Abstract
Propofol has been shown to exert cardioprotection, but the underlying mechanisms remain incompletely understood. We examined: (1) whether propofol-induced cardioprotection depended on the time and the dose of administration; (2) the role of mitochondrial adenosine triphosphate-sensitive potassium channels, nitric oxide synthase, and mitochondrial respiratory chain activity in propofol-induced cardioprotection. Human right atrial trabeculae were obtained during cardiopulmonary bypass for coronary artery bypass and aortic valve replacement. Isometric force of contraction of human right atrial trabeculae hanged in an oxygenated Tyrode’s solution was recorded during 30-min hypoxia and 60-min reoxygenation (Control). Propofol 0.1, 1, and 10 µM was administered: (1) 5 min before hypoxia until the end of the experiment; (2) 5 min followed by 5-min washout before hypoxia; (3) during the reoxygenation period, propofol 10 µM was administered in presence of 5-hydroxydecanoate (antagonist of mitochondrial adenosine triphosphate-sensitive potassium channels), and NG-nitro-L-arginine methyl ester (inhibitor of nitric oxide synthase). In addition, mitochondria were isolated from human right atrial at 15 min of reoxygenation. The effect of propofol on activity of the mitochondrial respiratory chain complexes was evaluated by spectrophotometry. The force of contraction (% of baseline) and the complex activity between the different groups were compared with an analysis of variance and post hoc test. Propofol 10 µM administered during the reoxygenation period significantly improved the recovery of force of contraction at the end of reoxygenation (82 ± 6% of baseline value vs. 49 ± 6% in Control; P < 0.001). The beneficial effects of propofol 10 µM were abolished by co-administration with 5-hydroxydecanoate (53 ± 8%) or NG-nitro-L-arginine methyl ester (57 ± 6%). Propofol 10 µM significantly increased enzymatic activities of the mitochondrial respiratory chain complexes, in reoxygenation period, compared to their respective untreated controls. In conclusion, in human myocardium, propofol-induced cardioprotection was mediated by mitochondrial adenosine triphosphate-sensitive potassium channels opening, nitric oxide synthase activation and stimulation of mitochondrial respiratory chain complexes, in early reoxygenation period.
Keywords: Propofol, cardioprotection, mitochondrial adenosine triphosphate-sensitive potassium channels, mitochondrial respiratory chain complexes
Introduction
The intravenous anesthetic propofol (2,6-diisopropylphenol) is widely used for the induction and maintenance of anesthesia. The cardioprotective effect of propofol has been shown in experimental studies on post-ischemic myocardial contractile dysfunction,1–3 arrhythmias,4 infarct size,2,3,5 and histological damage.3 However, results from clinical studies remain contradictory, suggesting indirect cardioprotective effects in pediatric cardiac surgery6 or lack of cardioprotective effect in adult cardiac and non-cardiac surgery.7–9
Besides the antioxydant activity of propofol,1,2,5 the mitochondria seem to play a key role in propofol-induced cardioprotection as suggested by the attenuation of reactive oxygen species production in early reoxygenation,10 inhibition of the mitochondrial permeability transition pore,11,12 and regulation of mitochondrial DNA transcription.13 However, the role of the mitochondrial adenosine triphosphate-dependent potassium channel remains unclear.2,14,15 Finally, propofol has been shown to inhibit mitochondrial respiration of rat liver,16 but only one study reported the effect of propofol on the mitochondrial respiratory chain activity during myocardial ischemia reperfusion.17 Altogether, these data suggest that propofol should exert its cardioprotective effect during reperfusion where mitochondria and the burst of reactive oxygen species play a key role in ischemia reperfusion injury.18
In the present study, using isolated human myocardium, we tested the hypothesis that propofol-induced cardioprotection could depend on the time of its administration. In addition, we examined the effect of propofol on the mitochondrial respiratory chain complexes, and the role of the nitric oxide synthase (NOS) and mitochondrial adenosine triphosphate-sensitive potassium channels on the cardioprotective effect of propofol.
Materials and methods
After the approval of local medical ethics committee, Comité de Protection des Personnes Nord Ouest III, Caen, France (ref.: DC-2013-1967 for Pr Jean-Luc Hanouz) and written informed consent, right atrial appendages were obtained during cannulation for cardiopulmonary bypass from patients scheduled for coronary artery bypass surgery and aortic valve replacement. Patients with chronic atrial arrhythmia and diabetes mellitus treated with insulin or oral hypoglycemic agents were excluded from the study because of myocardial remodeling and interference with cardioprotective mechanism.19,20 The patients in whom volatile anesthetics were administered before atrial appendage dissection have not been included in the present study, because it has been shown that volatile anesthetics may result in myocardial preconditioning in clinical studies.21,22
Contracting isolated human right atrial trabeculae
Right atrial trabeculae (one or two per appendage) were dissected and suspended vertically between an isometric force transducer (MLT0202; AD Instruments, Sydney, Australia) and a stationary stainless clip in a 200 mL jacketed reservoir filled with daily prepared Tyrode’s modified solution containing 0.12 M sodium chloride, 3.5 mM potassium chloride, 1.1 mM magnesium chloride, 1.8 mM sodium phosphate monobasic, 25.7 mM sodium bicarbonate, 2.0 mM calcium chloride, and 5.5 mM glucose. To ensure stability of the model throughout the experiment, the jacketed reservoir was maintained at 34 ± 0.5℃ by a thermostatic water circulator (Polystat micropros; Bioblock, Illkirch, France). The bathing solution was oxygenated with carbogen (95% dioxygen-5% Carbon dioxide), resulting in a pH of 7.40 ± 0.05 and a partial pressure of oxygen of 600 ± 50 mmHg. Isolated muscles were field-stimulated at 1 Hz by two platinum electrodes with rectangular wave pulses of 5 ms duration 20% above threshold (CMS 95107; Bionic Instrument, Paris, France).
Trabeculae were equilibrated for 60–90 min to allow stabilization of their optimal mechanical performance at the apex of the length active isometric tension curve (Lmax). The force developed was measured continuously, digitized at a sampling frequency of 400 Hz, and stored in a computer (PowerLab; ADInstruments). In all groups, hypoxia-reoxygenation was performed by replacing 95% dioxygen-5% carbon dioxide with 95% diazote-5% carbon dioxide in the buffer for 30 min, followed by a 60-min oxygenated recovery period. The primary endpoint of contracting experiments was the recovery of force of contraction at 60 min of reoxygenation (expressed as percent of baseline).
At the end of experiment, the muscle cross-sectional area was calculated from its weight and length assuming a cylindrical shape and a density of 1. To avoid core hypoxia, trabeculae included in the study must have a cross-sectional area less than 1.0 mm2, a force of contraction normalized per cross-sectional area greater than 5.0 mN/mm2, and a ratio of resting force/total force less than 0.50 otherwise they were excluded a posteriori.
Activity of complexes I, II, III, and IV of the mitochondrial respiratory chain
Preparation of isolated mitochondria from human right atrial myocardial
After 15 min of reoxygenation, atrial sample immediately placed in cold buffer A containing mannitol 0.21 M, sucrose 70 mM, Tris 50 mM, potassium EDTA 10 mM, pH 7.4. All operations were carried out in the cold at 4℃ Briefly, atrial sample (0.3–0.5 g) placed in isolation buffer A, then it was finely minced with scissors, and incubated at 37℃ with trypsin during 30 min, the enzymatic reaction is stopped with the addition of inhibitor of trypsin. Then, atrial sample is homogenized with a Polytron. The homogenate was centrifuged at 1500 g for 15 min. The supernatant was centrifuged at 8000 g for 20 min. The mitochondrial pellet was suspended in isolation buffer B containing mannitol 0.225 M, sucrose 75 mM, Tris 10 mM, potassium EDTA 0.1 mM, pH 7.2. Protein content was routinely assayed according to procedure with bovine serum albumin (BSA) used as a standard.
