Abstract
Mounting evidence indicates that physical activity and alcohol consumption are positively associated, but potential moderators of this relationship remain unclear. Both physical activity and alcohol drinking are potentially reinforcing and may be more strongly associated among individuals who tend to be higher in reward seeking and related processes governed by the prefrontal cortex. Thus, behaviors linked to the prefrontal cortex, such as impulsivity, may influence the association between physical activity and alcohol intake. The present study therefore evaluated dimensions of impulsivity as moderators of the association between physical activity and alcohol consumption. We surveyed 198 undergraduate students and obtained self-reports of their drinking habits, physical activity, and dimensions of impulsivity. We found that moderate but not vigorous physical activity was positively associated with drinking. Linear regression analyses were used to evaluate dimensions of impulsivity as moderators of the association between physical activity (vigorous or moderate) and drinks per week. Results revealed a consistent pattern of interactions between the positive urgency and sensation seeking dimensions of impulsivity and moderate physical activity on number of drinks per week. For both interactions, there was a significant positive association between moderate physical activity and drinking at higher but not lower levels of impulsivity. We conclude that impulsivity moderates the positive association between physical activity and alcohol consumption. These results have significant implications for the develop ment of prevention and treatment programs for alcohol use disorders.
Keywords: Impulsivity, Sensation seeking, Exercise, Drinking
Introduction
Like all rewarding behaviors, exercise and alcohol consumption share common underlying brain circuitry (Nestler, 2005; Olsen, 2011; Perrotti et al., 2008; Volkow & Wise, 2005; Werme et al., 2002). This suggests that exercise experience may influence the effects of alcohol. In support of this idea, it has been shown that voluntary exercise reduces behavioral intoxication in a rodent model (Leasure & Nixon, 2010). Overlapping neural circuitry also suggests that exercise experience may influence alcohol intake behaviors. In support of this idea, several large studies of non-institutionalized adults in the United States indicate a significant positive association between physical activity and alcohol consumption (French, Popovici, & Maclean, 2009; Lisha, Martens, & Leventhal, 2011).
In humans, the relationship between physical activity and drinking has been studied in adolescent and undergraduate athletes. Many of these studies indicate heavier drinking in athletes compared to non-athlete peers (Dunn & Wang, 2003; Kokotailo, Henry, Koscik, Fleming, & Landry,1996; Leichliter, Meilman, Presley, & Cashin,1998; Nattiv & Puffer,1991; Wechsler, Dowdall, Davenport, & Rimm,1995), although some do not (Elder, Leaver-Dunn, Wang, Nagy, & Green, 2000; Overman & Terry, 1991). Mixed findings suggest that the relationship betweenphysical activity and alcohol consumption may differ as a function of physiological, neural, cultural, and/or personality factors. However, other than gender and age, factors that moderate the relationship between physical activity and alcohol intake have been understudied (Lisha et al., 2011).
To pinpoint factors that may influence this relationship, it is helpful to consider neurocircuitry. With any rewarding and potentially addictive behavior (including alcohol consumption), the transition from experimentation to increased use appears to be governed by the prefrontal cortex (Bechara, 2005; Koob & Le Moal, 2005). Moreover, the processes which govern reward seeking and impulsivity are likely to affect behaviors which are potentially reinforcing. Sensation seeking, which is one component of impulsivity, has been suggested as a potential factor in the association between physical activity and alcohol consumption, in that a “sensation-seeking lifestyle” may be consistent with engaging in both behaviors (French et al., 2009). More generally, impulsivity is a set of behavioral tendencies (Cyders & Smith, 2007) that confer vulnerability to the development of drug addiction (de Wit, 2009), and rely heavily on prefrontal cortical function (Fineberg et al., 2010; Perry et al., 2011). The present study evaluated dimensions of impulsivity as moderators of the association between physical activity and alcohol consumption in a sample of college students.
Research on impulsivity has long been hindered by a lack of uniformity in the definition of the construct. Cyders and Smith (2007) have developed a new measure of impulsivity, the UPPS-P, based on compelling evidence that impulsivity, or “rash action” has three distinct components. The first is mood-based and is composed of positive urgency (action driven by positive emotion) and negative urgency (action driven by negative emotion). The second component involves lack of conscientiousness, and includes lack of planning and lack of perseverance. The last component is sensation seeking. In the present study, we have utilized this conceptualization of the construct of impulsivity.
