Abstract
Circulating tumor cells (CTCs) in the blood of cancer patients have been demonstrated to be of prognostic value regarding metastasis and survival. The CellSearch® system has been certified for the detection of CTCs and as a prognostic tool in patients with metastatic breast, colon and prostate cancer. Few studies have evaluated the detection of CTCs originating from esophagogastric or pancreatic cancer with the CellSearch® system. In the present small pilot study, a total of 16 patients with either esophagogastric (n=8) or pancreatic (n=8) adenocarcinomas at various disease stages were randomly screened and included. A total of 7.5 ml of blood was drawn from each patient and analyzed for CTCs using the CellSearch® device. CTCs could be detected in 1 out of 8 patients (12.5%) with esophagogastric and in 7 out of 8 patients (87.5%) with pancreatic cancer. The preliminary data obtained from this observational feasibility study suggested that the CellSearch® system may become a valuable tool for the detection of CTCs in patients with pancreatic adenocarcinoma, whereas the usefulness in patients with early-stage esophagogastric adenocarcinoma may be limited. This study clearly points towards a requirement for larger studies focusing on patients with pancreatic adenocarcinoma at various disease stages and assessing CTCs, whereas patients with esophagogastric adenocarcinomas should be part of further pilot studies.
Keywords: circulating tumor cells, pancreatic cancer, esophagogastric cancer
Introduction
Adenocarcinomas of the esophagogastric junction (AEGJ) are of particular interest nowadays, as numerous studies from a range of industrialized Western countries have reported an increased incidence of adenocarcinomas of the esophagus and the cardia over the last 30 years, which is in contrast to the decreasing incidence of gastric cancer (1). These tumors are sub-classified based on the anatomical-topographical location of the tumor center according to the Siewert classification (2). AEGJ are currently staged according to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer Tumor-Node-Metastasis (TNM) classification system (3). The overall survival (OS) rate has been reported to be 15–20% and the 5-year survival rates are ~40% following a complete (R0) resection (4,5).
Exocrine tumors are the most common type of pancreatic cancer, the majority presenting with the histological characteristics of adenocarcinoma, resembling the pancreatic ductal cell (6). Among all cancer-related mortalities in the United States, malignant tumors of the pancreas rank fourth (7) and the overall (global) mortality rate is 98% (8). As a number of patients already present with advanced disease or even metastasis at the time of diagnosis, only 10–20% of the patients are eligible for surgical resection. These patients have a 5-year survival rate of 10–24% for cases with R0 resection (9,10). This poor prognosis reflects the particularly aggressive and lethal nature of this type of cancer. Recurrence rates of almost 80% after have also been observed following R0 resections (10).
Over the past decade, the detection of circulating tumor cells (CTCs) in the peripheral blood of cancer patients has gained more and more attention. An increasing number of trials have suggested that those patients who tested positive for CTCs experienced shorter survival times than those who tested negative for CTCs. For example, primary breast cancer patients who tested ‘positive’ for CTCs (i.e., ≥5 CTCs/7.5 ml of blood) had a shorter progression-free survival (PFS) time (2.7 vs. 7.0 months) and a shorter OS time (10.1 vs. >18 months) compared with those who tested ‘negative’ (i.e., <5 CTC/7.5 ml of blood) (11,12).
The CellSearch® system is a validated and widely accepted device for the detection of CTCs in human peripheral blood (13), which has already gained approval by the American Food and Drug Administration (FDA) for the detection of CTCs and as a prognostic tool to predict PFS and OS in patients with metastatic disease originating from the prostate, breasts and colon (14).
However, limited data regarding the feasibility of detection of CTCs in patients with pancreatic or esophagogastric adenocarcinoma is available. Therefore, the aim of the present small study was to determine the feasibility and frequency of the detection of CTCs by applying the CellSearch® system in these two tumor entities, with the primary endpoint being the detection of CTCs (yes or no). We hypothesized that CellSearch® would be able to detect CTCs in the blood of patients from the two disease groups regardless of the stage of the disease.
Patients and methods
Study population
Following approval by the local ethics committee (Kantonale Ethikkommission Zurich, Zurich, Switzerland), 16 consecutive patients (>18 years old) with either AEGJ or pancreatic cancer (8 patients each) at various disease stages and undergoing various treatments were included in the study. Patients were only excluded in instances of any ethical contraindications or the inability of the patient to understand the language of the center where the study was performed (i.e., German). Written informed consent was obtained from all patients.
