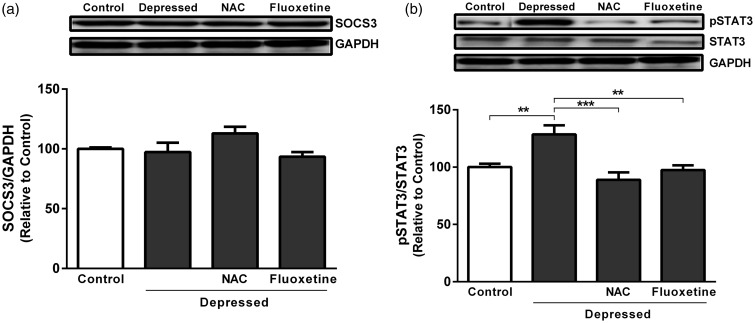
Effect of NAC and fluoxetine on SOCS3 and STAT3 gene expression
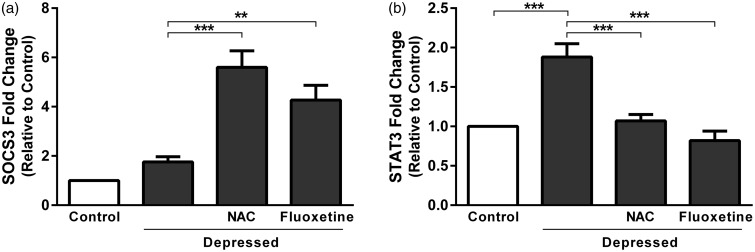
Gene expression levels were assessed in the hippocampus and prefrontal cortex of control and experimental rats using qRT-PCR (Figure 4). SOCS3 mRNA expression revealed non-significant (P > 0.05) difference in depressed rats when compared with the control group. Treatment of the depressed rats with NAC produced significant (P < 0.001) up-regulation of SOCS3 mRNA expression as depicted in Figure 4(a). Also, fluoxetine significantly (P < 0.01) up-regulated SOCS3 in the hippocampus and prefrontal cortex of rats exposed to FST.
Figure 4.
Effect of NAC and fluoxetine on mRNA expression of (a) SOCS3 and (b) STAT3 in rats subjected to the FST. Data are mean ± SEM (N = 12). **P < 0.01 and ***P < 0.001.
SOCS: suppressor of cytokine signaling; STAT: signal transducer and activator of transcription.
STAT3 was significantly (P < 0.001) up-regulated in hippocampus and prefrontal cortex of the depressed rats as compared to the corresponding control rats (Figure 4(b)). Treatment of the depressed rats with either NAC or fluoxetine produced significant (P < 0.001) decrease in STAT3 expression.
Effect of NAC and fluoxetine on SOCS3 protein expression and STAT3 phosphorylation
FST did not significantly affect SOCS3 protein expression levels when compared with non-depressed control rats as represented in Figure 5(a). Unlike gene expression data, treatment of the FST-exposed rats with either NAC or fluoxetine produced non-significant (P > 0.05) effect on SOC3 protein expression. On the other hand, FST-exposed rats exhibited significant (P < 0.01) increase in STAT3 phosphorylation when compared with the corresponding control rats (Figure 5(b)). Treatment with NAC significantly (P < 0.001) decreased STAT3 phosphorylation. In addition, fluoxetine supplementation produced a marked (P < 0.01) decline in phosphorylation of STAT3, as outlined in Figure 5(b).
Figure 5.
Effect of NAC and fluoxetine on protein expression of (a) SOCS3 and (b) pSTAT3 in rats subjected to the FST. Data are mean ± SEM (N = 12). **P < 0.01 and ***P < 0.001.
SOCS: suppressor of cytokine signaling; STAT: signal transducer and activator of transcription.
Discussion
NAC targets diverse aspects of nervous system functioning contribute to the pathophysiology of major depression including glutamatergic transmission, the antioxidant GSH, neurotrophins, apoptosis, mitochondrial function, and inflammatory pathways.48 NAC exhibits a better tolerability profile compared to other treatments.48 Recent evidence has suggested that NAC may exert therapeutic benefits in multiple neuropsychiatric disorders, including major depression.49 Therefore, the precise analysis of how NAC works is crucial to both understanding the core biology of MDD and investigating promising novel therapies that act on relevant pathways.
