Abstract
Objective
To date, no approved clinical intervention successfully prevents the progressive degradation of injured articular cartilage that leads to osteoarthritis (OA). Stem/progenitor cell populations within tissues of diarthrodial joint have shown their therapeutic potential in treating OA. However, this potential has not been fully realized due in part to the heterogeneity of these subpopulations. Characterization of clonal populations derived from a single cell may help identify more homogenous stem/progenitor populations within articular cartilage. Moreover, chondrogenic potential of clonal populations from different zones could be further examined to elucidate their differential roles in maintaining articular cartilage homeostasis.
Method
We combined FACS (Fluorescence-activated cell sorting) and clonogenicity screening to identify stem/progenitor cells cloned from single cells. High-efficiency colony-forming cells (HCCs) were isolated, and evaluated for stem/progenitor cell characteristics. HCCs were also isolated from different zones of articular cartilage. Their function was compared by lineage-specific gene expression, and differentiation potential.
Results
A difference in colony-forming efficiency was observed in terms of colony sizes. HCCs were highly clonogenic and multipotent, and overexpressed stem/progenitor cell markers. Also, proliferation and migration associated genes were over-expressed in HCCs. HCCs showed zonal differences with deep HCCs more chondrogenic and osteogenic than superficial HCCs.
Conclusion
Our approach is a simple yet practical way to identify homogeneous stem/progenitor cell populations with clonal origin. The discovery of progenitor cells demonstrates the intrinsic self-repairing potential of articular cartilage. Differences in differentiation potential may represent the distinct roles of superficial and deep zone stem/progenitor cells in the maintenance of articular cartilage homeostasis.
Keywords: single cell, stem/progenitor cell, articular cartilage
1. Introduction
Cartilage lesions are a fairly common problem in orthopaedic practice. However, as an avascular and aneural tissue, articular cartilage has minimal intrinsic healing ability [1]. More often, most macroscopic cartilage lesions not only cause local tissue damage, but initiate whole joint progressive cartilage degeneration, which will ultimately leads to osteoarthritis (OA) [2, 3]. Stem cell-based treatments have been explored for enhancing cartilage repair in degenerating joint for the past few years [4–6]. Evidence has emerged on the existence of MSCs-like cells from the synovium, articular cartilage, infrapatellar fat pat [7–9], and other tissues within articular joints. These cells can be primed towards chondrogenic differentiation both in vitro and in vivo, thus might represent possible candidates to maintain normal turnover of cartilage as well as to restore damaged cartilage upon joint lesions. Nevertheless, more complete understanding of their reparative behaviors is needed to further explore their therapeutic potential.
Adult mesenchymal stem cells (MSCs), or cartilage chondroprogenitors, are known to reside residing in hyaline tissue and have been shown to be highly clonogenic, multipotent, and chemotactic [10–12]. These tissue stem/progenitor cells are able to migrate towards local injury sites, where they proliferate and differentiated as needed to replace damaged tissue [13, 14]. Unlike MSCs, which are able to differentiate into multiple tissue types in different organ systems, tissue progenitor/stem cells are typically only capable of generating limited tissue types for local tissue regeneration, especially the tissue of their origin. Stem/progenitor cells in articular cartilage are an example of the latter cell type, one that is able to undergo multi lineage differentiation, but in situ and in normal physiological conditions is lineage restricted to differentiate into hyaline cartilage-producing chondrocytes.
CPCs were first discovered by Dowthwaite et al, who identified them to be a subpopulation of superficial zone cells for appositional growth of articular cartilage [15], which have enhanced affinity to fibronectin and highly expressed stem cell-associated factor Notch-1. Koelling et al have also found chondrogenic progenitor cells (CPCs) in articular cartilage during later stages of human osteoarthritis [16], these cells were highly migratory towards damaged cartilage tissue and repopulated in repair tissue. Grogan et al later examined the distribution of stem cells markers (Notch-1, Stro-1, VCAM-1), and found inconsistency between stem-cell marker expression and stem cells distribution, thus concluded that these stem cell markers may not be useful to identify progenitors in cartilage. Some other studies also showed stem/progenitor cells overexpressed stem cell surface markers (CD105, CD166) [17] and were capable of Hoechst 33342 dye exclusion as a side population, characteristic of stem cells [18]. Moreover, we previously found migrating CPCs strikingly proliferating on the articular surface post traumatic injuries in an in vitro bovine osteochondral explant impact model in response to multiple alarmins released by necrotic cells [19]. Another study also showed that injured bovine cartilage induces migration of Notch-1 positive cells to the surface damaged region [20].