Detection of CTCs using the CellSearch system
In total, 7.5 ml of blood was drawn from each patient into CellSave Preservative tubes (Janssen Diagnostics, Raritan, NJ, USA). Blood collection from pancreatic cancer patients was conducted during an office visit. For esophagogastric cancer patients, two different time points were established to collect the blood samples: i) Prior to chemotherapy or surgery (if eligible; t0); and ii) 60 min after the surgical en bloc resection of the tumor (if eligible; t1). Details about the timing of blood collection for each patient are provided in Table I.
Table I.
Characteristics of all 8 patients with esophagogastric adenocarcinoma.
| Case no. | Age/gender | Siewert type | Time point | No. of CTCs | TNM stage (34) | Metastasis | Prior chemotherapy | Prior radiation | Prior surgery | Relevant medical history (ref) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41/M | I | t0 | 0 | ypT3 ypN1 M0 | None | None | None | None | None |
| 2 | 69/M | II | t1 | 0 | cT4 cN1 cM0 | None | 4 cycles of FLOT | None | Diagnostic laparoscopy | COPD GOLD III (35) |
| 3 | 52/F | I | t1 | 0 | cT3 cN+ cM1 | Deep cervical lymph nodes | 5 cycles of carboplatin/paclitaxel | 42 Gy | None | Diffuse large cell lymphoma (stage IIIb), arterial HTN |
| 4 | 62/M | III | t1 | 0 | ypT3 ypN3 ycM0 | None | 4 cycles of FLOT | None | Diagnostic laparoscopy | Endocarditis of the aortic valve, CAD/NSTEMI |
| 5 | 72/F | I | t0 | 3 | uT2 uN0 cM1 | Bone (singular) | None | None | None | Stroke |
| 6 | 82/M | I | t0+t1 | 0 | cT3 cN1 cM0 | None | None | None | None | Aortic regurgitation |
| 7 | 76/F | I | t0+t1 | 0 | cT3 N1 M0 | None | None | None | None | Bladder carcinoma, hypertensive CM |
| 8 | 65/M | I | t0+t1 | 0 | cT2 N+ M0 | None | 4 weekly doses of cisplatin/docetaxel | 45 Gy | Diagnostic laparoscopy | OSA, arterial HTN; hypothyroidism, TIA |
FLOT, 5-fluorouracil, leucovorin, oxaliplatin, docetaxel; COPD, chronic obstructive pulmonary disease; HTN, hypertension; CAD, coronary artery disease; NSTEMI, non-ST elevation myocardial infarction; CM, cardiomyopathy; OSA, obstructive sleep apnea; TIA, transient ischemic attack; F, female; M, male; TNM, Tumor-Node-Metastasis; t0, prior to chemotherapy/surgery; t1, 60 min post-en bloc resection; c, staging via clinical examination or small surgical/diagnostic procedures; (y)p, staging via histopathologic examination (following neoadjuvant chemotherapy); u, staging via ultrasound examination.
CTC analysis was conducted in accordance with the manufacturer's instructions (Janssen Diagnostics). Briefly, the cells were initially centrifuged at room temperature at 800 × g for 10 min. Subsequently, further processing of the blood cells was performed with the automated CellTracks® Autoprep® System (Janssen Diagnostics), which uses ferrofluid nanoparticles coated with an antibody against epithelial cell adhesion molecule (EpCAM) for the enrichment of CTC, as well as fluorescent staining against cytokeratin and the cell surface marker cluster of differentiation 45 (CD45). Cell nuclei were stained using 4′,6-diamidino-2-phenylindole. Finally, cells captured in the MagNest cassette were analyzed with CellTracks Analyzer II®. Nucleated cells, which stained positive for cytokeratin and negative for CD45 (Fig. 1) were counted as CTCs by two specially trained individuals.
Figure 1.