Due to the complex pathophysiology and symptoms of depressive disorders in humans, the creation of animal models has been difficult so far. Additionally, investigation of the antidepressant-like activity of novel compounds is sensitive for predictive validity. The FST remains one of the most common antidepressant screening tools with good reliability and strong predictive validity. This test considered to be a suitable model to detect antidepressant activity due to its ability to detect the majority of antidepressants and to discriminate antidepressants from neuroleptics and anxiolytics.50 For these reasons, a range of studies have used FST as a behavioral model for the evaluation of depressive-like behavior and the prediction of antidepressant drug efficacy in laboratory rodents.36,37 In this study, we used the FST as a reliable screening test to assess antidepressant effect of NAC. The present results demonstrated that NAC exerts an antidepressant effect that can be observed following its acute administration. NAC decreased the immobility time in the FST. This result supports the findings of Ferreira et al.28 who demonstrated that the acute administration of NAC (15–150 mg/kg) induced antidepressant-like effects in adult male Wistar rats subjected to the FST. Also, a recent clinical study conducted by Berk et al.27 suggested the antidepressant-like effects of NAC. As NAC and fluoxetine did not induce any significant change in exploratory activity in the OFT, these results demonstrate the antidepressant-like effects of NAC.
Strong evidence suggests a link between MDD and the hypothalamic–pituitary–adrenal (HPA) axis dysfunction.51 Indeed, the current study showed marked increase in serum corticosterone concentrations occurred immediately after the 5 min FST session. In agreement with our results, swim stressors have been reported to elevate corticosterone plasma levels immediately after the FST.52,53 In contrast, Karandrea et al.54 demonstrated that acute single stress episodes raised corticosterone levels more than repeated chronic stress did. Furthermore, medications that normalize the HPA axis system are hypothesized to play an important role in mediating antidepressant activity.55 Interestingly, for the first time, the present data indicated that acute treatment with the antioxidant NAC significantly reduced serum corticosterone levels of depressed rats subjected to the FST compared to the depressed control rats. The mechanism of action that NAC utilizes to lower serum corticosterone levels is unclear and requires further investigation. However, to a lesser extent, NAC may contribute to the negative feedback mechanism of the corticotropin-releasing factor, which is under the control of monoamergic systems.
In contrast, acute treatment with fluoxetine did not attenuate corticosterone levels significantly in depressed rats. Fluoxetine is a classical antidepressant that was used in this study as a positive control. Its action is mediated by blocking serotonin (5-hydroxytryptamine, 5-HT) reuptake at the synapse, resulting in an elevation of extracellular 5-HT concentrations in the limbic regions of brain that can act through various postsynaptic 5-HT receptors.56,57 This behavioral profile elicited by fluoxetine is similar to that previously reported.38,58 The normalization of serum corticosterone levels and the negative feedback mechanism have been reported to be more effective after chronic fluoxetine treatment.59 Additionally, Chen et al.60 reported that the abnormalities in the HPA axis system induced in mice by swimming stress could be reversed by chronic, but not acute fluoxetine treatment. This might be attributed to the desensitization of hypothalamic post-synaptic receptors and/or the up-regulation of central corticosteroid receptors involved in the negative feedback mechanism.61 On the contrary, Connor et al.62 confirmed that chronic treatment with the selective serotonin reuptake inhibitor (SSRI) paroxetine exhibited no effect on corticosterone levels. The reason for this discrepancy is that fluoxetine may mediate HPA axis stimulation by increasing serotonergic activity.63 In addition, many factors such as the duration of stress and treatment, as well as the time of blood collection, may influence the effect of antidepressant drugs on HPA axis activity.
