Abstract
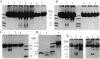
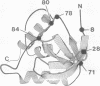
We report the synthesis and evaluation of (EDTA-2-aminoethyl) 2-pyridyl disulfide. By using this easily prepared cysteine-specific hydrophilic reagent, an ethylenediaminetriacetic acid-Fe3+ complex (EDTA-Fe) was covalently attached to a single genetically engineered cysteine residue in staphylococcal nuclease. Upon addition of the iron reductant ascorbate, the nuclease-EDTA-Fe conjugate underwent a protein self-cleavage reaction mediated by reactive oxygen species. Sequence analysis of the products indicated that cleavage occurs close in tertiary structure to the EDTA-Fe attachment site. In the presence of denaturants, the cleavage pattern changes and the reaction is limited to residues proximal in sequence to the cysteine attachment site. These results indicate that intramolecular protein cleavage reactions mediated by EDTA-Fe can be used to evaluate changes in protein conformation. The reagent described should be a useful tool in the structural mapping of nonnative protein states populated at equilibrium, such as the molten globule, that are frequently refractory to conventional structure analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Bateman R. C., Jr, Youngblood W. W., Busby W. H., Jr, Kizer J. S. Nonenzymatic peptide alpha-amidation. Implications for a novel enzyme mechanism. J Biol Chem. 1985 Aug 5;260(16):9088–9091. [PubMed] [Google Scholar]
- Blond S., Goldberg M. E. Kinetic characterization of early intermediates in the folding of E. coli tryptophan-synthase beta 2 subunit. Proteins. 1986 Nov;1(3):247–255. doi: 10.1002/prot.340010307. [DOI] [PubMed] [Google Scholar]
- Blond S., Goldberg M. Partly native epitopes are already present on early intermediates in the folding of tryptophan synthase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1147–1151. doi: 10.1073/pnas.84.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Evans P. A., Kautz R. A., Fox R. O., Dobson C. M. A magnetization-transfer nuclear magnetic resonance study of the folding of staphylococcal nuclease. Biochemistry. 1989 Jan 10;28(1):362–370. doi: 10.1021/bi00427a050. [DOI] [PubMed] [Google Scholar]
- Freskgård P. O., Carlsson U., Mårtensson L. G., Jonsson B. H. Folding around the C-terminus of human carbonic anhydrase II. Kinetic characterization by use of a chemically reactive SH-group introduced by protein engineering. FEBS Lett. 1991 Sep 2;289(1):117–122. doi: 10.1016/0014-5793(91)80922-p. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Semisotnov G. V., Friguet B., Kuwajima K., Ptitsyn O. B., Sugai S. An early immunoreactive folding intermediate of the tryptophan synthease beta 2 subunit is a 'molten globule'. FEBS Lett. 1990 Apr 9;263(1):51–56. doi: 10.1016/0014-5793(90)80703-l. [DOI] [PubMed] [Google Scholar]
- Hynes T. R., Fox R. O. The crystal structure of staphylococcal nuclease refined at 1.7 A resolution. Proteins. 1991;10(2):92–105. doi: 10.1002/prot.340100203. [DOI] [PubMed] [Google Scholar]
- Hynes T. R., Kautz R. A., Goodman M. A., Gill J. F., Fox R. O. Transfer of a beta-turn structure to a new protein context. Nature. 1989 May 4;339(6219):73–76. doi: 10.1038/339073a0. [DOI] [PubMed] [Google Scholar]
- JAYKO M. E., GARRISON W. M. Formation of >C=O bonds in the radiation-induced oxidation of protein in aqueous systems. Nature. 1958 Feb 8;181(4606):413–414. doi: 10.1038/181413a0. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. A centrifuged-column procedure for the measurement of ligand binding by beef heart F1. Methods Enzymol. 1979;56:527–530. doi: 10.1016/0076-6879(79)56050-9. [DOI] [PubMed] [Google Scholar]
- Ramzan M. A., Cookson E. J., Beynon R. J. Limited proteolysis as a probe for protein folding. Biochem Soc Trans. 1991 Aug;19(3):296S–296S. doi: 10.1042/bst019296s. [DOI] [PubMed] [Google Scholar]
- Rana T. M., Meares C. F. Transfer of oxygen from an artificial protease to peptide carbon during proteolysis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10578–10582. doi: 10.1073/pnas.88.23.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. The protein folding problem. Sci Am. 1991 Jan;264(1):54-7, 60-3. doi: 10.1038/scientificamerican0191-54. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. I. Linear order of the fragments produced by cleavage with cyanogen bromide. J Biol Chem. 1966 Oct 10;241(19):4366–4385. [PubMed] [Google Scholar]
- Tullius T. D. Physical studies of protein-DNA complexes by footprinting. Annu Rev Biophys Biophys Chem. 1989;18:213–237. doi: 10.1146/annurev.bb.18.060189.001241. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]