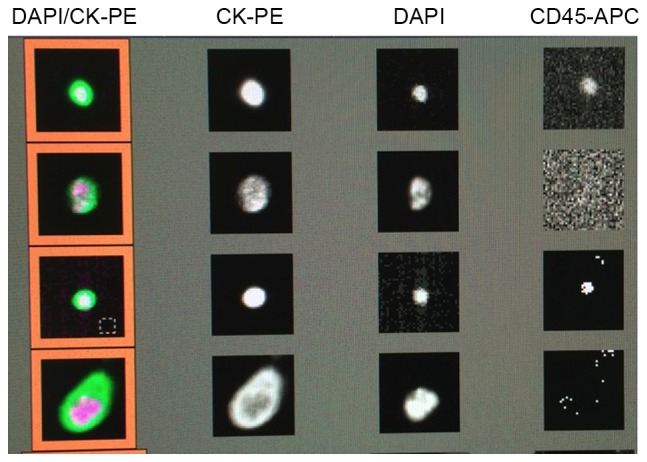
Representative images of circulating tumor cells (CTCs) detected with the CellTracks Analyzer II® after enrichment of whole blood samples using nanoparticles with an antibody directed against epithelial cell adhesion molecule and subsequent staining with 4′,6-diamidino-2-phenylindole (DAPI; for nuclei), the epithelial marker cytokeratin (CK) and the hematopoetic cell surface marker cluster of differentiation 45 (CD45). Nucleated cells that stained positive for CK and negative for CD45 were counted as CTCs. PE, phycoerythrin; APC, antigen-presenting cell.
Results
Patient characteristics
A total of 8 patients (3 females and 5 males), with locally-advanced (cT2-4, N any, M any) AEGJ and a median age of 67 years (range, 41–82 years) were included in the study (Table I). Loco-regional lymph nodes (cN+) were detected in 7 out of 8 (87.5%) patients and distant metastases (lymph node or bone) were present in 2 out of 8 patients (25.0%) (Table I). Additionally, 4 out of 8 (50.0%) patients received chemotherapy prior to the blood collection for CTC detection. Of these, 2 patients received 4 cycles of a 5-fluorouracil, leucovorin, oxaliplatin and docetaxel regimen, 1 patient underwent pre-treatment with 5 cycles of carboplatin and paclitaxel, and 1 patient received 4 weekly doses of a cisplatin and docetaxel-based regimen. Of the 8 patients, 2 (25.0%) were subjected to concurrent pre-operative local radiotherapy (42–45 Gy), and 3 (37.5%) had undergone a diagnostic laparoscopy during the staging process (Table I).
The characteristics of the patients with pancreatic adenocarcinoma are summarized in Table II. For this group, 8 consecutive patients (2 females and 6 males) at different disease stages were enrolled in the study. The patients had a median age of 60 years at the time of study entry (range, 35–73 years). In total, 6 out of 8 (75.0%) patients presented with loco-regional lymph node metastases and 4 out of 8 (50.0%) patients exhibited distant metastases, as specified in Table II. With regard to treatment, 5 out of 8 patients (62.5%) received chemotherapy prior to CTC detection. The majority of patients received the folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) regimen; in 4 patients, a median of 8 cycles (range, 1–13 cycles) were used, representing the standard of care for metastatic pancreatic adenocarcinoma. Prior to FOLFIRINOX, 2 patients had received 6 cycles of gemcitabine (weekly), whereas 1 patient had undergone 5 cycles of gemcitabine + chloroquine (within a phase II clinical trial) only. Radiation therapy was administered to 3 (37.5%) patients prior to the CTC measurement (median, 30 Gy; range, 20–50 Gy). Surgical interventions had been conducted in 5 out of 8 patients (62.5%), with the majority of those being strictly palliative (Table II).
Table II.
Characteristics of all 8 patients with pancreatic adenocarcinoma included in the study.