Despite the evidence that these cells might represent a putative cartilage progenitor cell maintaining the homeostasis of the articular joint, only a few studies thus far have identified homogeneous single cell-derived clonal sub-population within the normal articular cartilage [21]. Full characterization of stem/progenitor cell potential requires the generation genetically identical populations from a single progenitor [22]. Otherwise, the phenotypic “stemness” may actually result from a heterogeneous pool of cells with different origins. Williams et al. has demonstrated clonal cartilage progenitor cells have distinct phenotype from full-depth chondrocytes, as well as different telomerase activity [23]. In addition, where progenitors from articular cartilage normally reside within extracellular matrix is still not clear and worthy further investigation.
In the present study, we describe, for the first time, a single cell clonogenicity screening technique to identify progenitor cells in healthy articular cartilage. This technique allows isolation of progenitors from the superficial 1/3 as well as deep 2/3 of full thickness cartilage, with distinct differences in differentiation potency. Genetic and functional characteristics of the high-efficiency colony-forming cells (HCCs) reveal their similarities with adult stem/progenitor cells.
2. Materials and Methods
Cartilage Tissue Harvesting and Cell Isolation
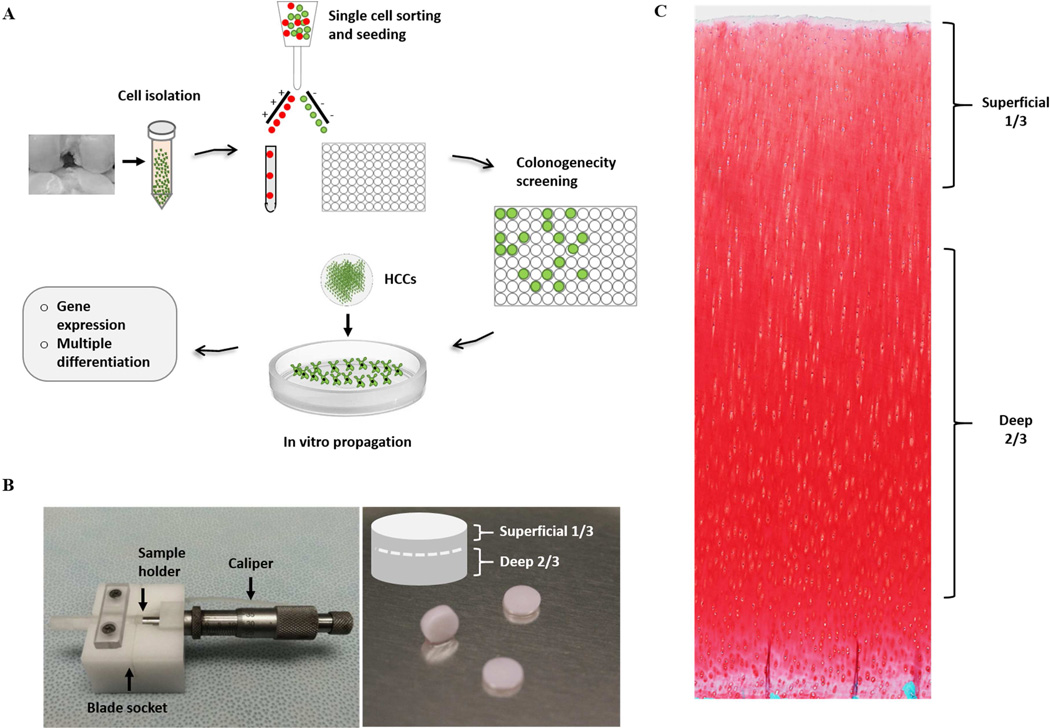
Fresh stifle joints from young adult cattle (15–24 months old) were obtained from a local abattoir (Bud`s Custom Meats). Articular cartilage was harvested from the femur condyle using a 6mm biopsy punch (Figure 1B) and rinsed in Hank’s Balanced Salt Solution (Invitrogen, California, USA) supplemented with 100 U/µl penicillin, 100 µg/ml streptomycin, and 2.5 µg/µl fungizone. Full thickness cartilage biopsy samples were minced into fine pieces and digested overnight with 0.25mg/ml collagenase type 1 and pronase E (1:1) (Sigma-Aldrich, St. Louis, MO) dissolved in culture medium in a shaking incubator overnight (0.25 mg/ml each). When needed, a customized apparatus was used to separate the superficial 1/3 (including superficial zone) and deep 2/3 part of the cartilage biopsies prior to digestion (Figure 1B). The next day, the digestion solution was neutralized by culture medium and passed through different sized cell strainers (BD Falcon™, BD bioscience, Maryland, USA) serially (100µm, 70µm, and 30µm) to obtain a single cell solution, which was confirmed by hemocytometer. 5–10 × 106 cells were suspended in 2ml Hank`s Balanced Salt Solution (Invitrogen, California, USA) in 5ml Falcon Polystyrene Tube (BD bioscience, Maryland, USA) for FACS.