Measurement of respiratory chain enzyme activities
The mitochondrial respiratory chain enzymatic activities were determined by spectrophotometry from isolated mitochondria of human atria as previously described by Medja et al.23 The data of each complex (I, II, III, IV) were expressed as a ratio between activities of a given complex and the citrate synthase.23
Measurement of citrate synthase activity
Citrate synthase activity was determined by the reduction of DTNB at 412 nm. The assay mixture contained Tris 0.1 M pH = 7.5, 5,5′-dithio-bis (2-nitrobenzoic acid) 100 µM, acetyl CoA 0.3 mM, Triton 0.1%. The mitochondrial sample was added to the assay mixture and the reaction was triggered by the addition of oxaloacetic acid 500 µM. Activity was calculated using an extinction coefficient of ɛ = 13.5 e3 L.mol−1 for 5,5′-dithio-bis (2-nitrobenzoic acid). Citrate synthase activity corresponds to the difference between the activity with and without oxaloacetic acid used as substrate.
Measurement of complex I enzymatic activity
The complex I (nicotinamide adenine dinucleotide –CoQ reductase) activity was evaluated by measuring rotenone-sensitive decrease in the absorbance of nicotinamide adenine dinucleotide at 340 nm. The assay mixture contained potassium phosphate monobasic buffer 25 mM pH 7.5, BSA 5%, dichloroindophénol 75 µM, decylubiquinone 100 µM, antimycin 0.1% 1 mg/ml, nicotinamide adenine dinucleotide 0.2 mM. The mitochondrial sample was added to the assay mixture and the reaction was triggered by the addition of 60 mM nicotinamide adenine dinucleotide. Activity was calculated using an extinction coefficient ɛ = 6.2.e3 L.mol−1 for nicotinamide adenine dinucleotide. Specific activity of the enzyme was expressed as the amount of nicotinamide adenine dinucleotide oxidized (10−9 mol.min−1.mg−1). The complex I activity represents the difference between the complex I activity without rotenone and with rotenone 25 µM measured in parallel in spectrophotometer.
Measurement of complex II enzymatic activity
The complex II (succinate deshydrogenase) activity was measured at 600 nm corresponding to the reduced form of 2,6-dichloroindophenol. The assay mixture contained potassium phosphate monobasic buffer 25 mM pH 7.5, BSA 5%, potassium cyanide 1 mM, succinate 20 mM pH 7.4, 2,6-dichloroindophénol 50 µM, ATP 0.1 M. The reaction was triggered by the addition of decylubiquinone 100 µM. Complex II activity was calculated using an extinction coefficient of ɛ = 19.1.e3 L.mol−1 for dichloroindophénol. The complex II activity represents the difference between the complex II activity with decylubiquinone and without decylubiquinone.
Measurement of complex III enzymatic activity
The antimycin-sensitive complex III activity (decylubiquinol/ferricytochrome c oxido-reductase) was evaluated by measuring the increase in reduced cytochrome c absorbance at 550 nm. The assay mixture contained potassium phosphate monobasic buffer 0.1 M pH 7.5; EDTA 250 µM; cytochrome c 50 µM final, antimycin 12.5 µM. The mitochondrial sample was added to assay mixture and reaction was started by addition of 200 µM decylubiquinol. Activity was calculated using an extinction coefficient ɛ = 18.5.e3 L.mol−1 for cytochrome c. Specific activity of the enzyme was expressed as the amount of cytochrome c reduced (nmol.min−1.mg−1). The complex III activity represents the difference between the complex III activity without antimycin and the complex III activity with antimycin.
Measurement of complex IV enzymatic activity
The complex IV activity (cytochrome c oxydase) was evaluated by measuring the decrease of reduced cytochrome c absorbance at 550 nm. The assay mixture contained potassium phosphate monobasic buffer 100 mM pH 7.5; EDTA 250 µM; cytochrome c bovin 50 µM final, antimycin 12.5 µM. The sample was added to assay mixture and reaction was started by addition of 200 µM decylubiquinol. Activity was calculated using an extinction coefficient ɛ = 18.5.e3 L.mol−1 for cytochrome c.
Experimental protocols
Isolated human right atrial contracting experiments
Trabeculae were randomly assigned to one of the experimental groups (Figure 1(a)).
Figure 1.
Schematic diagram depicting the experimental protocol. (a) All isolated human right atrial trabeculae were exposed to 30-min hypoxic period followed by 60-min reoxygenation period, alone in “Control” group (n = 6). In “Propofol All” groups, propofol at 0.1 µM, 1 µM, and 10 µM was administered 5 min before hypoxia until the end experiment n = 6 for each concentration. In “Propofol Pre” groups, propofol at 0.1 µM, 1 µM and 10 µM was administered before hypoxia during 5 min followed by 5-min washout before hypoxia (n = 6 for each concentration). In “Propofol Post” groups, propofol at 0.1 µM, 1 µM, and 10 µM was administered during reoxygenation: 5 min before reoxygenation until the end experiment (n = 6 for each concentration). In “Propofol-Post 10 µM + inhibitors” groups and “inhibitors” groups, 5-hydroxydecanoate and NG-nitro-L-arginine methyl ester were infused 10 min before reoxygenation and throughout reoxygenation (n = 6 in each group). In “Intralipid-All” group, intralipid 0.01% was administered all along the experiment (started 5 min before hypoxia) and in “Intralipid-Post” group, intralipid 0.01% was administered during the reoxygenation period (n = 6 in each group). (b) Mitochondria are isolated from atrial appendages: (1) without hypoxia-reoxygenation protocol (“Sham” group; n = 6), (2) after 30-min hypoxia followed by 15 min of reoxygenation (“Control” group; n = 6), (3) exposed to propofol 10 µM, 5 min before reoxyenation and during the 15 min reoxygenation period (“Propofol-Post 10 µM” group; n = 6) and exposed to intralipid 0.01%, 5 min before reoxyenation and during the 15 min reoxygenation period (“Intralipid-Post 0.01%” group; n = 6)
In the control group (“Control”; n = 6), trabeculae were exposed to the hypoxia-reoxygenation protocol alone.
In independent groups, propofol at 0.1 µM, 1 µM, and 10 µM was administered.
Throughout the experiment: 5 min before hypoxia until the end experiment (“Propofol All-0.1 µM,” “Propofol All-1 µM,” and “Propofol All-10 µM” groups; n = 6 for each concentration).
Before hypoxia during 5 min followed by 5-min washout (“Propofol Pre-0.1 µM,” “Propofol Pre-1 µM,” and “Propofol Pre-10 µM” groups; n = 6 for each concentration, Figure 1(a)).
During reoxygenation: 5 min before reoxygenation until the end experiment (“Propofol Post-0.1 µM,” “Propofol Post-1 µM,” and “Propofol Post-10 µM” groups; n = 6 for each concentration, Figure 1(a)).
The concentrations tested were based on plasma propofol concentrations measured during anesthesia and reported from 0.7 to 20 µg.mL−1 (4 µM to 100 µM).24 Because 97–99% of propofol is bound to proteins, free plasma propofol concentrations should be less than 3 µM.