The objective of the present study was to determine whether impulsivity influences the relationship between physical activity and alcohol intake. Stated another way, we were interested in determining the extent to which impulsivity is characteristic of individuals who both drink and exercise. Accordingly, participants completed self-report measures of alcohol consumption, physical activity, and impulsivity. We hypothesized that the relationship between physical activity and alcohol intake would be strongest in individuals that score highly on the subscales of the UPPS-P, including sensation-seeking, negative and positive urgency, lack of premeditation, and lack of perseverance.
Materials and methods
Participants
Participants included 198 (173 women and 25 men) undergraduate psychology students at a large Southwestern university. The average age of participants was 24.11 years (SD = 6.92). The race distribution was 39.27% Caucasian, 19.37% Asian/Asian American, 16.23% Black/African American, 1.57% Native Alaskan/Pacific Islander, 6.81% multi-racial, and 16.75% other. The ethnicity distribution was 30.61% Hispanic and 69.39% non-Hispanic. With the exception of gender, this sample was demographically similar to the university's undergraduate population. Written informed consent was obtained from all subjects, and all experimental procedures followed were in accordance with the ethical standards of the University of Houston's Committee for the Protection of Human Subjects and with the Helsinki Declaration of 1975, as revised in 1983.
Procedure
After providing informed consent, participants completed a web-based survey which included measures of impulsivity, physical activity, and drinking. Participants received extra course credit in exchange for participation.
Measures
Impulsivity
Impulsivity was assessed by the UPPS-P (Cyders & Smith, 2007). The UPPS-P is a revised version of the UPPS Impulsive Behavior scale (Whiteside & Lynam, 2001). The original UPPS assessed urgency (negative), lack of premeditation, lack of perseverance, and sensation seeking. The UPPS-P adds the dimension of positive urgency. The scale consists of 59 items. Negative urgency refers to a tendency to engage in impulsive behaviors in response to negative effect and is assessed by 12 items, e.g., “When I am upset I often act without thinking.” Lack of premeditation refers to the absence of consideration of consequences prior to engaging in an act and is assessed by 11 items, e.g., “Before making up my mind, I consider all the advantages and disadvantages”; Reversed. Lack of perseverance assesses an inability to remain focused on boring or difficult tasks and is measured by 10 items, e.g., “I tend to give up easily.” Sensation seeking refers to a tendency to pursue exciting activities and an openness to trying new experiences that are potentially dangerous and is assessed by 12 items, e.g., “I welcome new and exciting experiences and sensations, even if they are a little frightening and unconventional.” Finally, positive urgency refers to a tendency to engage in impulsive behaviors in response to positive effect and is measured by 14 items, e.g., “When I am in a great mood, I tend to get into situations that could cause me problems.” Cronbach's alphas in the present data were 0.89, 0.86, 0.85, 0.87, and 0.94, for negative urgency, lack of premeditation, lack of perseverance, sensation seeking, and positive urgency, respectively.
Physical activity
Physical activity was assessed using items from the CDC Behavioral Risk Factor Surveillance System (BRFSS) (Fisher, Spicer, Race, & Melnik, 2003). Participants were asked to report 1) the number of minutes per day they engaged in moderate activities on days when they engaged in moderate activities and 2) the number of minutes per day they engaged in vigorous activities on days when they engaged in vigorous activities. Responses ranged from 0 to 180 min for each item. The BRFSS also includes items regarding how many days per week participants exercise; however, preliminary analyses revealed that frequency was not related to drinking. Moreover, analyses examining exercise frequency for both moderate and vigorous exercise indicated that neither was related to drinking nor did either interact with any dimension of impulsivity in predicting drinking. Thus, the present results focus specifically on amount of exercise per occasion.