Recently, less than two-thirds of patients who received treatment with the currently available SSRI antidepressant drugs achieve remission.6 Therefore, exploration of new antidepressants with different mechanisms of action is essential to ameliorate treatment outcome. Many studies support a crucial role for oxidative stress in the pathophysiology of major depression.6,8 In the current study, FST generated an increase in H2O2 and a decrease in GSH levels in the prefrontal cortex and hippocampus of depressed rats. The involvement of endogenous ROS H2O2 and its scavenging antioxidant enzymes, catalase and GSH peroxidase together with the antioxidant GSH, has long been recognized in major depression.6,8 Accordingly, the disturbances in oxidative stress parameters observed in this experiment may reflect a decline in the activities of free radical scavenging enzymes. This oxidative damage likely further exacerbates lipid peroxidation and neuronal damage.64 In addition, oxidative damage may interfere with critical GSH-related processes such as antioxidant defense, the detoxification of electrophilic xenobiotics, the storage and transport of cysteine, and the regulation of cell proliferation and signaling pathways.65 Similar findings were reported for recent preclinical66,67 and clinical trials.68,69
Antioxidants are of great importance in preventing oxidative stress-related disorders such as major depression, and recently, they have been considered as promising therapeutic agents for treating such disorders. In the present study, treatment of rats subjected to the FST with NAC significantly reduced the oxidative damage caused by ROS and restored the depleted GSH levels in the prefrontal cortex and hippocampus. These results are in accordance with similar findings obtained from previous preclinical studies.30,66 Furthermore, several preclinical studies have suggested that NAC protects the brain from oxidative stress due to its antioxidant properties.30,66 NAC acts as an antioxidant by modulating the redox status of cells; the compound augments the synthesis of the endogenous antioxidant GSH, which is usually depleted as a result of increased oxidative stress.70 Moreover, NAC acts as a direct scavenger of free radicals in the cells.71 Both of these antioxidant activities of NAC may be related to protective effects against oxidative stress and the consequent reduction in immobility time induced by the FST.
Similarly, treatment with the classical antidepressant fluoxetine produced a significant decrease in H2O2 and an increase in GSH levels. This investigation may have provided core evidence that fluoxetine not only reduces the immobility time by increasing the serotonin concentration at the synapse but also modulates oxidative stress pathways. Lobato et al.66 investigated the similar effects of acute and chronic fluoxetine treatment; however, these studies were performed in mice using the FST. Another study reported by Zafir et al.72 confirmed the neuroprotective effect of chronic treatment with certain antidepressants (fluoxetine, imipramine and venlafaxine) in rats through the modulation of oxidative stress pathways using the FST, along with other animal models of depression. In one clinical study, the authors demonstrated that combined therapy with fluoxetine and acetylsalicylic acid resulted in an improvement in oxidative stress parameters in patients treated for depression.73
Increased ROS and decreased antioxidant protection associated with MDD can provoke inflammatory responses. In addition, recent evidence suggests that inflammatory process, working in concert with ROS, may play a crucial role in the pathogenesis of depression.74,75 The JAK/STAT signaling pathway is an inflammatory pathway, and its components are widely expressed in several areas of the brain, such as the cerebral cortex and the hippocampus.14,16 Dysregulation of the JAK/STAT pathway has been found to be a key factor in a variety of neurodegenerative diseases, highlighting the importance of understanding how this pathway can influence the fate and function of brain cells.17 In this study, FST induced an increase in STAT3 mRNA expression, as well the protein phosphorylation in the hippocampus and prefrontal cortex of depressed rats. In consequence of FST-evoked oxidative stress, the released H2O2 activates STAT3 signaling pathway. The phosphorylation of STAT proteins at a specific tyrosine residue via an SH2 domain-phosphotyrosine interaction is required for the dimerization of the STAT proteins and their translocation to the nucleus.76 H2O2 has been demonstrated to stimulate tyrosine phosphorylation in some cells, as well as to inhibit tyrosine phosphatases.77 Therefore, the mechanism of H2O2 activation of STAT3 proteins likely involves the activation of cellular tyrosine kinases such as JAK2 and tyrosine kinase 2 (TYK2) that phosphorylate the STAT3 proteins or inactivates a STAT-3 phosphatase.78 Moreover, cytokines released by immune cells during inflammatory processes may activate the STAT3 transcription factor.79