The involvement of mitochondrial adenosine triphosphate-sensitive potassium channels and NOS was tested using 500 µM 5-hydroxydecanoate (“Post-10 µM + 5-hydroxydecanoate” group; n = 6) an antagonist of mitochondrial adenosine triphosphate-sensitive potassium channels and 200 µM L-NG-Nitroarginine méthyl ester (“Post-10 µM + NG-nitro-L-arginine methyl ester” group; n = 6) a non-selective NOS inhibitor, respectively, in presence of propofol 10 µM administered during reoxygenation period. The inhibitors were administered 10 min before and during the reoxygenation. In two additional groups, muscles were exposed to 500 µM 5-hydroxydecanoate alone (“5-hydroxydecanoate” group; n = 6) and 200 µM NG-nitro-L-arginine methyl ester alone (“NG-nitro-L-arginine methyl ester” group; n = 6), 10 min before and during the reoxygenation (Figure 1(a)). The concentrations of 5-hydroxydecanoate 500 µM and NG-nitro-L-arginine methyl ester 200 µM have been used in human myocardium in vitro.19,20
Finally, in order to evaluate the specific effect of propofol, we have tested the effect of intralipid used in the formulation of propofol solution. The selected dose of intralipid is equivalent to that administered during a 10 µM propofol infusion (less of intralipid 0.01%), administered in a first group all along the experiment (started 5 min before hypoxia; intralipid-All group; n = 6) and in the second group during the reoxygenation period (intralipid-Post group; n = 6).
Isolated mitochondria and measurement of respiratory chain complexes activity
Baseline activity of mitochondrial respiratory chain complexes was measured on mitochondria isolated from atrial appendages without hypoxia-reoxygenation protocol (“Sham” group; n = 6). The mitochondria were isolated from atrial appendages after 30-min hypoxia followed by 15 min of reoxygenation (“Control” group; n = 6). We have previously showed that 30-min hypoxia was sufficient to activate apoptotic cascade.20 Separate atrial appendages were exposed to propofol 10 µM, 5 min before reoxyenation and during the 15 min-reoxygenation period (“Propofol-Post 10 µM” group; n = 6) and in another group to intralipid 0.01%, 5 min before reoxyenation and during the 15-min reoxygenation period (“Intralipid-Post 0.01%” group; n = 6, Figure 1(b)).
Biochemicals
Propofol was purchased from B.Braun Medical (Boulogne-Billancourt, France). 5-hydroxy decanoate and NG-nitro-L-arginine methyl ester were purchase from Sigma Aldrich (Saint Quentin Fallavier, France). Intralipid 20% was purchased from Fresenius Kabi France, (Sèvre, France).
Statistics
Data are expressed as mean ± SD. Baseline values of main mechanical parameters, age, preoperative left ventricular ejection fraction, and the recovery of force of contraction at 60 min of reoxygenation were compared by univariate analysis of variance with group factor as the independent variable. Statistical analysis was performed with Statview v5.0 software (Deltasoft, Meylan, France). All P values were two-tailed and that a P value of less than 0.05 was considered significant. If the P value was less than 0.05, a Bonferroni post hoc analysis was performed.
For the mitochondrial respiratory chain, the enzymatic activity of each complex was normalized to that of the citrate synthase activity. The citrate synthase activity was measured for evaluating the quality of the mitochondria used.23 The ratio of each complex activity/citrate synthase activity was compared by univariate analysis of variance with group factor as the independent variable. If the P value was less than 0.05, a Bonferroni post hoc analysis was performed. Data are expressed as mean ± SEM.
Results
Patients’ characteristics and left ventricular ejection fraction were not different between groups (Table 1). Ninety-six right atrial trabeculae and 24 right atrial appendages were studied. There were no significant differences between the groups for trabeculae length at the apex of the length-active isometric tension curve, cross-sectional area, ratio of resting-to-total force (Table 2).
Table 1.
Patients demographic data, preoperative drug treatments, and preoperative left ventricular ejection fraction
| Groups and heart disease | Type of surgery |
Preoperative drug treatments |
Age (years) | Preoperative left ventricular ejection fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aortic valve replacement | Coronary artery bypass graft | ACE | Beta- adrenergic blocking drugs | Benzodiazepine | Calcium channel antagonists | Amiodarone | Statins | Nitroglycerin | |||
| (a) Contracting isolated human right atrial trabeculae: | |||||||||||
| Control (n = 6) | 3 | 3 | 2 | 3 | 0 | 1 | 0 | 3 | 0 | 64 ± 8 | 56 ± 11 |
| Propofol All-0.1 µM (n = 6) | 3 | 3 | 2 | 4 | 1 | 1 | 0 | 4 | 3 | 68 ± 9 | 67 ± 20 |
| Propofol All-1 µM (n = 6) | 3 | 3 | 3 | 3 | 1 | 1 | 0 | 3 | 1 | 71 ± 4 | 66 ± 4 |
| Propofol All-10 µM (n = 6) | 2 | 4 | 3 | 6 | 1 | 1 | 0 | 4 | 0 | 69 ± 6 | 75 ± 9 |
| Propofol Pre-0.1 µM (n = 6) | 2 | 4 | 2 | 4 | 0 | 2 | 2 | 2 | 1 | 71 ± 5 | 53 ± 19 |
| Propofol Pre-1 µM (n = 6) | 2 | 4 | 3 | 3 | 0 | 3 | 0 | 3 | 0 | 50 ± 9 | 50 ± 10 |
| Propofol Pre-10 µM (n = 6) | 2 | 4 | 3 | 6 | 1 | 1 | 0 | 4 | 0 | 72 ± 8 | 68 ± 11 |
| Propofol Post-0.1 µM (n = 6) | 3 | 3 | 2 | 3 | 1 | 1 | 1 | 4 | 1 | 65 ± 5 | 53 ± 15 |
| Propofol Post -1 µM (n = 6) | 4 | 2 | 4 | 2 | 0 | 2 | 2 | 3 | 0 | 68 ± 8 | 65 ± 5 |
| Propofol Post -10 µM (n = 6) | 3 | 3 | 2 | 4 | 0 | 1 | 0 | 2 | 1 | 70 ± 8 | 61 ± 9 |
| Post-10 µM + 5-Hydroxy-Decanoate (n = 6) | 4 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 73 ± 7 | 64 ± 7 |
| Post-10 µM + NG-nitro-L-arginine methyl ester (n = 6) | 2 | 4 | 3 | 5 | 0 | 1 | 0 | 5 | 0 | 77 ± 9 | 55 ± 4 |
| 5-Hydroxy-Decanoate (n = 6) | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 5 | 0 | 72 ± 6 | 68 ± 13 |
| NG-nitro-L-arginine methyl ester (n = 6) | 3 | 3 | 3 | 4 | 0 | 0 | 0 | 3 | 0 | 75 ± 11 | 65 ± 11 |
| Intralipid-All (n = 6) | 2 | 4 | 3 | 2 | 1 | 2 | 2 | 1 | 1 | 64 ± 9 | 60 ± 14 |
| Intralipid-Post (n = 6) | 2 | 4 | 3 | 3 | 1 | 2 | 1 | 1 | 1 | 68 ± 12 | 60 ± 12 |
| (b) Activity of Complexes of the mitochondrial respiratory chain | |||||||||||
| Sham (n = 6) | 4 | 2 | 0 | 5 | 0 | 1 | 0 | 2 | 1 | 77 ± 8 | 57 ± 11 |
| Control-15 min reoxygenation (n = 6) | 2 | 4 | 1 | 4 | 0 | 1 | 0 | 3 | 1 | 67 ± 14 | 53 ± 13 |
| Propofol-Post 10 µM (n = 6) | 3 | 3 | 0 | 4 | 0 | 0 | 0 | 2 | 1 | 71 ± 13 | 66 ± 7 |
| Intralipid-Post 0.01% (n = 6) | 2 | 4 | 2 | 4 | 1 | 2 | 1 | 2 | 1 | 56 ± 12 | 56 ± 12 |
ACE: angiotensin-converting enzyme inhibitors.