Drinking
The Daily Drinking Questionnaire (Collins, Parks, & Marlatt, 1985) was used to assess average number of drinks consumed per week over the previous 3 months. Participants were asked, “Consider a typical week during the last 3 months. How much alcohol, on average (measured in number of drinks), do you drink on each day of a typical week?” Participants responded by noting the average number of drinks consumed on each day of the week. Drinks per week was calculated as the sum of responses for each day of the week. This measure has demonstrated good reliability and validity in comparison to other measures of drinking (Borsari & Carey, 2000; Neighbors, Oster-Aaland, Bergstrom, & Lewis, 2006).
Results
Relationships between physical activity, drinking, and impulsivity
Means, standard deviations, and correlations for all variables are presented in Table 1. All impulsivity subscales were significantly and positively associated with each other with the exception that sensation seeking was not correlated with lack of premeditation and was negatively correlated with lack of perseverance. Sensation seeking was positively associated with moderate and vigorous physical activity but none of the other impulsivity scales was associated with physical activity. With the exception of lack of perseveration, all of the other impulsivity subscales were positively associated with drinking, at least marginally. In addition, moderate but not vigorous exercise was positively associated with drinking.
Table 1.
Means, standard deviations, and correlations among variables.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|
| 1. Negative urgency | – | |||||||
| 2. Positive urgency | 0.74*** | – | ||||||
| 3. Lack of premeditation | 0.43*** | 0.41*** | – | |||||
| 4. Lack of perseverance | 0.46*** | 0.47*** | 0.62*** | – | ||||
| 5. Sensation seeking | 0.17* | 0.28*** | −0.05 | −0.18** | – | |||
| 6. Moderate physical activity | −0.09 | −0.00 | −0.02 | −0.06 | 0.15* | – | ||
| 7. Vigorous physical activity | −0.06 | 0.05 | −0.08 | −0.07 | 0.16* | 0.50*** | – | |
| 8. Drinks per week | 0.16* | 0.19** | 0.13† | 0.08 | 0.32*** | 0.19** | 0.07 | – |
| Mean | 2.09 | 1.71 | 1.85 | 1.84 | 2.54 | 36.02 | 36.94 | 5.90 |
| Standard deviation | 0.62 | 0.61 | 0.51 | 0.54 | 0.66 | 30.24 | 36.90 | 8.14 |
N's ranged from 188 to 195 depending on missing responses.
p < 0.001
p < 0.01
p < 0.05
p < 0.10.
Impulsivity moderates the association between moderate physical activity and drinking
Linear regression analyses were used to evaluate impulsivity as a moderator of the association between physical activity and drinking. Analyses were conducted separately for impulsivity subscales and for moderate and vigorous activity. In each model, the number of drinks per week was regressed on the relevant impulsivity subscale and physical activity variable as well as the product of impulsivity and physical activity. All variables were mean-centered (Cohen, Cohen, West, & Aiken, 2003). Significant interactions were graphed and interpreted using procedures described by Aiken and West (Aiken & West, 1991). In order to control for alpha inflation due to multiple testing, p values less than 0.01 are considered statistically significant. Regression results are presented in Table 2.
Table 2.
Regression analysis examining impulsivity as a moderator of the association between physical activity and drinking.
| Predictor | Moderate activity |
Vigorous activity |
||
|---|---|---|---|---|
| B (SEB) | β | B (SEB) | β | |
| Negative urgency | 2.611 (0.969) | 0.195** | 1.924 (1.000) | 0.143† |
| Activity | 0.073 (0.021) | 0.268*** | 0.017 (0.016) | 0.077 |
| Negative urgency × activity | 0.083 (0.039) | 0.164* | −0.006 (0.026) | −0.017 |
| Positive urgency | 4.063 (0.973) | 0.292*** | 2.899 (1.040) | 0.208** |
| Activity | 0.053 (0.018) | 0.196** | 0.012 (0.016) | 0.054 |
| Positive urgency × activity | 0.200 (0.040) | 0.354*** | 0.049 (0.031) | 0.120 |
| Lack of premeditation | 2.223 (1.146) | 0.138† | 2.166 (1.183) | 0.134† |
| Activity | 0.065 (0.020) | 0.240** | 0.022 (0.017) | 0.100 |
| Lack of premeditation × activity | 0.085 (0.039) | 0.161* | 0.064 (0.034) | 0.138† |
| Lack of perseverance | 1.329 (1.094) | 0.087 | 1.311 (1.126) | 0.086 |
| Activity | 0.058 (0.020) | 0.212** | 0.019 (0.017) | 0.085 |
| Lack of perseverance × activity | 0.050 (0.039) | 0.094 | 0.035 (0.035) | 0.073 |
| Sensation seeking | 3.389 (0.843) | 0.271*** | 3.992 (0.881) | 0.317** |
| Activity | 0.029 (0.018) | 0.105 | 0.002 (0.016) | 0.010 |
| Sensation seeking × activity | 0.092 (0.023) | 0.270*** | 0.053 (0.026) | 0.141* |
Degrees of freedom ranged from 183 to 184 depending on missing responses.