| Case no. | Age/gender | No. of CTCs | TNMa | Metastasis | Prior chemotherapy | Prior radiation | Prior surgery | Relevant medical history (ref) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73/M | 1 | cT4 cN1 M1 | Peritoneum | None | None | None | Dilatative CM, arterial HTN, DM type 2 | |
| 2 | 35/M | 25 | cT4 cN1 cM0 | None | 12 cycles of FOLFIRINOX | None | Nanoknife | None | |
| 3 | 47/M | 42 | cT2 cN0 cM0 | None | 6 cycles of gemcitabine (weekly), 4 cycles of FOLFIRINOX | 50 Gy | Gastric bypass (palliative) | Arterial HTN | |
| 4 | 64/M | 2 | pT3 pN1 M0 | None | 1 cycle of FOLFIRINOX | None | None | Arterial HTN, hypertensive CM, DVT | |
| 5 | 70/F | 2 | cT4 cNx cM0 | None | 5 cycles of gemcitabine + chloroquine | None | Y-Roux anastomosis, hepatico-jejunostomy, gastro-enterostomy | Cachexia, hyperthyreosis, malignant melanoma | |
| 6 | 66/M | 83 | cT4 cN+ M1 | Bone, lung, liver, brain | None | 20 Gy | None | DM type 2, PAOD, COPD GOLD II (35) | Succumbed 4 days after CTC analysis |
| 7 | 56/M | 0 | cT4 cN1 pM1 | Lung, liver | None | None | Wedge resection (metastasis) lower lobe left lung | HIV, hepatitis B (chronic) | |
| 8 | 53/F | 7 | pT3 pN1 M1 | Liver, brain, bone | 6 cycles of gemcitabine, 13 cycles of FOLFIRINOX | 30 Gy | Duodeno-pancreatectomy | PBC |
34). FOLFIRINOX, folinic acid, 5-fluorouracil, irinotecan and oxaliplatin; CM, cardiomyopathy; DM, diabetes mellitus; HTN, hypertension; PAOD, peripheral arterial occlusive disease; COPD, chronic obstructive pulmonary disease; PBC, primary biliary cirrhosis; TNM, Tumor-Node-Metastasis; M, male; F, female.; c, staging via clinical examination or small surgical/diagnostic procedures; (y)p, staging via histopathologic examination (following neoadjuvant chemotherapy); u, staging via ultrasound examination.
Detection of CTCs
CTCs were detected (CTC count ≥1/7.5 ml of blood) in the circulation of only 1 out of the 8 patients with AEGJ (12.5% of total; median CTC count of 3/7.5 ml of blood; Table I). However, CTCs were detected in 7 out of 8 patients (87.5%) with pancreatic adenocarcinoma, where a median overall CTC count of 4.5/7.5 ml of blood (range, 0–83/7.5 ml of blood; Table II) was recorded (Fig. 2).
Figure 2.
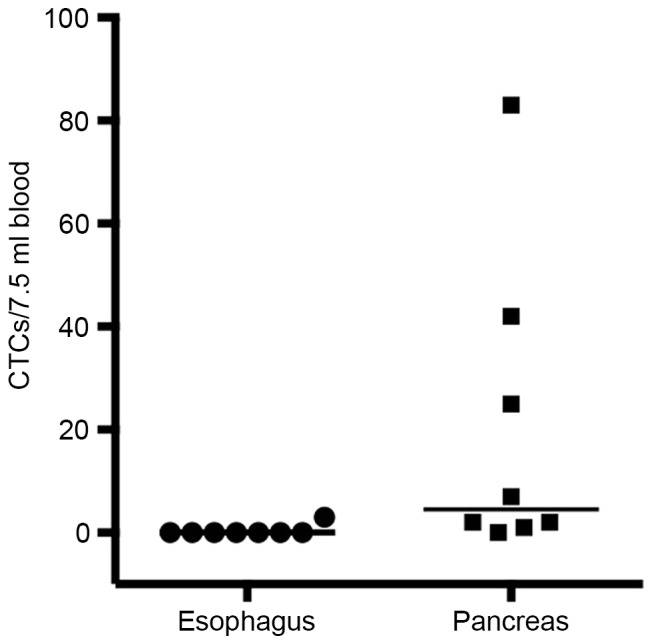
Scatter plot of the number of ciculating tumor cells (CTCs) found in 7.5 ml of blood drawn from patients with either esophagogastric or pancreatic adenocarcinoma. Horizontal lines indicate the median number of CTCs.