Note: The numbers in the columns “Type of Surgery” and “Preoperative drug treatments” indicate the number of patients undergoing this type of surgery, and the number of patients taking this type of preoperative drug treatments per group (on the total of six patients included in each group). Age and preoperative left ventricular ejection fraction are expressed as mean ± SD.
Table 2.
Control values of main mechanical parameters of human right atrial trabeculae
| Experimental groups | Lmax (mm) | CSA (mm2) | FoC (mN.mm−2) | RF/TF |
|---|---|---|---|---|
| Control (n = 6) | 6.0 ± 1.2 | 0.52 ± 0.17 | 24 ± 18 | 0.33 ± 0.14 |
| Propofol All-0.1 µM (n = 6) | 5.8 ± 1.7 | 0.33 ± 0.12 | 23 ± 12 | 0.40 ± 0.09 |
| Propofol All-1 µM (n = 6) | 6.3 ± 1.2 | 0.68 ± 0.38 | 29 ± 13 | 0.35 ± 0.12 |
| Propofol All-10 µM (n = 6) | 6.9 ± 2.0 | 0.46 ± 0.17 | 27 ± 5 | 0.29 ± 0.05 |
| Propofol Pre-0.1 µM (n = 6) | 5.8 ± 1.0 | 0.50 ± 0.16 | 19 ± 7 | 0.32 ± 0.15 |
| Propofol Pre-1 µM (n = 6) | 6.7 ± 1.8 | 0.51 ± 0.25 | 27 ± 11 | 0.35 ± 0.14 |
| Propofol Pre-10 µM (n = 6) | 5.5 ± 1.3 | 0.48 ± 0.24 | 19 ± 11 | 0.31 ± 0.12 |
| Propofol Post-0.1 µM (n = 6) | 7.0 ± 1.6 | 0.40 ± 0.09 | 17 ± 9 | 0.39 ± 0.10 |
| Propofol Post-1 µM (n = 6) | 6.5 ± 1.1 | 0.63 ± 0.24 | 18 ± 9 | 0.39 ± 0.05 |
| Propofol Post-1 0 µM (n = 6) | 5.5 ± 1.0 | 0.34 ± 0.08 | 30 ± 15 | 0.35 ± 0.12 |
| Post-10 µM + 5-Hydroxy-Decanoate (n = 6) | 4.7 ± 0.8 | 0.32 ± 0.12 | 24 ± 16 | 0.38 ± 0.11 |
| Post-10 µM + NG-nitro-L-arginine methyl ester (n = 6) | 5.3 ± 1.5 | 0.41 ± 0.15 | 26 ± 11 | 0.26 ± 0.07 |
| 5-Hydroxy-Decanoate (n = 6) | 5.9 ± 0.9 | 0.48 ± 0.07 | 17 ± 7 | 0.39 ± 0.07 |
| NG-nitro-L-arginine methyl ester (n = 6) | 5.6 ± 1.6 | 0.45 ± 0.05 | 21 ± 6 | 0.31 ± 0.06 |
| Intralipid-All (n = 6) | 5.5 ± 1.4 | 0.55 ± 0.16 | 25 ± 10 | 0.25 ± 0.07 |
| Intralipid-Post (n = 6) | 6.2 ± 1.7 | 0.37 ± 0.11 | 21 ± 10 | 0.39 ± 0.10 |
Lmax: maximal length at the apex of the length-active force curve; CSA: cross-sectional area; FoC: isometric force of contraction normalized per cross-sectional area; RF/TF: ratio of resting force on total force; LVEF: preoperative left ventricular ejection fraction.
Note: Data are mean ± SD.
Effects of propofol on contractile force of human right atrial trabeculae submitted to hypoxia-reoxygenation
In the Control group, the recovery of force of contraction at 60 min of reoxygenation was 49 ± 6% of baseline.
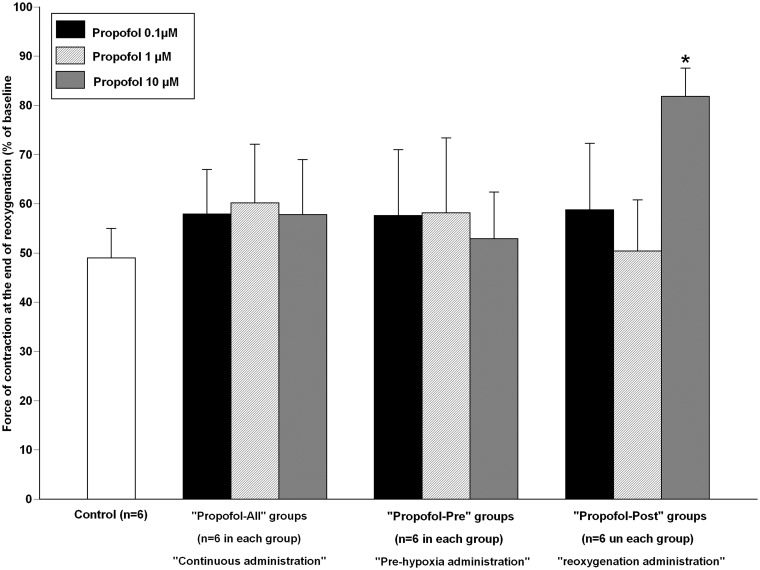
Propofol throughout the experiment did not significantly modify the force of contraction at 60 min of reoxygenation (“Propofol All-0.1 µM”: 58 ± 9% of baseline; P = 0.13 vs. “Control”; “Propofol All-1 µM”: 60 ± 12%; P = 0.52 vs. “Control” and “Propofol All-10 µM”: 58 ± 11%; P = 0.12 vs. “Control,” Figure (2)).
Figure 2.
Recovery of force of contraction of isolated human right atrial trabeculae at the end of the 60-min reoxygenation period after the 30-min hypoxic challenge in groups exposed to propofol, Control group (n = 6). In “Propofol All” groups, propofol at 0.1 µM, 1 µM, and 10 µM was administered 5 min before hypoxia until the end experiment (n = 6 for each concentration). In “Propofol Pre” groups, propofol at 0.1 µM, 1 µM and 10 µM was administered before hypoxia during 5 min followed by 5-min washout period before hypoxia (n = 6 for each concentration). In “Propofol Post” groups, propofol at 0.1 µM, 1 µM and 10 µM was administered during reoxygenation: 5 min before reoxygenation until the end experiment (n = 6 for each concentration).
Data are mean ± SD. *P < 0.001 versus “Control” group
Administration of propofol before hypoxia did not significantly modify the force of contraction at 60 min of reoxygenation at 0.1 µM (“Propofol Pre-0.1 µM”: 53 ± 13% of baseline; P = 0.48 vs. “Control”), 1 µM (“Propofol Pre-1 µM”: 56 ± 12%; P = 0.11 vs. “Control”), and 10 µM (“Propofol Pre-10 µM”: 53 ± 10%; P = 0.46 vs. “Control,” Figure (2)).
Administration of propofol during reoxygenation at 0.1 µM and 1 µM did not significantly modify the force of contraction at 60 min of reoxygenation (“Propofol Post-0.1 µM”: 59 ± 8% of baseline; P = 0.10 vs. “Control”; and “Propofol Post-1 µM”: 50 ± 10%; P = 0.78 vs. “Control”).