p < 0.001
p < 0.01
p < 0.05
p < 0.10.
Results for analyses examining impulsivity as a moderator of the association between moderate physical activity and drinking are presented on the left of Table 2. The main effects of impulsivity largely duplicate the zero-order correlations between subscales and drinking. Similarly, moderate physical activity was significantly associated with drinking in all models except for the model examining sensation seeking as a moderator of physical activity and drinking. This was likely due to a larger amount of shared variance between sensation seeking and moderate physical activity relative to the other impulsivity subscales in combination with the relatively strong association between sensation seeking and drinking.
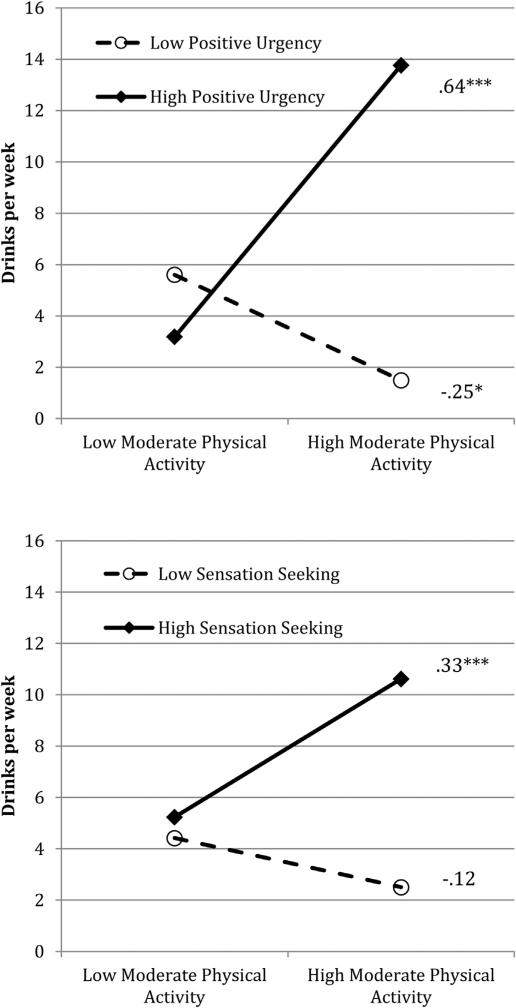
More importantly, results revealed a consistent pattern of interactions between subscales of impulsivity and moderate physical activity in their association with drinking. After controlling for alpha inflation, these interactions were only significant for positive urgency and sensation seeking. Graphs of each interaction are presented in Fig. 1 with simple slopes, derived from the regression equations, where high and low values of each predictor were specified as one standard deviation above and below the mean, respectively. Results indicated that for both interactions, there was a significant positive association between moderate physical activity and drinking at higher but not lower levels of impulsivity. At lower levels of impulsivity, there was no significant association between moderate physical activity and drinking. Interactions with negative urgency and lack of premeditation with moderate physical activity approached significance and had identical patterns as those presented in Fig. 1.
Fig. 1.
Impulsivity subscales moderate the association between moderate physical activity and drinks per week. *p < 0.05; ***p < 0.001.