In the present work, we tested the hypothesis that NAC may induce anti-inflammatory effects by inhibiting the activation of the JAK/STAT pathway. We found that treatment with NAC significantly decreased STAT3 mRNA expression and STAT3 protein phosphorylation levels in depressed rats. NAC reduced the FST-induced ROS, inhibited the activation of STAT3, and suppressed STAT3 transcription factor expression. The underlying mechanisms that mediate NAC’s reduction of STAT3 expression in the rat hippocampus and prefrontal cortex may include the inhibition of STAT3 phosphorylation at Tyr705 by blocking the phosphorylation and thus the tyrosine kinase activity of the upstream proteins JAK1, JAK2, JAK 3, and Tyk2.80 In addition, NAC is well known to function as GSH precursor and is a major regulator of the redox system in the cells, which may mediate its antioxidant properties.81 The glutathionylation of STAT3 inhibits its activation through JAK proteins.82 Moreover, NAC may modulate cytokines receptors that signal through the JAK/STAT pathway.83
More interestingly, there was diminished expression of STAT3 mRNA and protein phosphorylation in fluoxetine-treated rats. These novel findings may be involved in the mechanisms through which fluoxetine is able to improve depressive symptoms. Chronic fluoxetine administration has been reported to inhibit ERK1/2 phosphorylation in the rat brain84 and interferon alpha (IFN-α)-induced 5-HT uptake by interfering with ERK1/2.85 Inhibition of the ERK1/2 pathway mimics the function of an antidepressant.86 In addition, fluoxetine may inhibit glycogen synthase kinase-3β activity by increasing glycogen synthase kinase-3β phosphorylation at Ser9.87
Suppressors of cytokine signaling (SOCS), key physiological regulators of inflammation, are considered to be the main negative feedback loop that suppresses the JAK/STAT signaling pathway.19 SOCS3 can be markedly up-regulated by different cytokines and in a variety of neuroendocrine and neuroinflammatory states.19 Our results indicated that no differences exist between the depressed and non-depressed control groups in the induction of SOCS3 mRNA expression. These results suggest that at least one other negative regulatory pathway is involved in the regulation of the STAT3 transcription factor. In contrast to our findings, Mingmalairak et al.88 reported that the SOCS3 gene is up-regulated in non-depressed mice compared with depressed mice. Treatment with either NAC or fluoxetine evoked a remarkably significant increase in SOCS3 mRNA expression. SOCS3 regulates JAK/STAT signaling by inhibiting JAK kinase activity, facilitating the proteasomal degradation of JAK and STAT proteins, and competing with STATs for binding to cytokine receptors.89 On the other hand, neither the FST nor any of the treatment agents affect the SOCS3 protein levels. Since mRNA is eventually translated into protein, this result possibly indicates that SOCS3 mRNA is not expressed at a sufficiently high level to result in a more correlated level of protein abundance. Other possible explanation could be due to post-translational modification.
In conclusion, our study provides new findings suggesting the possible anti-inflammatory mechanisms through which NAC is able to treat MDD. The inhibition of excessive STAT3 protein activation and SOCS3 gene induction contribute to the anti-inflammatory activity of NAC. In addition, this study provides new evidence of the involvement of the STAT3/SOCS3 signaling pathway in the pathogenesis of depression. Therefore, depression research may target the JAK/STAT signaling pathway to provide a novel effective therapy for treating MDD. However, additional preclinical studies and clinical trials are needed to better elucidate the effects of this therapeutic strategy.
Acknowledgements
This research project was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
Authors’ contributions
MMA and IHH carried out the animal experimentation and biochemical estimations, MMA and ND conducted the gene and protein analysis, Nouf MA, NMA, and SA conceived of the study, and participated in its design and coordination, AMM and IHH analyzed the data, AMM drafted the manuscript and all authors read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med 1998; 4: 1241–3. [DOI] [PubMed] [Google Scholar]