Figure 1.
Experimental schematics and customized apparatus. A) Flow diagram representing the methodology for isolating and characterizing cartilage progenitor cells. B) A customize-made apparatus for separating superficial and deep cartilage from 6mm cartilage biopsy, with a caliper, and sample holder, and a blade socket. C) Histological image showing full thickness bovine articular cartilage (Scale bar represents 500µm).
FACS and Single Cell Plating
Prior to single cell sorting, 96-well culture plates were coated with 0.1% gelatin solution (Bio-Rad, CA, USA) to give optimal cell attachment. The single cell solution was subjected to 1µg/ml Propidium iodide staining (Life technologies, NY, USA) for excluding dead cells during fluorescence-activated cell sorting (FACS) (Becton Dickinson Aria II, BD, Maryland, USA). Viable cells were sorted into 96-well plates, one cell per well, sequentially, in DMEM-based culture medium (Figure 1A). The rest of the cells were re-plated and cultured in Dulbecco`s modified Eagle`s medium (DMEM) and Ham`s F12 (1:1 mixture) supplemented with 10% KnockOut serum replacement (Life Technology, Grand Island, NY), 50 µg/µl L-ascorbate, 100 U/µl penicillin, 100 µg/ml streptomycin, and 2.5 µg/µl fungizone at 37° C with 5% CO2.
Clonogenicity Screening
After FACS and single cell plating, cells were cultured for 48 hours at the same culture condition using DMEM-based medium. Beginning on day 3, culture wells were examined every other day under microscope to check the availability, location and size of colonies. At day 10, cultures were stained with 1 µg/ml Calcein-AM (green fluorescent for live cells). Colonies were analyzed based on green fluorescent detection using Olympus IX81 Inverted Light Microscope (Olympus, PA, USA). Colony sizes and numbers were measured by ImageJ according to the users` manual (http://rsb.info.nih.gov/ij). Colonies were categorized into confluent colonies (CCs), big colonies (BCs) that covered over ½ of the surface area of the well, and small colonies (SCs) according to their relative size (Figure 1A).
Colony Isolation and In Vitro Expansion
Big colonies (BCs) and confluent colonies (CCs) were manually picked and passaged (Figure 1A) serially to 24-well or 6-well culture plates (BD Bioscience, Maryland, USA) pre-coated with 0.1% gelatin solution (Bio-Rad, CA, USA). Cells were expanded in DMEM/F12 with GlutaMax (Life technologies, NY, USA) supplemented with 10% KnockOurt serum replacement (Life Technology, Grand Island, NY), 50 µg/µl L-ascorbate, 100 µg/µl penicillin, 100 µg/ml streptomycin, and 2.5 µg/µl fungizone at 5% CO2, 37 °C. Media were replenished upon needed.