Vigorous physical activity, sensation seeking, and drinking
Results for analyses examining impulsivity as a moderator of the association between vigorous physical activity and drinking are presented on the right portion of Table 2. The main effects of impulsivity were again consistent with the zero-order correlations between subscales and drinking. Vigorous activity was not significantly associated with drinking in any of the models. Moreover, the interaction between impulsivity and vigorous physical activity in association with drinking only approached significance for 2 of the 5 subscales, i.e., sensation seeking and lack of premeditation. For both of these the pattern of findings was similar to those shown in Fig. 1.
Discussion
This research was designed to examine the association of physical activity and drinking among young adults and to evaluate whether this association might vary as a function of impulsivity. Despite a small sample size, our results are consistent with prior findings showing a positive association between physical activity and drinking (French et al., 2009; Lisha et al., 2011) and between impulsivity and drinking (Bickel & Marsch, 2001; Lyvers, Duff, Basch, & Edwards, 2012; Martens, Pedersen, Smith, Stewart, & O'Brien, 2011; Redish, Jensen, & Johnson, 2008). Moreover, results provided some support for impulsivity as a moderator of the association between physical activity and drinking. This was primarily true for moderate physical activity and less so for vigorous activity.
Impulsivity provides a useful framework in which to consider the link between physical activity and alcohol intake, as it is associated with dysfunction in and/or immaturity of the prefrontal cortex (Crews & Boettiger, 2009; Fineberg et al., 2010), which in turn has been implicated in risky behaviors such as heavy drinking (Bechara, 2005; Lyvers, 2000; Lyvers et al., 2012). In the present study, we found that the interactions between positive urgency and sensation seeking with moderate physical activity are positively associated with drinking only when self-reported levels of impulsivity were high (see Fig. 1). Of these facets of impulsivity, sensation seeking may be particularly informative. We found that the sensation-seeking subscale correlated with both moderate and vigorous physical activity. It has been suggested that the positive association between physical activity and alcohol consumption is due in part to sensation-seeking, in that heavy drinking may be a component of a “sensation-seeking lifestyle” (French et al., 2009). In humans, novelty seeking is a behavioral trait that is a component of “reward deficiency syndrome,” which is also characterized by impulsive and compulsive behaviors (Blum et al., 1996) as well as alcoholism (Bowirrat & Oscar-Berman, 2005). This constellation of behaviors has been linked with the neural phenotype of decreased density and/or dysfunction of the D2 subtype of dopamine receptor (DRD2). In human subjects, there is an inverse relationship between DRD2 density and pleasurable response to stimulant drugs (Volkow et al., 2002). In rats, DRD2 density can be manipulated using viral vectors. If DRD2 density is decreased, alcohol consumption rises, then dwindles as DRD2 density rises (Thanos et al., 2001). Thus, while speculative, it is possible that impulsivity is a characteristic of people who engage in both physical activity and alcohol drinking because all three behaviors stem from the low DRD2 phenotype.
While neurological correlates provide useful insights into the relationship between physical activity, impulsivity, and drinking, there are also psychological explanations. For instance, the work hard, play hard mentality (Martens, Watson, Royland, & Beck, 2005) is unique to humans. Thus, it may be that impulsivity is a characteristic of young adults who are both energetic and physically active and also more likely to drink relatively heavily. It is also possible that heavy drinkers who also engage in healthy behaviors might do so as a means of justification. Thus, heavy partying might seem less problematic among those who are also physically active or also engaging in healthy behaviors.
From a clinical standpoint, given that exercise has been touted as a potentially useful component of prevention and/or treatment programs for alcohol use disorders (Brown et al., 2009; Palmer, Vacc, & Epstein, 1988; Ussher, Sampuran, Doshi, West, & Drummond, 2004; Weinstock, 2010; Werch et al., 2003), it is important to pinpoint moderators of the association between physical activity and drinking. One implication of this is that physical activity may be unsuitable as a prevention component, at least for some individuals. This may be especially true if the target population is relatively high in impulsivity, as is the case with adolescents, who are higher in impulsivity due to immaturity of the frontal cortex (Giedd, 2008; Spear, 2002). Results also suggest that prevention programs for young adults who are engaged in regular physical activity (e.g., athletes) may require special attention to the links between physical activity and alcohol consumption. Treatment programs, however, may be another matter. Problem drinking has profound effects on the brain (Koob, 2003), which could in turn alter the neural effects of exercise. There is some evidence that increased physical activity attenuates drinking in heavy consumers (Brown et al., 2009; Murphy, Pagano, & Marlatt, 1986). It is important that future studies further examine the complex interactive effects of exercise and alcohol on the brain. It would also be worth examining other potential moderators of associations between exercise and consumption. For example, alcohol expectancies, which have been found to correlate with gray matter volume in social drinkers (Ide et al., 2014), might be good candidates for potential moderators of the association between moderate exercise and drinking.