- 2.Szabo ST, Gould TD, Manji HK. Neurotransmitters, receptors, signal transduction, and second messengers in psychiatric disorders. In: Schatzberg AF, Nemeroff CB. (eds). Textbook of psychopharmacology, 4th ed Arlington, VA: American Psychiatric Publishing, 2004, pp. 3–52. [Google Scholar]
- 3.Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand 2007; 433: 90–103. [DOI] [PubMed] [Google Scholar]
- 4.Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 2010; 7: 231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP task force on biological markers: biological markers in depression. World J Biol Psychiatry 2007; 8: 141–74. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett 2009; 30: 470–6. [PubMed] [Google Scholar]
- 7.Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev 2012; 2012: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 676–92. [DOI] [PubMed] [Google Scholar]
- 9.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2’ deoxyguanosine in clinical depression. Psychosom Med 2006; 68: 1–7. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasmalevels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res 2008; 161: 59–66. [DOI] [PubMed] [Google Scholar]
- 11.Galecki P, Szemraj J, Bienkiewicz M, Florkowski A, Galecka E. Lipid perox-idation and antioxidant protection in patients during acute depressive episodesand in remission after fluoxetine treatment. Pharmacol Rep 2009; 61: 436–47. [DOI] [PubMed] [Google Scholar]
- 12.Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology 2010; 35: 1356–62. [DOI] [PubMed] [Google Scholar]
- 13.Nunes SOV, Reiche EMV, Morimoto HK, Matsuo T, Itano EN, Xavier EC, Yamashita CM, Vieira VR, Menoli AV, Silva SS, Costa FB, Reiche FV, Silva FL, Kaminami MS. Immune and hormonal activity in adults suffering from depression. Braz J Med Biol Res 2002; 35: 581–7. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev 2000; 11: 199–207. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat Immunol 2007; 8: 1281–3. [DOI] [PubMed] [Google Scholar]
- 16.Soebiyanto RP, Sreenath SN, Qu CK, Loparo KA, Bunting KD. Complex systems biology approach to understanding coordination of JAK-STAT signaling. Biosystems 2007; 90: 830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S. The role of JAK-STAT signaling within the CNS. JAKSTAT 2013; 2: e22925–e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 1995; 269: 81–3. [DOI] [PubMed] [Google Scholar]
- 19.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res 2007; 13: 1362–6. [DOI] [PubMed] [Google Scholar]
- 20.Simon R, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol 1998; 275: 1640–52. [DOI] [PubMed] [Google Scholar]
- 21.Carballo M, Conde M, El Bekay R, Martín-Nieto J, Camacho MJ, Monteseirín J, Conde J, Bedoya FJ, Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem 1999; 274: 17580–6. [DOI] [PubMed] [Google Scholar]
- 22.Gorina R, Sanfeliu C, Galitó A, Messeguer A, Planas AM. Exposure of glia to pro-oxidant agents revealed selective Stat1 activation by H2O2 and Jak2-independent antioxidant features of the Jak2 inhibitor AG490. Glia 2007; 55: 1313–24. [DOI] [PubMed] [Google Scholar]
- 23.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. N-acetyl cysteine for depressive symptoms in bipolar disorder – a double-blind randomized placebo-controlled trial. Biol Psychiatry 2008; 64: 468–75. [DOI] [PubMed] [Google Scholar]
- 24.Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol 1992; 43: 667–9. [DOI] [PubMed] [Google Scholar]
- 25.Holdiness MR. Clinical pharmokinetics of N-acetylcysteine. Clin Pharmacokinet 1991; 20: 123–34. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuénod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 2008; 33: 2187–99. [DOI] [PubMed] [Google Scholar]
- 27.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia – a double-blind randomized placebo-controlled trial. Biol Psychiatry 2008; 64: 361–8. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira FR, Biojone C, Joca SR, Guimarães FS. Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behav Pharmacol 2008; 19: 747–50. [DOI] [PubMed] [Google Scholar]
- 29.Linck VM, Costa-Campos L, Pilz LK, Garcia CRL, Elisabetsky E. AMPA glutamate receptors mediate the antidepressant-like effects of N-acetylcysteine in the mouse tail suspension test. Behav Pharmacol 2012; 23: 171–7. [DOI] [PubMed] [Google Scholar]
- 30.Smaga I, Pomierny B, Krzyżanowska W, Pomierny-Chamioło L, Miszkiel J, Niedzielska E, Ogórka A, Filip M. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: behavioral and biochemical analyses in rats. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 280–7. [DOI] [PubMed] [Google Scholar]