Discussion
Thus far, the CellSearch® device has only been approved by the FDA for the detection of CTCs and as a prognostic tool to predict PFS and OS times in metastatic breast, colon and prostate cancer (14). However, as there is only limited literature available regarding the use of CellSearch® in patients with esophagogastric or pancreatic cancer (15–18), the present study aimed to determine whether CTCs are detectable by the CellSearch® system in these particular patient groups. AEGJ and pancreatic cancer were chosen due to their epithelial cell characteristic, an increasing incidence in the Western world and the rather high mortality even after surgical and/or chemotherapeutic treatments. Therefore, developing a prognostic tool would be of high clinical importance, maybe even in order to guide therapeutic decisions. CTCs were identified in 7 out of 8 pancreatic cancer patients (87.5%), while detection of CTCs originating from an AEGJ was only possible in 1 out of 8 patients (12.5%). The current study, even though the number of subjects included was low, offered the advantage of no inclusion bias, as the patients were randomly screened. At the same time, it allowed a 1:1 comparison of the two tumor entities. CTCs were determined using one single device under similar circumstances within the same period of time at a single university center.
The impact of the presence of CTCs in the circulation on patient outcome and survival has been studied extensively over the last years. A recently published meta-analysis pooling >6,800 patients investigated the prognostic value of CTCs in breast cancer (19): CTCs were associated with an increased risk of recurrence of the disease [hazard ratio (HR), 2.86; 95% confidence interval (CI), 2.19–3.75] and with significantly higher mortality rates (HR, 2.78; 95% CI, 2.22–3.48). The analysis also provided evidence that the presence of CTCs was associated with a poorer prognosis in early-stage and metastatic breast cancer, regardless of the detection method [CellSearch® assay or reverse-transcriptase polymerase chain reaction (RT-PCR)] (19). There have been numerous studies trying to determine a cutoff-value for the CTC count as a prognostic decision point. Studies have suggested a CTC count of ≥5/7.5 ml of blood to be valid for metastatic breast cancer (11,20), and a count of ≥3/7.5 ml of blood for metastatic colorectal cancer (21). However, the statistical determination method of these cutoff-values along with their prognostic and clinical importance are sources of controversy (22).
Thus far, the detection of CTCs in the circulation of patients with AEGJ has been based on anecdotal findings and case reports (15). A recent study reported the successful detection of CTCs (≥2 CTCs/7.5 ml of blood) in 8 out of 18 patients (44.4%) with gastric or esophageal cancer (16), which could not be confirmed by the present small series. Notably, the only patient who tested positive in the current study presented with distant metastases, whereas CTCs could not be detected in any of the 6 patients staged M0 [in accordance with the TNM classification (3)]. We may therefore hypothesize that the stage of the disease could have an impact on the detection (or even the presence) of CTCs in patients with AEGJ. This would also be in accordance with the results of a recent study in patients with esophageal squamous cell carcinoma demonstrating a correlation between the stage of the disease, the presence of distant metastasis and the detection of CTCs (16), which has also been shown in patients with colorectal cancer (23).
For pancreatic cancer, there are also only a few studies evaluating CTCs. There is certain supporting evidence that CTCs originating from pancreatic adenocarcinoma can not only be detected, but may have a prognostic value as well (17): Kurihara et al (17) clearly showed a correlation between survival times and the presence of CTCs (≥1 CTC/7.5 ml of blood) in patients with advanced pancreatic carcinoma: In cases where CTCs were detected, patients with disease stage IV had a mean survival time of only 53 days, as opposed to 308 days for the CTC-negative patients (0 CTCs/7.5 ml of blood). Notably, the patient with the highest number of CTCs (105 CTCs/7.5 ml of blood) succumbed to the disease 5 days after the measurement. In the present study, the highest CTC value found was 83 CTCs/7.5 ml of blood. This patient also succumbed to the disease 4 days after the CTC measurement.
In a larger study (79 patients), Bidard et al (18) not only showed that CTC-positive patients with locally advanced pancreatic cancer (tested prior to and after chemotherapy) experienced shorter survival times, but also that the tumors of these patients were poorly-differentiated compared with those of CTC-negative patients. It has been shown that pancreatic endocrine tumors usually present with a relatively stable expression of EpCAM, thus making it possible to detect CTCs of these tumors with any EpCAM-based enrichment method (24).
A recent study has suggested that the site where the blood for CTC determination is drawn could also be of importance. Patients undergoing surgery for pancreatic cancer who presented with CTCs in portal venous blood exhibited a significantly higher rate of liver metastases 3 years after surgery compared with CTC-negative patients, whereas the presence of CTCs in the systemic circulation had no impact (25).