Propofol at 10 µM during reoxygenation significantly enhanced the force of contraction at 60 min of reoxygenation (“Propofol Post-10 µM”: 82 ± 6% of baseline; P < 0.001 vs. “Control”). As compared to “Control” group, intralipid 0.01% alone did not significantly modify the recovery of the force of contraction at 60 min of reoxygenation (55 ± 5 % of baseline in “Intralipid-All” and 52 ± 10% in “Intralipid-Post,” respectively P = 0.27 and 0.62 vs. “Control”).
Effect of 5-hydroxydecanoate and NG-nitro-L-arginine methyl ester treatment on propofol 10 µM administered during reoxygenation
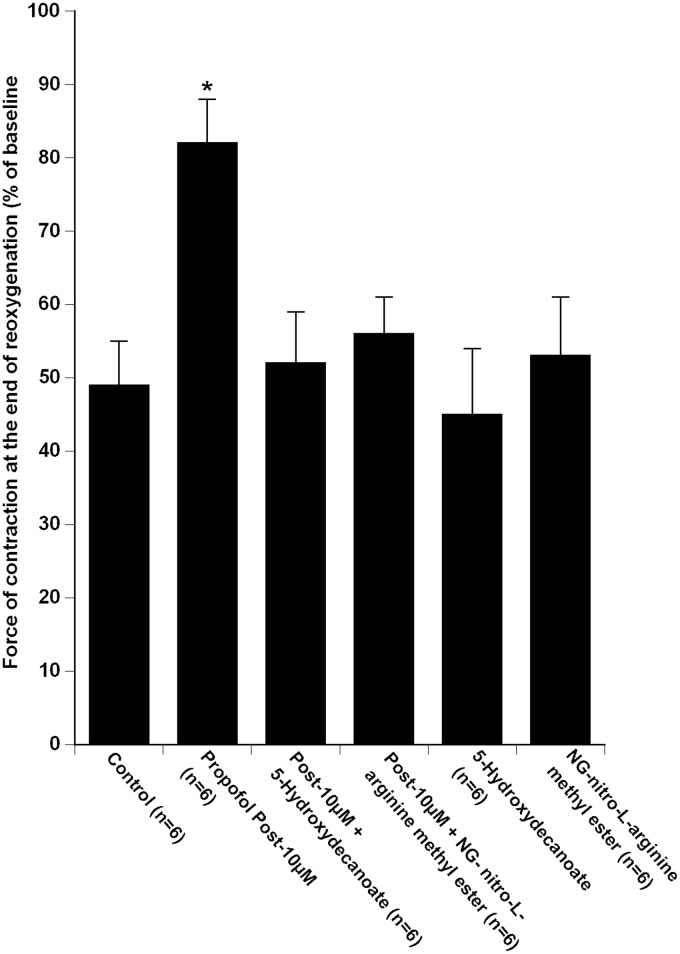
Pre-treatment with 5-hydroxydecanoate and NG-nitro-L-arginine methyl ester 5 min prior to propofol 10 µM inhibited the propofol-induced increase in the force of contraction at 60 min of reoxygenation (53 ± 8% in “Post-10 µM + 5-hydroxydecanoate” group and 57 ± 6% in “Post-10 µM + NG-nitro-L-arginine methyl ester” group of baseline; P < 0.001 vs. “Propofol Post-10 µM,” Figure 3).
Figure 3.
Recovery of force of contraction of isolated human right atrial trabeculae at the end of the 60-min reoxygenation period after the 30-min hypoxic challenge in groups exposed to propofol 10 µM during reoxygenation alone (Propofol Post-10 µM group, n = 6) and in the presence of 5-Hydroxydecanoate (“Post-10 µM + 5-Hydroxydecanoate” group, n = 6) and NG-nitro-L-arginine methyl ester (“Post -10 µM + NG-nitro-L-arginine methyl ester” group, n = 6).
Data are mean ± SD. *P < 0.0001 versus “Control,” “Post-10 µM + 5 - Hydroxydecanoate,” “Post-10 µM + NG-nitro-L-arginine methyl ester,” “5 - Hydroxydecanoate,” “NG-nitro-L-arginine methyl ester” groups
Administration of 5-hydroxydecanoate alone and NG-nitro-L-arginine methyl ester alone did not modify the force of contraction at 60 min of reoxygenation as compared to “Control” group (respectively 45 ± 9% of baseline in “5-hydroxydecanoate” group; P = 0.55 vs. “Control” and 52 ± 8% in “NG-nitro-L-arginine methyl ester” group; P = 0.44 vs. “Control”, Figure 3).
Effect of propofol 10 μM and intralipid on mitochondrial respiratory chain activity
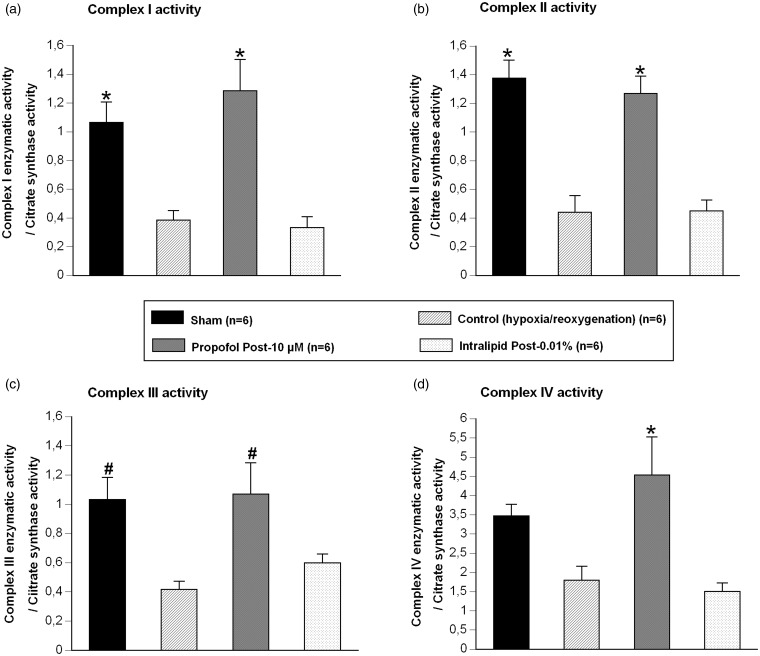
Mitochondria isolated after 30-min hypoxia followed by 15 min of reoxygenation exhibited a significant decrease in the ratio of complex I/citrate synthase (2.76 fold vs. “Sham”; P < 0.01), complex II/citrate synthase (3.12 fold vs. “Sham”; P < 0.001), and complex III/citrate synthase (2.48 fold vs. “Sham”; P < 0.05), but not for the complex IV/citrate synthase (1.93 fold vs. “Sham”; P = 0.21, Figure 4).
Figure 4.
Activity of complexes of the mitochondrial respiratory chain: the ratio complex 1 enzymatic activity / citrate synthase activity (a), the ratio complex 2 enzymatic activity / citrate synthase activity (b), the ratio complex 3 activity / citrate synthase activity (c), the ratio complex 4 activity / citrate synthase activity (d); in Sham group (n = 6), in group exposed to 30-min hypoxia followed by 15-min reoxygenation period alone (“Control” group, n = 6), in presence of propofol 10 µM during reoxygenation (“Propofol Post-10 µM” group, n = 6), and in presence of intralipid 0.01% during reoxygenation (“Intralipid Post-0.01%” group, n = 6).