- 31.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alphalipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 2003; 84: 1173–83. [DOI] [PubMed] [Google Scholar]
- 32.Dean O, van den Buuse M, Copolov D, Berk M, Bush A. N-acetylcysteine inhibits depletion of brain glutathione levels in rats: implications for schizophrenia. Int J Neuropsychopharmacol 2004; 7: S262–S262. [Google Scholar]
- 33.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 2005; 25: 6389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gere-Paszti E, Jakus J. The effect of N-acetylcysteine on amphetamine-mediated dopamine release in rat brain striatal slices by ion-pair reversed-phase high performance liquid chromatography. Biomed Chromatogr 2009; 23: 658–64. [DOI] [PubMed] [Google Scholar]
- 35.Beloosesky R, Weiner Z, Ginsberg Y, Ross MG. Maternal N-acetyl-cysteine (NAC) protects the rat fetal brain from inflammatory cytokine responses to lipopolysaccharide (LPS). J Matern Fetal Neonatal Med 2012; 25: 1324–8. [DOI] [PubMed] [Google Scholar]
- 36.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatment. Nature 1977; 266: 730–2. [DOI] [PubMed] [Google Scholar]
- 37.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 1978; 47: 379–91. [DOI] [PubMed] [Google Scholar]
- 38.Cryan JF, Lucki I. 5-HT4 receptors do not mediate the antidepressant-like behavioral effects of fluoxetine in a modified forced swim test. Eur J Pharmacol 2000; 409: 295–9. [DOI] [PubMed] [Google Scholar]
- 39.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 140–4. [DOI] [PubMed] [Google Scholar]
- 40.Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radiolig and [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci 2004; 24: 5420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull 1976; 83: 482–504. [PubMed] [Google Scholar]
- 42.Brenes Sáenz JC, Villagra OR, Fornaguera Trías J. Factor analysis of forced swimming test, sucrose preference test and open field test on enriched, social and isolated reared rats. Behav Brain Res 2006; 169: 57–65. [DOI] [PubMed] [Google Scholar]
- 43.Karuri AR, Engelking LR, Kumar MSA. Effects of halothane and methoxyflurane on the hypothalamic-pituitary-adrenal axis in rat. Brain Res Bull 1998; 47: 205–9. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 1997; 253: 162–8. [DOI] [PubMed] [Google Scholar]
- 45.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70–7. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 47.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–54. [DOI] [PubMed] [Google Scholar]
- 48.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 2013; 34: 167–77. [DOI] [PubMed] [Google Scholar]
- 49.Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 2011; 36: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacol 1988; 94: 147–60. [DOI] [PubMed] [Google Scholar]
- 51.Dinan TG. Glucocorticoids and the genesis of depressive illness. A psychobiologicalmodel. Br J Psychiatry 1994; 164: 365–71. [DOI] [PubMed] [Google Scholar]
- 52.Duncan GE, Knapp DJ, Carson SW, Breese GR. Differential effects of chronic antidepressant treatment on swim stress- and fluoxetine-induced secretion of corticosterone and progesterone. J Pharmacol Exp Ther 1998; 285: 579–89. [PMC free article] [PubMed] [Google Scholar]
- 53.Connor TJ, Kelliher P, Harkin A, Kelly JP, Leonard BE. Reboxetine attenuates forced swim test-induced behavioural and neurochemical alterations in the rat. Euro J Pharmacol 1999; 379: 125–33. [DOI] [PubMed] [Google Scholar]
- 54.Karandrea D, Kittas C, Kitraki E. Forced swimming differential affects male and female brain corticosteroid receptors. Neuroendocrinology 2002; 75: 217–26. [DOI] [PubMed] [Google Scholar]
- 55.Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, Price LH, Carpenter LL. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology 2006; 31: 1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fava M, Kendler KS. Major depressive disorder. Neuron 2000; 28: 335–41. [DOI] [PubMed] [Google Scholar]
- 57.Walker FR. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 2013; 67: 304–17. [DOI] [PubMed] [Google Scholar]
- 58.Page M.E, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology 1999; 147: 162–7. [DOI] [PubMed] [Google Scholar]
- 59.Armario A, Garcia-Marquez C. Tricyclic antidepressants activate the pituitary–adrenal axis in the rat. Tolerance to repeated drug administration. Eur J Pharmacol 1987; 140: 239–44. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Kong LD, Xia X, Kung HF, Zhang L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J Ethnopharmacol 2005; 96: 451–9. [DOI] [PubMed] [Google Scholar]