Immunofluorescence and immunocytochemistry staining
HCCs were isolated and seeded onto chamber slides for immunostaining. For Abcg2 (ab 3380, Abcam, Cambridge, MA) and Notch-1 (sc-6014, Santa Cruz Biotechnology, Inc., Dallas, TX), immunofluorescence staining was used. Primary antibodies was labeled at 1:400 and 1:200 dilution respectively, followed by Alexa 488 secondary antibody (Jackson Immunoresearch, West Grove, PA), and imaged by an Olympus FluoView™ FV1000 laser scanning confocal microscope (LSCM) (Olympus NDT Inc., MA). Lubrin staining was also performed on isolated HCCs from both superficial 1/3 and deep 2/3 cartilage using a mouse monoclonal antibody (ab 28484, Abcam, Cambridge, MA), and detected with and a Vectastain ABC kit (Vector, Burlingame, CA).
Gene Expression analysis
For gene expression analysis, RNA was extracted directly from passage 2 BCs and CCs. Passage 2 normal chondrocytes (NCs), isolated from the same specimens and cultured for the same period of time, were used as controls. Cells were homogenized in TRIzol® reagent (InvitrogenTM Life Technologies, Carlsbad, CA) and total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer`s instructions. A previous study found that progenitor cells usually overexpress genes commonly expressed in MSCs (Mesenchymal stromal cells). Thus, markers examined were: ATP-binding cassette sub family G member 2 (ABCG2), which is a characteristic gene of “side population” identified by flow cytometry to be stem/progenitor cells; Telomerase reverse transcriptase (TERT) gene is an indicator of length of telomeres, which is a genetic marker for both stem cells and cancer cells. Both of ABCG2 and TERT were previous shown to have increased expression in chondrogenic progenitor cells [24]. Sox-9 is a chondrogenic transcription factor, which is related to chondrogenic potential; RunX-2 is an osteogenic marker for progenitor cells. In addition, an array of clusters of differentiation (CD) markers (CD105, CD90, and CD133, etc.) commonly seen in stem/progenitor cells, were also examined. Moreover, PRG4 (lubricin encoding gene) was analyzed to compare its expression between HCCs from superficial 1/3 and deep 1/3. RT-PCR and qRT-PCR were used to compare the expression of these markers in normal chondrocytes (NCs), BCs, and CCs, essentially as described [25, 26]. Relative expression levels compared to the house-keeping gene were calculated using the e 2ΔCt method. Primers were purchased from Integrated DNA Technologies (Coralville, IA). Table 1 summarizes the primers used in the PCR analysis.
Table 1.
Primer information for PCR
| Forward | Reverse | |
|---|---|---|
| ABCG2 | CCTTGGTTGTCATGGCTTCA | AGTCCTGGGCAGAAGTTTTGTC |
| CD 105 | CCACTGCCCCAGAGACTGCGC | GCCCCCACAGTGAGTGCTTAGGT |
| CD 90 | CGGTGGTGTTTGGCCATGTAATGA | GAGAGAGGGGAGTCCTATCCTGGT |
| CD 73 | AGCTTTCCCAGCCTTCCATGCG | GGGTGTCCTCTTGAGTCCTGCA |
| CD 29 | GCGGCCTCCGGGTGGATTCC | GCCGGGAAGGTCCAGGGGC |
| Runx-2 | GCATGAAGCCCTATCCAGAGTCT | GCTGATGGAGCTGTTGGTGTAG |
| Sox-9 | CGGTGGTGTTTGGCCATGTAATGA | GAGAGAGGGGAGTCCTATCCTGGT |
| B-actin | TCGACACCGCCAACCAGTTCGC | CATGCCGGAGCCGTTGTCGA |
| CXCL-12 | AGATGCCCTTGCCGATTC | TCTTCAGCCTTGCCACGA |
| Dock-10 | ATCCCAGTAGCAACGAGC | ATCATGTGGTCAGCGAAG |
Cell Migration/Chemotaxis Assay
Cell migration/ chemotaxis assays were performed using a CytoSelect 24-Well Cell Invasion Assay kit (Cell Biolabs) according to manufacturer`s instructions. HCCs (High-efficiency colony-forming cells) or NCs (normal chondrocytes) suspensions from full thickness cartilage (5×105 cells in serum-free medium) were added to the upper Transwell and placed in reservoirs containing serum-free medium alone or serum-free medium (SF) with 20 nM HMGB-1 (High-mobility group protein B1), a nuclear alarmin released by necrotic cells post injury. The plates were incubated for 24 hours prior to processing. Cell lysates from the culture plate were then transferred to fluorescence plates and read on a micro-plate reader (Molecular Devices, California, USA). The data are presented as the relative fold-change regarding fluorescent intensity readings.