Data are mean ± SEM. *P < 0.01 versus “Control” and “Intralipid Post 0.01%” groups; # P < 0.05 versus “Control” group
As compared to mitochondria from atria submitted to 30-min hypoxia followed by 15 min of reoxygenation alone (“Control”), administration of propofol 10 µM during the reoxygenation period (“Propofol-Post 10 µM”) increased the ratio of complex I/citrate synthase (3.34 fold vs. “Control”; P < 0.001), complex II/ citrate synthase (2.87 fold vs. “Control”; P < 0.01), complex III/citrate synthase (2.57 fold vs. “Control”; P < 0.05), and complex VI/citrate synthase (2.52 fold vs. “Control”; P < 0.01, Figure 4).
For all mitochondrial respiratory complexes reported to citrate synthase activity, no significant difference was observed between “Sham” and “Propofol-Post 10 µM” groups (P = 0.12, P = 0.54, P = 0.87, P = 0.51, respectively, for complex I, II, III, and IV).
Compared to “Control” group (mitochondria from atria submitted to 30-min hypoxia followed by 15 min of reoxygenation alone), administration of intralipid 0.01% during the reoxygenation period (“Intralipid-Post”) did not significantly modify the ratio of complex I/citrate synthase (P = 0.78 vs. “Control”), complex II/citrate synthase (P = 0.95 vs. “Control”), complex III/citrate synthase (P = 0.82 vs. “Control”), and complex VI/citrate synthase (P = 0.71 vs. “Control”).
Discussion
The present study showed that, in human myocardium: (1) propofol administered before and during hypoxia-reoxygenation had no effect on the recovery of contractile force following hypoxia-reoxygenation; (2) administration of propofol 10 µM during the reoxygenation period enhanced the contractile force through opening of mitochondrial adenosine triphosphate-sensitive potassium channels and activation of NOS; (3) propofol 10 µM during the early phase of reoxygenation enhanced the activity of mitochondrial respiratory chain.
Several experimental evidences suggest that propofol could exert cardioprotective effects. A continuous administration of propofol improved post-ischemic functional recovery of isolated perfused rat hearts12 and decreased the incidence of ventricular arrhythmias during acute coronary occlusion in vivo.25 In anesthetized pigs, administration of propofol (6 mg.kg−1.h−1) during cardiopulmonary bypass, attenuated the accumulation of lactate, preserved ATP content, decreased cardiac troponin I release, and reduced hemodynamic dysfunction after cardioplegic arrest.26 Additionally, propofol-induced myocardial preconditioning has been shown to decrease infarct size, left ventricular function and coronary flow in isolated heart,2,15,27 and arrhythmia occurrence in rat heart, in vivo.25 A study of Zuurbier et al.,28 in heart mouse model, showed that pharmacologic conditioning (high-dose folic acid) was ineffective to protect against myocardial ischemia reperfusion injuries, when the animals were anesthetized by a mix of fentanyl-propofol, nevertheless, the results of this study are limited by the fact that the authors used simultaneously two anesthesic, in consequence it is difficult to determine if the lack of cardioprotection was due to propofol or fentanyl.28 In contrast, the present results showed that propofol did not trigger myocardial preconditioning and that continuous administration of propofol during hypoxia-reoxygenation did not modify the recovery of contractile force of isolated human right atrial myocardium submitted to hypoxia-reoxygenation. Several hypotheses could explain, at least in part, these results:
First, in previous experimental studies concentrations of propofol were either not reported or higher than those tested in the present study. Thus, propofol concentrations administered in the present study were 3 to 100-fold lower than those reported in animal studies15,27 and represent clinically relevant concentrations. Because our results suggest a concentration dependent effect of propofol it cannot be ruled out that higher concentration of propofol may induce pharmacological preconditioning. A concentration-effect relationship has been reported for the cardioprotection induced by opioids29 and halogenated volatile anesthetics.30 Second, there are several differences between end points (from in vivo ventricular function and arrhythmias to troponin release and in vitro isometric contractile force) and experimental settings (from in vivo cardiopulmonary bypass to isolated myocardial strip) which hinder comparisons between studies. Third, species differences in myocardial physiopathology cannot be ruled out.31 Studies on animal models mainly examined healthy heart or myocardium whereas in the present study the effect of chronic diseases and treatments cannot be ruled out. Fourth, in anesthetized animals, the effect of additional anesthetic agents such as opioids and ketamine cannot be excluded.32 Finally, clinical studies have shown that as compared with volatile anesthetics, propofol based anesthesia had less or no cardioprotective effect.8,9
It has been established that propofol exerts antioxidant effect.1,5,33 In isolated rat heart, it has been shown that propofol was protective against peroxidative damage induced by exogenous H2O2.1 During surgery, propofol inhibited the oxidative damage and increased glutathione content in platelets,34 and decreased lipid peroxidation in right atrial myocardium.35 These data strongly suggest that propofol could exert cardioprotection during the reperfusion where burst of reactive oxygen species occurs. The present results showed that propofol at 10 µM administered during the reoxygenation period enhanced the recovery of force of contraction of isolated human myocardium. Altogether our results and the previous results suggest that propofol limits the bust of reactive oxygen species in early reoxygenation period, permitting a cardioprotective effect. Moreover, we showed that the intralipid emulsion (the vehicle used to deliver propofol) alone was not protective against myocardial hypoxia-reoxygenation injuries; in accordance of previous studies in animal models,3,5,34 suggesting the cardioprotective effect of propofol was not due to an excipient.
The mechanisms involved in propofol-induced cardioprotection at reperfusion remain incompletely understood. It was already demonstrated that propofol-induced postconditioning is mediated through decrease in cardiomyocyte apoptosis and NF-κB nucleus translocation potentially via ERK signaling pathways.36 Although, nitric oxide has been shown to mediate ischemic pre and postconditioning,37 its role in propofol-induced cardioprotection remains poorly studied. At the present time, one study showed that the cardioprotective effect of propofol was mediated through increased in NOS activity and Nitric Oxide production, in the isolated rat hearts.27 The present results showed that the propofol-induced cardioprotection during reoxygenation phase was abolished in the presence of NG-nitro-L-arginine methyl ester, in human myocardium suggesting that nitric oxide production was, at least in part, involved. A growing body of evidences suggests that mitochondrion is a major component of cellular cardioprotective pathways.
The role of mitochondrial adenosine triphosphate-sensitive potassium channels in the cardioprotective effect of propofol remains controversial. It has been shown that opening of mitochondrial adenosine triphosphate-sensitive potassium channels mediated the effect of preconditioning by propofol, in rat in vivo.14 In contrast, in isolated guinea pig heart submitted to ischemia-reperfusion, 5-hydroxydecanoate and glyburide (non-specific adenosine triphosphate-sensitive potassium channels antagonist) did not modify the reduction in infarct volume induced by propofol.15 Finally, 5-hydroxydecanoate has been shown to exert metabolic effects independently of adenosine triphosphate-sensitive potassium channels (i.e. inhibition of respiratory chain complexes).38 Our study shows that 5-hydroxydecanoate inhibited the enhanced recovery of myocardial contractile force induced by propofol, suggesting mitochondrial adenosine triphosphate-sensitive potassium channels could be involved, for precise the mechanism, we have then examined the effect of propofol during the reoxygenation on the respiratory chain complex of isolated mitochondria isolated from human myocardium.