- 61.Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary- adrenocortical regulation. Endocrin Rev 1996; 17: 187–205. [DOI] [PubMed] [Google Scholar]
- 62.Connor TJ, Kelliher P, Shen Y, Harkin A, Kelly JP, Leonard BE. Effect of subchronic antidepressant treatments on behavioral, neurochemical, and endocrine changes in the forced-swim test. Pharmacol Biochem Behav 2000; 65: 591–7. [DOI] [PubMed] [Google Scholar]
- 63.Feldman S, NewmanME, Gur E, Weidenfeld J. Role of serotonin in the amygdale in hypothalamo-pituitaryadrenocortical receptors. Neuroreport 1998; 9: 2007–9. [DOI] [PubMed] [Google Scholar]
- 64.Matés JM, Sánchez-Jiménez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosc 1999; 4: 339–45. [DOI] [PubMed] [Google Scholar]
- 65.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 1996; 10: 709–20. [DOI] [PubMed] [Google Scholar]
- 66.Lobato KR, Cardoso CC, Binfaré RW, Budni J, Wagner CL, Brocardo PS, de Souza LF, Brocardo C, Flesch S, Freitas AE, Dafré AL, Rodrigues AL. α-Tocopherol administration produces an antidepressant-like effect in predictive animal models of depression. Behav Brain Res 2010; 209: 249–59. [DOI] [PubMed] [Google Scholar]
- 67.Moretti M, Budni J, Ribeiro CM, Rodrigues AL. Involvement of different types of potassium channels in the antidepressant-like effect of ascorbic acid in the mouse tail suspension test. Eur J Pharmacol 2012; 687: 21–7. [DOI] [PubMed] [Google Scholar]
- 68.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord 2010; 125: 287–94. [DOI] [PubMed] [Google Scholar]
- 69.Gawryluk JW1, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 2011; 14: 123–30. [DOI] [PubMed] [Google Scholar]
- 70.Grinberg L, Fibach E, Amer J, Atlas D. N-acetylcysteine amide, a novel cell permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med 2005; 38: 136–45. [DOI] [PubMed] [Google Scholar]
- 71.Sadowska AM, Manuel-y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007; 20: 9–22. [DOI] [PubMed] [Google Scholar]
- 72.Zafir A, Ara A, Banu N. In vivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 220–8. [DOI] [PubMed] [Google Scholar]
- 73.Gałecki P, Szemraj J, Bieńkiewicz M, Zboralski K, Gałecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol 2009; 24: 277–86. [DOI] [PubMed] [Google Scholar]
- 74.Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl 2011; 88: 48–54. [DOI] [PubMed] [Google Scholar]
- 75.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012; 36: 764–85. [DOI] [PubMed] [Google Scholar]
- 76.Shuai K1, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signaling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature 1993; 366: 580–3. [DOI] [PubMed] [Google Scholar]
- 77.Gamou S, Shimizu N. Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett. 357: 161–4. [DOI] [PubMed] [Google Scholar]
- 78.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon-a/b signaling pathway. Cell 1992; 70: 313–22. [DOI] [PubMed] [Google Scholar]
- 79.Parker-Athill E1, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, Legradi G, Tan J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol 2009; 217: 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci 2004; 117: 1281–3. [DOI] [PubMed] [Google Scholar]
- 81.Kojer K1, Bien M, Gangel H, Morgan B, Dick TP, Riemer J. Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state. EMBO J 2012; 31: 3169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y1, Kole S, Precht P, Pazin MJ, Bernier M. S-glutathionylation impairs signal transducer and activator oftranscription 3 activation and signaling. Endocrinology 2009; 150: 1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray PJ. The JAK–STAT signaling pathway: input and output integration. J Immunol 2007; 178: 2623–9. [DOI] [PubMed] [Google Scholar]
- 84.Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem 2005; 93: 1551–60. [DOI] [PubMed] [Google Scholar]
- 85.Tsao CW, Lin YS, Cheng JT, Lin CF, Wu HT, Wu SR, Tsai WH. Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J Psychopharmacol 2008; 22: 753–60. [DOI] [PubMed] [Google Scholar]
- 86.Einat H1, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 2003; 23: 7311–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 2004; 29: 1426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mingmalairak S, Tohda M, Murakami Y, Matsumoto K. Possible involvement of signal transducers and activators of transcription 3 system on depression in the model mice brain. Biol Pharm Bull 2010; 33: 636–40. [DOI] [PubMed] [Google Scholar]
- 89.O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol 2007; 44: 2497–506. [DOI] [PubMed] [Google Scholar]