Multi-lineage Differentiation Assay
The multi-potency of HCCs (BCs and CCs) was examined by performing chondrogenic, osteogenic and adipogenic differentiation, respectively. For chondrogenic induction, 1.5×106 cells from each group were pelleted in 15 ml conical tubes at 300×G for 5 minutes, and then cultured in chondrogenic medium (DMEM containing 10 ng/ml TGF-β1, 0.1 µM dexamethasone, 25 µg/ml L-ascorbate, 100 µg/ml pyruvate, 50 mg/ml ITS+Premix and antibiotics) at 5% CO2, 37 °C for 3 weeks. The resulting pellets were cryosectioned and analyzed for extra cellular matrix (ECM) formation by Safranin-O/fast green staining. For osteogenesis, HCCs were seeded into 12-well plates at 2×104 cells/well and cultured in osteogenic medium (DMEM/F-12 containing 0.1 µM dexamethasone, 100 mM β-glycerophosphate, 50 µg/ml L-ascorbate and antibiotics) 5% CO2, 37 °C for 3 weeks. Alizarin Red staining was used to detect calcium phosphate deposition. STEMPRO® Adipogenesis differentiation kit (GIBCO, Grand Island, NY) was used to induce adipogenesis according to the manufactures instructions. 3 weeks post-induction, cells were subjected to Oil Red O staining and imaged on a Nikon XB inverted microscope.
Statistical analysis
For clonogenicity screening, results were pooled from cartilage harvested from 6 animals with experiments done in duplicates. For gene expression studies, three different colonies for each group from each animal was tested. Statistical analysis was performed by Student`s t-test for each target gene. Migration assay was done by pooling HCCs from each animal (n=6) and run in triplicates and Student`s t-test was used confirm statistical significance between each treatment group. All statistical analysis was completed by the GraphPad Prism software package (Lajolla, California, USA)
Results
Discovery of High-efficiency Colony-forming Cells (HCCs)