Increasing evidences suggested that the mitochondrial respiratory chain is involved in myocardial reperfusion injury through initiating and promoting oxidative stress at the onset of reoxygenation period.18 It has been suggested that propofol-induced cardioprotection may partly result from a direct effect on myocardial calcium influx,39 or from inhibition of mitochondrial permeability transition.40 The proposed mechanism is that the mPTP opening modifies the fluidity and rigidity of the inner mitochondrial membrane, which influences the transport of electrons, exacerbates mitochondrial dysfunction which leads to a vicious circle including inhibition of neutrophil accumulation and inactivation of superoxide radicals.41.Shao et al.17 showed that the decrease of complexes I and III activity observed in isolated mitochondria during myocardial reperfusion was attenuated by propofol (20 to 50 µM) resulting in a reduced reactive oxygen species production.17 The present study confirms and extends these results showing that, in isolated human mitochondria, propofol 10 µM during early reoxygenation restored the activity of complex I, II, III, and IV which was decreased following hypoxia-reoxygenation (Figure 4). This result must be interpreted with the emerging concept that modulation of mitochondrial oxidative metabolism during early reperfusion protects mitochondrial function and could decrease myocardial cell injury.35
Several limitations must be considered in the interpretation of the present results. First, the effects of anesthetic drugs, the patient’s chronic diseases, and preoperative treatments, including statins, cannot be ruled out. Nevertheless, the control group was also exposed to this limit and we have shown that signaling pathways activated during cardioprotection could be studied using the present experimental model.19,20 Additionally, because in clinical practice patients received chronic treatments, it is of importance to study the cardioprotective effect of pharmacologic agents in conditions as close as possible to “real life”. Second, age has been shown to impair the effects of cardioprotective strategies. However, it has been suggested that there was no age-related difference in hypoxic preconditioning between myocardium obtained from 60–69-year and 70–89-year-old patients.42 Third, our experiments were performed under moderate hypothermia (34℃) which may have decrease the adenosine triphosphate-sensitive potassium channels sensitivity43; however, during surgical procedures moderate hypothermia may occur. Fourth, our results showed a beneficial effect of propofol on the activity of respiratory chain mitochondrial after 15 min of reoxygenation, nevertheless, we cannot speculated that the effect was maintained all along the 60 min of reoxygenation. Fifth, the specificity of the antagonist used in the present study must be considered. Thus, 5-hydroxydecanoate has been shown to exert metabolic effects independently of adenosine triphosphate-sensitive potassium channels.38 The specificity of the antagonist used in the present study must be discussed: NG-nitro-L-arginine methyl ester is a widely used non-specific inhibitor of NO synthases (NOS), mainly its inducible (iNOS or NOS2) and endothelial (eNOS or NOS3) forms. It has been shown that both iNOS and eNOS were implicated in the complex and intricated cardioprotective pathways either through direct NO effects or through indirect reactive oxygen species production (see for review Weerateerangkul et al.44; Burwell LS & Brookes PS 2008).44,45 It has been shown that NG-nitro-L-arginine methyl ester could have inhibitory effects on iron containing systems (largely present in mitochondria which play central role in cardioprotective pathways) apart from their inhibition of NO synthesis and may exerted antioxidant actions.
In conclusion, during reoxygenation, high concentration of propofol (10 µM) enhanced the contractile force of isolated human myocardium through opening of mitochondrial adenosine triphosphate-sensitive potassium channels and activation of NOS. Furthermore, the decrease in mitochondrial respiratory chain activity during hypoxia-reoxygenation was restored by propofol, supporting the importance of mitochondria in propofol-induced cardioprotection. Our results and these of others’ experimental studies show that ischemic postconditioning and propofol-induced postconditioning share the same signaling pathway46; taken together these arguments are very encouraging for clinical applicability of postconditioning by propofol in cardiac surgery, since in contrast to ischemic postconditioning, anesthetic postconditioning is a completely non-invasive maneuver.
ACKNOWLEDGEMENTS
We wish to thank Dr Babatasi, Dr Buklas, Dr Caprio, Dr Ivascau and Dr Saplacan, cardiac surgeons, for their help. Financial support was provided solely from institutional and/or departmental sources.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. SL and JLH designed the research study, reviewed the literature. SL, SA and JLH drafted the manuscript. SL and LZ conducted the experiments. SG, JLG, SA contributed to the interpretation of the data.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kokita N, Hara A. Propofol attenuates hydrogen peroxide-induced mechanical and metabolic derangements in the isolated rat heart. Anesthesiology 1996; 84: 117–27. [DOI] [PubMed] [Google Scholar]
- 2.Kamada N, Kanaya N, Hirata N, Kimura S, Namiki A. Cardioprotective effects of propofol in isolated ischemia-reperfused guinea pig hearts: role of KATP channels and GSK-3beta. Can J Anaesth 2008; 55: 595–605. [DOI] [PubMed] [Google Scholar]
- 3.Ko SH, Yu CW, Lee SK, Choe H, Chung MJ, Kwak YG, Chae SW, Song HS. Propofol attenuates ischemia-reperfusion injury in the isolated rat heart. Anesth Analg 1997; 85: 719–24. [DOI] [PubMed] [Google Scholar]
- 4.Hanouz JL, Yvon A, Flais F, Rouet R, Ducouret P, Bricard H, Gérard JL. Propofol decreases reperfusion-induced arrhythmias in a model of “border zone” between normal and ischemic-reperfused guinea pig myocardium. Anesth Analg 2003; 97: 1230–8. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi I, Kokita N, Namiki A. Propofol attenuates ischaemia-reperfusion injury in the rat heart in vivo. Eur J Anaesthesiol 2008; 25: 144–51. [DOI] [PubMed] [Google Scholar]
- 6.Xia WF, Liu Y, Zhou QS, Tang QZ, Zou HD. Protective effect of propofol and its relation to postoperation recovery in children undergoing cardiac surgery with cardiopulmonary bypass. Pediatr Cardiol 2011; 32: 940–6. [DOI] [PubMed] [Google Scholar]
- 7.De Hert SG, Cromheecke S, ten Broecke PW, Mertens E, De Blier IG, Stockman BA, Rodrigus IE, Van der Linden PJ. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology 2003; 99: 314–23. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm EE, Aune E, Norén CB, Seljeflot I, Hayes T, Otterstad JE, Kirkeboen KA. The anesthesia in abdominal aortic surgery (ABSENT) study: a prospective, randomized, controlled trial comparing troponin T release with fentanyl-sevoflurane and propofol-remifentanil anesthesia in major vascular surgery. Anesthesiology 2013; 119: 802–12. [DOI] [PubMed] [Google Scholar]
- 9.Lurati Buse GA, Schumacher P, Seeberger E, Studer W, Schuman RM, Fassl J, Kasper J, Filipovic M, Bolliger D, Seeberger MD. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation 2012; 126: 2696–704. [DOI] [PubMed] [Google Scholar]
- 10.McDermott BJ, McWilliams S, Smyth K, Kelso EJ, Spiers JP, Zhao Y, Bell D, Mirakhur RK. Protection of cardiomyocyte function by propofol during simulated ischemia is associated with a direct action to reduce pro-oxidant activity. J Mol Cell Cardiol 2007; 42: 600–8. [DOI] [PubMed] [Google Scholar]