However, it is possible that CTCs escape the detection process, even in patients with advanced and metastatic disease, due to a loss in EpCAM expression, e.g., as found in breast cancer (26). This downregulation of EpCAM expression by CTCs is most likely due to the process of epithelial-to-mesenchymal transition (27), which is a crucial step during the process of the liberation of CTCs into the circulation (28). This may also explain the fact that the detection rate of CTCs differs significantly between the different methods used. The isolation by size of epithelial tumors technique, which is based on the filtration of cellular blood components through a membrane microfilter device (29), was able to detect CTCs originating from pancreatic adenocarcinomas not only more frequently, but also at higher numbers than the CellSearch® system [frequency, 93 vs. 40%; median, 9 CTCs/7.5 ml of blood (range, 0–240) vs. 0 CTCs/7.5 ml of blood (range, 0–144, respectively) (30). Similar findings were reported for CTCs from esophagogastric adenocarcinoma, where another size-based enrichment method (MetaCell®) was able to detect CTCs in 15 out of 20 patients (75.0%) (31).
The number of patients included in the present preliminary study was small (n=8). It was chosen based on a reasonable balance between cost effectiveness and the opportunity to test our hypothesis. No solid conclusions can therefore be drawn from this finding in comparison with previous studies reporting larger sample sizes ranging from 16 to 79 patients (17,18,25,30,32). However, this was also not the overall goal of this study. Instead, the study aimed to evaluate whether it is generally possible to detect CTCs in patients with either AEGJ or pancreatic adenocarcinoma, regardless of the stage of the disease. In addition, due to the randomly screened nature and the fact that patients were chosen at various stages of the respective disease, any form of inclusion bias can definitely be excluded for the current study, which clearly demonstrates that CTCs originating from pancreatic adenocarcinoma can be detected by the CellSearch® device at various stages of the disease. A direct comparison between the detection rates of CTCs in the AEGJ and pancreatic patients in the present study may be inadequate, as the two groups were different in terms of the stage of the disease (only 2 out of 8 AEGJ patients with distant metastases vs. 4 out of 8 patients in the pancreatic cancer group). However, it can be concluded that the detection of CTCs in patients with AEGJ at early stages may be difficult.
Due to the small number of patients in the present study, we can only speculate about a correlation between the presence and detection of CTCs in these patients with the stage of the disease. Therefore, the feasibility of the CellSearch® system for this particular type of cancer remains questionable at this point. Recent studies in combination with the results from the present study suggest that size-based filtration methods may be superior to the CellSearch® method for the detection of CTCs in patients with esophagogastric cancer (31).
Despite its limitations, CellSearch® offers a good method for the detection of CTCs in several tumor entities. In addition to the FDA-approved application in breast, colorectal and prostate cancer, the present study underlines the possibility that pancreatic adenocarcinoma may be another tumor entity with solid CTC detection rates (17). The main advantage of the system is its easy practicability: the blood sample can be stored up to 96 h at room temperature. In addition, the majority of the steps during the detection are automated and therefore less prone to human error. This clearly offers the possibility of performing large multicenter trials. However, as already discussed, the price for this increase in practicability may be a loss in sensitivity when compared with size-based filtration methods (30) or RT-PCR (33).
In summary, the results of this small pilot study may be important for the design of future studies with regard to the number of patients to be included. The preliminary data for this observational feasibility study suggested that the CellSearch® system may become a valuable tool for the detection of CTCs in patients with pancreatic adenocarcinoma, whereas the usefulness in patients with early-stage esophagogastric adenocarcinoma may be limited. While large trials focusing on patients with pancreatic adenocarcinomas may be feasible, further pilot studies are warranted for esophagogastric tumors.