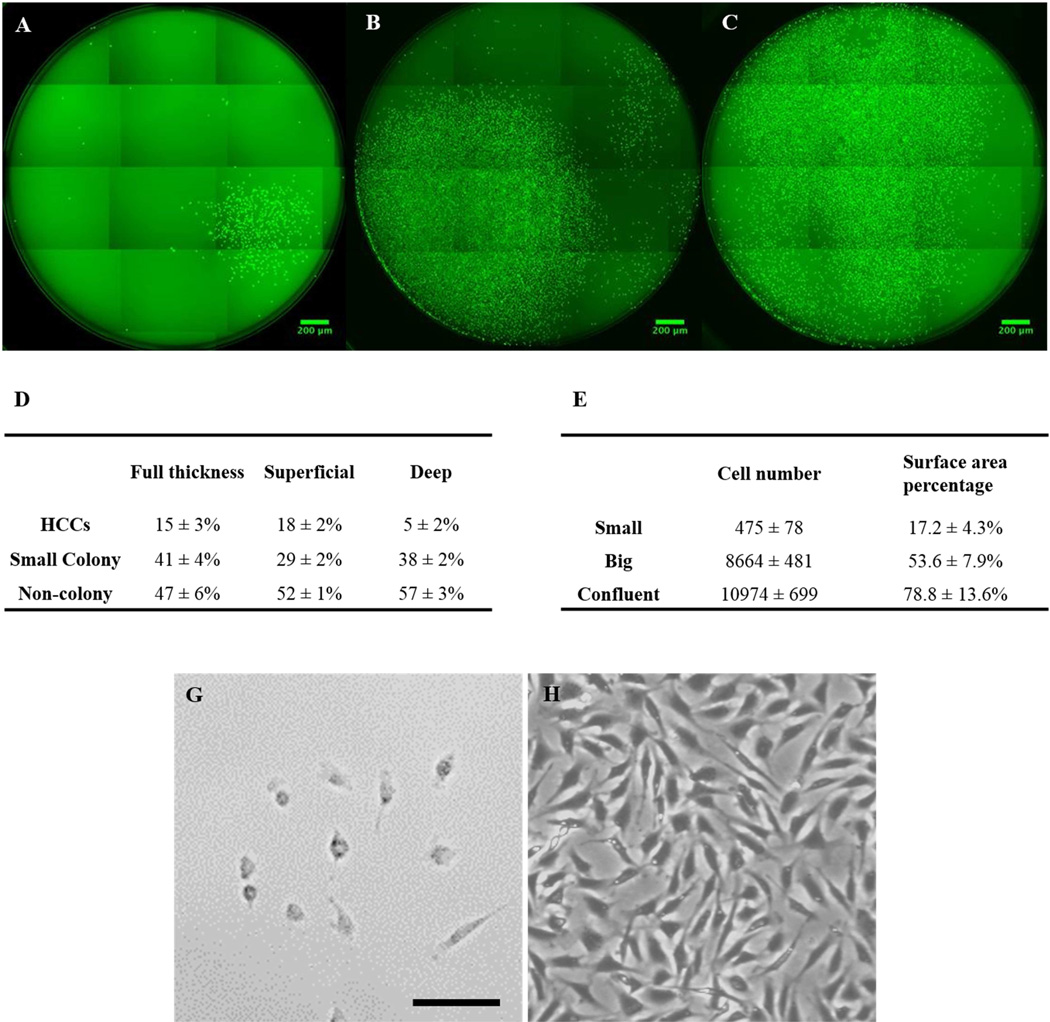
The number of cells and the size of the colonies varied from each individual single cell as determined by comparing the images taken at different time points. Individual cells grew at different rates with some single cells forming very big colonies very rapidly, while others were only able to give rise to small colonies or did not form colonies at all. Tiled fluorescent images of individual wells with different sized colonies at day 10 post initial single cell sorting are presented in figure 2. We found high efficiency colony-forming cells (HCCs), which either reached confluence (>2/3 surface area, 5 ± 1%) (Figure 2C), or had big-colony formation (1/3–2/3 surface area, 10±2%) (Figure 2B). The percentage of HCCs was relatively small (about 15±3%). Most of the cells (41±4% of all cells) formed small colonies (<1/3 surface area). HCCs started to form colonies (>50 cells) as early as day 5, unlike most cells, which showed only a limited number (<50) of cells growing at that time. Even at day 10, most of the wells had only small colony formation (Figure 2A); with 47±6% showing no colony formation, even after 14 days culture (data not shown). Some cells proliferated within the first couple days, but failed to continue growing; instead keeping the same cell numbers throughout prolonged culture. Figure 2D summarizes the average percentage of cells that formed each type of colony (n=6). Figure 2E summarizes the average cell number and surface area percentage of the colonies. Besides colony-forming efficiency, noticeable morphological differences were also observed between HCCs and Non-HCCs. Most HCCs displayed a fibroblast-like morphology, with a stretched and flat shape (Figure 2H), while non-HCCs showed the characteristic cobblestone-like shape of normal chondrocyte cultures (Figure 2G).
Figure 2.