The present research should be viewed in light of several limitations. The data come from a relatively small sample, primarily women. In and of itself, the relatively small sample is not a serious limitation and underscores the significance of the association between physical activity and drinking, given that many of the other studies which have documented this association have been extremely large. However, in combination with the gender disparity, the small sample precluded our ability to effectively examine gender differences. Thus, future research is needed to evaluate whether findings are similar across men and women. Note, however, that impulsivity and sensation seeking tend to be stronger among male, compared to female young adults (Nolen-Hoeksema & Hilt, 2006). Thus, in a future, more representative sample we are likely to observe even stronger associations between impulsivity, exercise, and alcohol intake.
Another limitation was the measure of physical activity, which was limited to single items. Though the measure employed has been widely used, it would seem prudent to examine associations using more multidimensional measures of physical activity in subsequent research. Furthermore, the assessment of exercise reflected the amount of time during a typical occasion rather than a measure of frequency of exercise or composite of frequency and quantity. As noted in the measures section, preliminary analyses seemed to rule out associations between frequency of exercise and drinking. In addition, the present study was cross-sectional, precluding our ability to make causal inferences. Future research examining physical drinking and drinking longitudinally would not allow causal inferences but would allow for examination of temporal precedence. Thus, longitudinal research would allow us to examine whether changes in exercise are associated with changes in drinking but would not directly inform whether deliberate changes in exercise would cause more drinking. Longitudinal data would also allow for evaluation of possible mediators of the association, which might differ depending on temporal precedence. In athletes, both coping and celebration models have been suggested to account for excessive drinking (Martens et al., 2005; Martens et al., 2011). In the general population, we might examine whether compensatory exercise follows drinking relative to the possibility that drinking serves as a reward for physical activity. Finally, the age restriction of the sample precluded our ability to examine whether findings vary by development. The vast majority of our participants was under age 25 and were still developing with respect to pre-frontal cortical function. The effect of impulsivity on the association between physical activity and drinking may be developmentally specific but further research is needed to evaluate this possibility.
Despite a small sample size, our results are consistent with larger studies showing a positive association between physical activity and drinking, as well as between impulsivity and drinking. Our study extends prior findings by providing a unique preliminary contribution in identifying impulsivity as a moderator of the association between physical activity and drinking among young adults.