References
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 3.Sobin LH, Gospodarowicz MK, Wittekind C. International union against cancer: TNM classification of malignant tumours. Wiley-Blackwell; Chichester, West Sussex, UK: 2010. [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Schiesser M, Schneider PM. Surgical strategies for adenocarcinoma of the esophagogastric junction. Recent Results Cancer Res. 2010;182:93–106. doi: 10.1007/978-3-540-70579-6_8. [DOI] [PubMed] [Google Scholar]
- 6.Esposito I, Konukiewitz B, Schlitter AM, Klöppel G. Pathology of pancreatic ductal adenocarcinoma: Facts, challenges and future developments. World J Gastroenterol. 2014;20:13833–13841. doi: 10.3748/wjg.v20.i38.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 9.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: An overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 10.Arvold ND, Ryan DP, Niemierko A, Blaszkowsky LS, Kwak EL, Wo JY, Allen JN, Clark JW, Wadlow RC, Zhu AX, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118:3026–3035. doi: 10.1002/cncr.26633. [DOI] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 12.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 13.de Wit S, van Dalum G, Terstappen LW. Detection of circulating tumor cells. Scientifica (Cairo) 2014;2014:819362. doi: 10.1155/2014/819362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother. 2013;62:931–939. doi: 10.1007/s00262-012-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu Q, Bittencourt Mde C, Cai H, Bastien C, Lemarie-Delaunay C, Bene MC, Faure GC. Case Report: Detection and quantification of tumor cells in peripheral blood and ascitic fluid from a metastatic esophageal cancer patient using the CellSearch (R) technology. F1000Res. 2014;3:12. doi: 10.12688/f1000research.3-12.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita D, Uenosono Y, Arigami T, Yanagita S, Nishizono Y, Hagihara T, Hirata M, Haraguchi N, Arima H, Kijima Y, et al. Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2015;22:3674–3680. doi: 10.1245/s10434-015-4392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, et al. Detection of circulating tumor cells in patients with pancreatic cancer: A preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 18.Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: The ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057–2061. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701–5710. doi: 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 20.Botteri E, Sandri MT, Bagnardi V, Munzone E, Zorzino L, Rotmensz N, Casadio C, Cassatella MC, Esposito A, Curigliano G, et al. Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122:211–217. doi: 10.1007/s10549-009-0668-7. [DOI] [PubMed] [Google Scholar]
- 21.Sastre J, Vidaurreta M, Gómez A, Rivera F, Massutí B, López MR, Abad A, Gallen M, Benavides M, Aranda E, et al. Prognostic value of the combination of circulating tumor cells plus KRAS in patients with metastatic colorectal cancer treated with chemotherapy plus bevacizumab. Clin Colorectal Cancer. 2013;12:280–286. doi: 10.1016/j.clcc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Fehm T, Sauerbrei W. Information from CTC measurements for metastatic breast cancer prognosis-we should do more than selecting an ‘optimal cut point’. Breast Cancer Res Treat. 2010;122:219–220. doi: 10.1007/s10549-010-0762-x. [DOI] [PubMed] [Google Scholar]
- 23.Romiti A, Raffa S, Di Rocco R, Roberto M, Milano A, Zullo A, Leone L, Ranieri D, Mazzetta F, Medda E, et al. Circulating tumor cells count predicts survival in colorectal cancer patients. J Gastrointestin Liver Dis. 2014;23:279–284. doi: 10.15403/jgld.2014.1121.233.arom1. [DOI] [PubMed] [Google Scholar]
- 24.Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, Luong TV, Caplin M, Meyer T. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res. 2011;17:337–345. doi: 10.1158/1078-0432.CCR-10-1776. [DOI] [PubMed] [Google Scholar]
- 25.Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991–996. doi: 10.1007/s13277-014-2716-0. [DOI] [PubMed] [Google Scholar]
- 26.Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M, Dittrich C. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011;50:700–710. doi: 10.3109/0284186X.2010.549151. [DOI] [PubMed] [Google Scholar]
- 27.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, Board R, Clack G, Hughes A, Blackhall F, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–516. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobek V, Matkowski R, Gürlich R, Grabowski K, Szelachowska J, Lischke R, Schützner J, Harustiak T, Pazdro A, Rzechonek A, Kolostova K. Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol. 2014;52:171–177. doi: 10.5603/FHC.2014.0020. [DOI] [PubMed] [Google Scholar]
- 32.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 33.Gervasoni A, Sandri MT, Nascimbeni R, Zorzino L, Cassatella MC, Baglioni L, Panigara S, Gervasi M, Di Lorenzo D, Parolini O. Comparison of three distinct methods for the detection of circulating tumor cells in colorectal cancer patients. Oncol Rep. 2011;25:1669–1703. doi: 10.3892/or.2011.1231. [DOI] [PubMed] [Google Scholar]
- 34.Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer: TNM Classification of Malignant Tumours. Wiley-Blackwell; Chichester: 2010. [Google Scholar]
- 35.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]