Fluorescent images of different type of colonies and their relative ratio and dimension. A) Small colony. B) Big colony. C) Confluent colony. D) The percentage of different type of colonies from full thickness cartilage, and superficial 1/3 and deep 2/3, respectively. E) The criteria used for categorized colonies in regard to their size and cell number. (Scale bar represents 200 µm) G) Non-colony forming cells with characteristic cobblestone-like shape same as chondrocytes. H) Colony-forming cells displayed more stretched fibroblast-like morphology similar as mesenchymal stem cells. (Scale bar represents 50µm)
HCCs were isolated from both the superficial 1/3 and the deep 2/3 articular cartilage. Even though the total number of colony-forming cells was not significantly different between the two sites, with the superficial 1/3 having 46 ± 4% and the deep 2/3 having 46 ± 1%, by day 10, the superficial 1/3 site produced more HCCs formation (18 ± 2%) than did the deep 2/3 site (5 ± 2%)(Figure 2D).
Stem/Progenitor cell marker expression
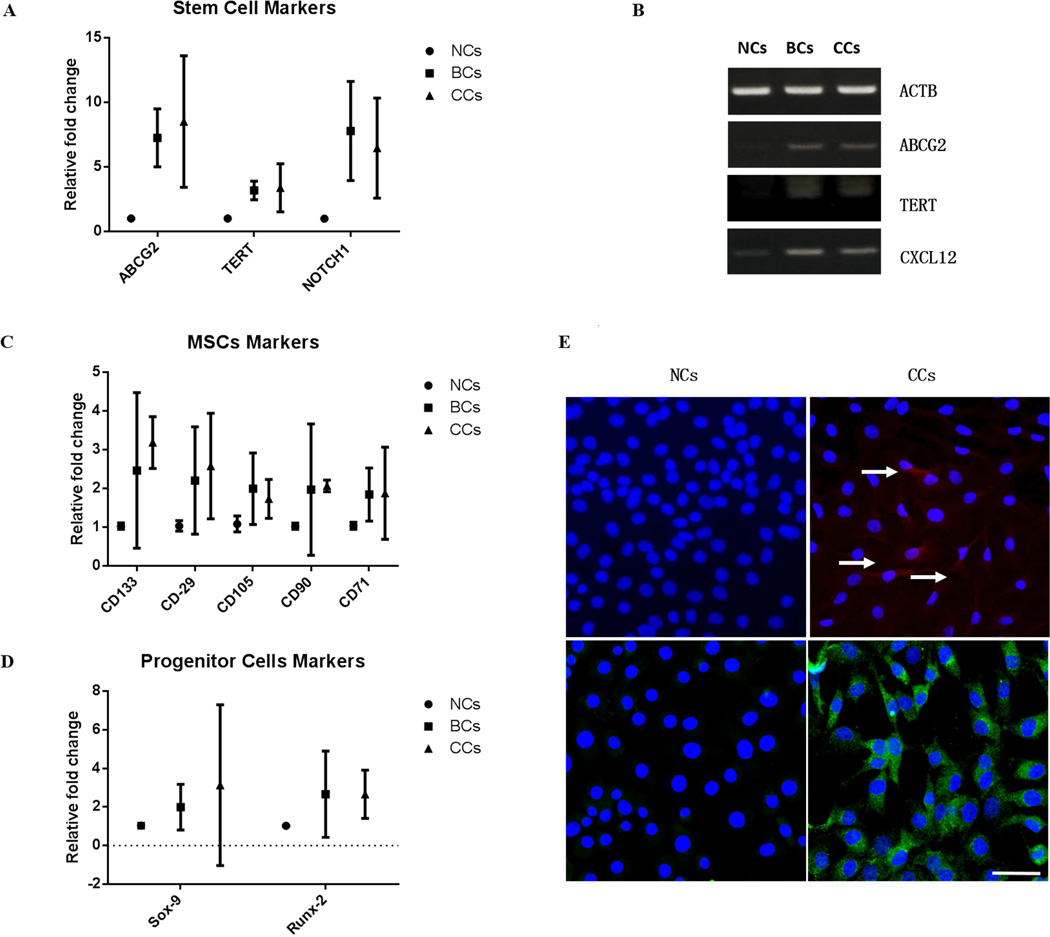
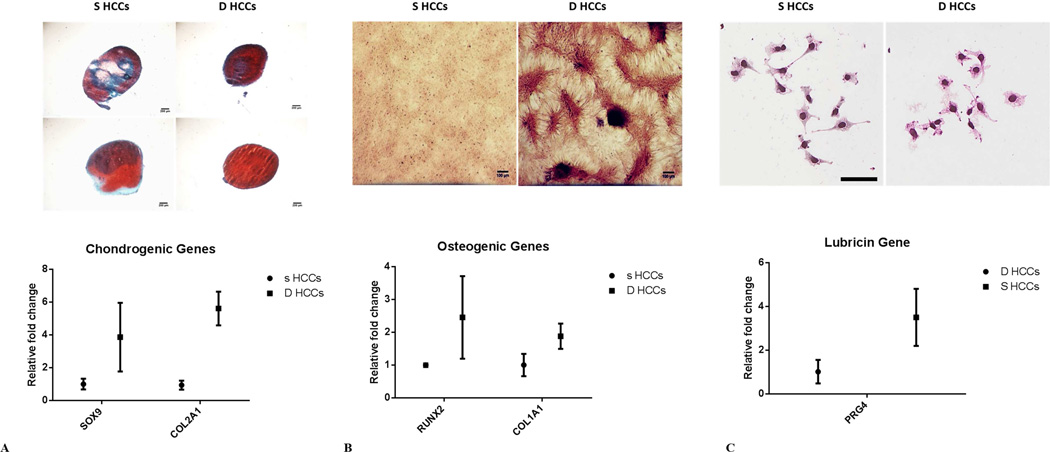
Gene expression analysis revealed substantially higher expression of stem/progenitor cell marker genes in the HCCs versus the NCs. qRT-PCR showed that ABCG2 was increased by over 8-fold, and TERT by 3.2-fold. Moreover, agarose gel electrophoresis showed much stronger bands for ABCG2 and TERT in HCCs compared with NCs (Figure 3A). Sox-9 was increased by 3-fold, Runx-2 by 2.5-fold (Figure 3D) and CD105, CD90, CD71, CD29, were increase around 2-fold in the HCCs over the NCs (Figure 3C). HCCs from the deep 2/3 showed higher RUNX-2, SOX-9, and COL1A1, COL2A1 expression, than colonies from the superficial 1/3 (Figure 5 A and B). In addition, gene expression also confirmed higher level of lubricin was expressed in HCCs from superficial 1/3 with over 3-fold increase