- 11.He W, Zhang FJ, Wang SP, Chen G, Chen CC, Yan M. Postconditioning of sevoflurane and propofol is associated with mitochondrial permeability transition pore. J Zhejiang Univ Sci B 2008; 9: 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res 2000; 45: 360–9. [DOI] [PubMed] [Google Scholar]
- 13.Jovic M, Stancic A, Nenadic D, Cekic O, Nezic D, Milojevic P, Micovic S, Buzadzic B, Korac A, Otasevic V, Jankovic A, Vucetic M, Velickovic K, Golic I, Korac B. Mitochondrial molecular basis of sevoflurane and propofol cardioprotection in patients undergoing aortic valve replacement with cardiopulmonary bypass. Cell Physiol Biochem 2012; 29: 131–42. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Yao JY, Qian C, Chen R, Li XY, Liu SW, Sun BG, Song LS, Hong J. Effects of propofol on ischemia-induced ventricular arrhythmias and mitochondrial ATP-sensitive potassium channels. Acta Pharmacol Sin 2012; 33: 1495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur S, Farhangkhgoee P, Karmazyn M. Cardioprotective effects of propofol and sevoflurane in ischemic and reperfused rat hearts: role of K(ATP) channels and interaction with the sodium-hydrogen exchange inhibitor HOE 642 (cariporide). Anesthesiology 1999; 91: 1349–60. [DOI] [PubMed] [Google Scholar]
- 16.Rigoulet M, Devin A, Averet N, Vandais B, Guerin B. Mechanisms of inhibition and uncoupling of respiration in isolated rat liver mitochondria by the general anesthetic 2,6-diisopropylphenol. Eur J Biochem 1996; 241: 280–5. [DOI] [PubMed] [Google Scholar]
- 17.Shao H, Li J, Zhou Y, Ge Z, Fan J, Shao Z, Zeng Y. Dose-dependent protective effect of propofol against mitochondrial dysfunction in ischaemic/reperfused rat heart: role of cardiolipin. Br J Pharmacol 2008; 153: 1641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–35. [DOI] [PubMed] [Google Scholar]
- 19.Lemoine S, Beauchef G, Zhu L, Renard E, Lepage O, Massetti M, Khayat A, Galera P, Gérard JL, Hanouz JL. Signaling pathways involved in desflurane-induced postconditioning in human atrial myocardium in vitro. Anesthesiology 2008; 109: 1036–44. [DOI] [PubMed] [Google Scholar]
- 20.Lemoine S, Allouche S, Coulbault L, Cornet V, Massetti M, Galera P, Gérard JL, Hanouz JL. Mechanisms involved in cardioprotective effects of pravastatin administered during reoxygenation in human myocardium in vitro. Anesthesiology 2012; 116: 824–33. [DOI] [PubMed] [Google Scholar]
- 21.De Hert SG, Van der Linden PJ, Cromheecke S, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology 2004; 101: 299–310. [DOI] [PubMed] [Google Scholar]
- 22.Frässdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, Weber R, Müllenheim J, Weber NC, Preckel B, Schlack W. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg 2009; 137: 1436–42. 1442.e1–2. [DOI] [PubMed] [Google Scholar]
- 23.Medja F, Allouche S, Frachon P, Jardel C, Malgat M, Mousson de Camaret B, Slama A, Lunardi J, Mazat JP, Lombès A. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 2009; 9: 331–9. [DOI] [PubMed] [Google Scholar]
- 24.Shafer A, Doze VA, Shafer SL, White PF. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology 1988; 69: 348–56. [DOI] [PubMed] [Google Scholar]
- 25.Hirata N, Kanaya N, Kamada N, Kimura S, Namiki A. Differential effects of propofol and sevoflurane on ischemia-induced ventricular arrhythmias and phosphorylated connexin 43 protein in rats. Anesthesiology 2009; 110: 50–7. [DOI] [PubMed] [Google Scholar]
- 26.Lim KH, Halestrap AP, Angelini GD, Suleiman MS. Propofol is cardioprotective in a clinically relevant model of normothermic blood cardioplegic arrest and cardiopulmonary bypass. Exp Biol Med (Maywood) 2005; 230: 413–20. [DOI] [PubMed] [Google Scholar]
- 27.Sun HY, Xue FS, Xu YC, Li CW, Xiong J, Liao X, Zhang YM. Propofol improves cardiac functional recovery after ischemia-reperfusion by upregulating nitric oxide synthase activity in the isolated rat hearts. Chin Med J (Engl) 2009; 122: 3048–54. [PubMed] [Google Scholar]
- 28.Zuurbier CJ, Heinen A, Koeman A, Stuifbergen R, Hakvoort TB, Weber NC, Hollmann MW. Cardioprotective efficacy depends critically on pharmacological dose, duration of ischaemia, health status of animals and choice of anaesthetic regimen: a case study with folic acid. J Transl Med 2014; 12: 325–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Irwin MG, Wong TM. Remifentanil preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology 2004; 101: 918–23. [DOI] [PubMed] [Google Scholar]
- 30.Nasu I, Yokoo N, Takaoka S, Takata K, Hoshikawa T, Okada M, Miura Y. The dose-dependent effects of isoflurane on outcome from severe forebrain ischemia in the rat. Anesthesiology 2006; 103: 413–8. [DOI] [PubMed] [Google Scholar]
- 31.Riou B. Halogenated anesthetics and human myocardium. Anesthesiology 2000; 92: 1–2. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig LM, Patel HH, Gross GJ, Kersten JR, Pagel PS, Warltier DC. Morphine enhances pharmacological preconditioning by isoflurane: role of mitochondrial K(ATP) channels and opioid receptors. Anesthesiology 2003; 98: 705–11. [DOI] [PubMed] [Google Scholar]
- 33.Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG. The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth 1992; 68: 613–8. [DOI] [PubMed] [Google Scholar]
- 34.De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg 1999; 89: 1050–5. [DOI] [PubMed] [Google Scholar]
- 35.Sayin MM, Ozatamer O, Taşöz R, Kilinç K, Unal N. Propofol attenuates myocardial lipid peroxidation during coronary artery bypass grafting surgery. Br J Anaesth 2002; 89: 242–6. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Tan J, Zou Z, Huang CG, Shi XY. Propofol post-conditioning protects against cardiomyocyte apoptosis in hypoxia/reoxygenation injury by suppressing nuclear factor-kappa B translocation via extracellular signal-regulated kinase mitogen-activated protein kinase pathway. Eur J Anaesthesiol 2011; 28: 525–34. [DOI] [PubMed] [Google Scholar]
- 37.Strijdom H, Chamane N, Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr 2009; 20: 303–10. [PMC free article] [PubMed] [Google Scholar]
- 38.Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol 2002; 542: 735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buljubasic N, Marijic J, Berczi V, Supan DF, Kampine JP, Bosnjak ZJ. Differential effects of etomidate, propofol, and midazolam on calcium and potassium channel currents in canine myocardial cells. Anesthesiology 1996; 85: 1092–9. [DOI] [PubMed] [Google Scholar]
- 40.Sztark F, Ichas F, Ouhabi R, Dabadie P, Mazat JP. Effects of the anaesthetic propofol on the calcium-induced permeability transition of rat heart mitochondria: direct pore inhibition and shift of the gating potential. FEBS Lett 1995; 368: 101–4. [DOI] [PubMed] [Google Scholar]
- 41.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298: 229–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg 2000; 120: 660–7. [DOI] [PubMed] [Google Scholar]
- 43.Saito W, Noguchi K, Okazaki K, Matsuda T, Kato Y, Tanaka H, Shigenobu K. Temperature-sensitive effects of potassium channel openers on isolated guinea pig myocardium and aorta. J Cardiovasc Pharmacol 1998; 31: 327–9. [DOI] [PubMed] [Google Scholar]
- 44.Weerateerangkul P, Chattipakorn S, Chattipakorn N. Roles of the nitric oxide signaling pathway in cardiac ischemic preconditioning against myocardial ischemia-reperfusion injury. Med Sci Monit 2011; 17: RA44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal 2008; 10: 579–99. [DOI] [PubMed] [Google Scholar]
- 46.Rohilla A, Rohilla S, Kushnoor A. Myocardial postconditioning: next step to cardioprotection. Arch Pharm Res 2011; 34: 1409–15. [DOI] [PubMed] [Google Scholar]