Acknowledgments
Preparation of this article was supported in part by National Institute on Alcohol Abuse and Alcoholism Grant R01AA014576 (CN).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Vol. 1991. Sage Publications; Thousand Oaks, CA: 1991. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsari B, Carey KB. Effects of a brief motivational intervention with college student drinkers. Journal of Consulting and Clinical Psychology. 2000;68:728–733. [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behavior Modification. 2009;33:220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/ correlation analysis for the behavioral sciences. 3rd ed. Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: the effects of social interaction and model status on the self-administration of alcohol. Journal of Consulting and Clinical Psychology. 1985;53:189–200. doi: 10.1037//0022-006x.53.2.189. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Mood-based rash action and its components: positive and negative urgency. Personality and Individual Differences. 2007;43:839–850. [Google Scholar]
- Dunn MS, Wang MQ. Effects of physical activity on substance use among college students. American Journal of Health Studies. 2003;18:126–132. [Google Scholar]
- Elder C, Leaver-Dunn D, Wang MQ, Nagy S, Green L. Organized group activity as a protective factor against adolescent substance use. American Journal of Health Behavior. 2000;24:108–113. [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BD, Spicer D, Race PO, Melnik T. Physical activity. Behavioral Risk Factor Surveillance System. 2003;10:1–8. [Google Scholar]
- French MT, Popovici I, Maclean JC. Do alcohol consumers exercise more? Findings from a national survey. American Journal of Health Promotion: AJHP. 2009;24:2–10. doi: 10.4278/ajhp.0801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. The Journal of Adolescent Health: Official Publication of the Society of Medicine. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CS. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug and Alcohol Dependence. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotailo PK, Henry BC, Koscik RE, Fleming MF, Landry GL. Substance use and other health risk behaviors in collegiate athletes. Clinical Journal of Sport Medicine: Official Journal of Canadian Academy of Sport Medicine. 1996;6:183–189. doi: 10.1097/00042752-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism, Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcoholism, Clinical and Experimental Research. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichliter JS, Meilman PW, Presley CA, Cashin JR. Alcohol use and related consequences among students with varying levels of involvement in college athletics. Journal of American College Health. 1998;46:257–262. doi: 10.1080/07448489809596001. [DOI] [PubMed] [Google Scholar]
- Lisha NE, Martens M, Leventhal AM. Age and gender as moderators of the relationship between physical activity and alcohol use. Addictive Behaviors. 2011;36:933–936. doi: 10.1016/j.addbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Duff H, Basch V, Edwards MS. Rash impulsiveness and reward sensitivity in relation to risky drinking by university students: potential roles of frontal systems. Addictive Behaviors. 2012;37:940–946. doi: 10.1016/j.addbeh.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Martens MP, Pedersen ER, Smith AE, Stewart SH, O'Brien K. Predictors of alcohol-related outcomes in college athletes: the roles of trait urgency and drinking motives. Addictive Behaviors. 2011;36:456–464. doi: 10.1016/j.addbeh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Martens MP, Watson JC, 2nd, Royland EM, Beck NC. Development of the athlete drinking scale. Psychology of Addictive Behaviors: Journal of Society of Psychologists in Addictive Behaviors. 2005;19:158–164. doi: 10.1037/0893-164X.19.2.158. [DOI] [PubMed] [Google Scholar]
- Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: effects of aerobic exercise and meditation. Addictive Behaviors. 1986;11:175–186. doi: 10.1016/0306-4603(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Nattiv A, Puffer JC. Lifestyles and health risks of collegiate athletes. The Journal of Family Practice. 1991;33:585–590. [PubMed] [Google Scholar]
- Neighbors C, Oster-Aaland L, Bergstrom RL, Lewis MA. Event- and context-specific normative misperceptions and high-risk drinking: 21st birthday celebrations and football tailgating. Journal of Studies on Alcohol. 2006;67:282–289. doi: 10.15288/jsa.2006.67.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. The Journal of General Psychology. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman SJ, Terry T. Alcohol use and attitudes: a comparison of college athletes and nonathletes. Journal of Drug Education. 1991;21:107–117. doi: 10.2190/KF20-4LER-J9N1-7Q3F. [DOI] [PubMed] [Google Scholar]
- Palmer J, Vacc N, Epstein J. Adult inpatient alcoholics: physical exercise as a treatment intervention. Journal of Studies on Alcohol. 1988;49:418–421. doi: 10.15288/jsa.1988.49.418. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, et al. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Research Reviews. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. The Behavioral and Brain Sciences. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. Journal of Studies on Alcohol. 2002;(Supplement):71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. Journal of Neurochemistry. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99:1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Rimm EB. A gender-specific measure of binge drinking among college students. American Journal of Public Health. 1995;85:982–985. doi: 10.2105/ajph.85.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J. A review of exercise as intervention for sedentary hazardous drinking college students: rationale and issues. Journal of American College Health. 2010;58:539–544. doi: 10.1080/07448481003686034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch C, Moore M, DiClemente CC, Owen DM, Jobli E, Bledsoe R. A sport-based intervention for preventing alcohol use and promoting physical activity among adolescents. The Journal of School Health. 2003;73:380–388. doi: 10.1111/j.1746-1561.2003.tb04181.x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, et al. Delta FosB regulates wheel running. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]