compared with deep HCCs (Figure 5C). Using immunostaining analysis, we showed that HCCs from full thickness cartilage showed positive staining for both ABCG2 and Notch-1, in comparison to NCs, which mostly are negative for these two stem/progenitor cells markers (Figure 3E). Also, for lubricin expression, HCCs from superficial 1/3 showed relatively intense lubricin staining signal with dark color for the cytoplasm, while HCCs from the deep 1/3 has only mildly stained cytoplasm with much brighter color mainly from H&E counter staining (Figure 5C).
Figure 3. Gene expression of high-efficiency colony-forming cells (BCs and CCs).
A, B) Real-time qRT-PCR and/or agarose gel electrophoresis showed significant overexpression of stem/progenitor cell makers ABCG2, TERT and Notch1 in BCs and CCs compared with NCs. C) A array of MSCs markers were significantly overexpressed level in BCs and CCs versus NCs. D) Higher expression of chondrogenic transcription factor SOX-9 and osteogenic transcription factor RUNX-2 in BCs and CCs relative to NCs. E) CCs showed positive staining for both ABCG2 (upper right) and Notch-1 (lower right) in comparison to NCs. (scale bar represent 50µm). The error bars in each plot represent the 95% confidence interval (CI).
Figure 5. Functional difference of superficial and deep HCCs.
A) Deep HCCs showed superior chondrogenic potential with stronger Safranin-O staining for proteoglycan on both day 7 (left) and day 21 (Right), and also showed higher chondrogenic genes expression. B) Deep HCCs had stronger osteogenic ability than superficial HCCs, with more calcium phosphate deposition on Alizarin red staining and osteogenic markers higher expression. C) Superficial HCCs displayed higher level of lubricin both for immunostaining and gene expression than deep HCCs. The error bars in each plot represent the 95% confidence interval (CI).
Chemotactic cell migration
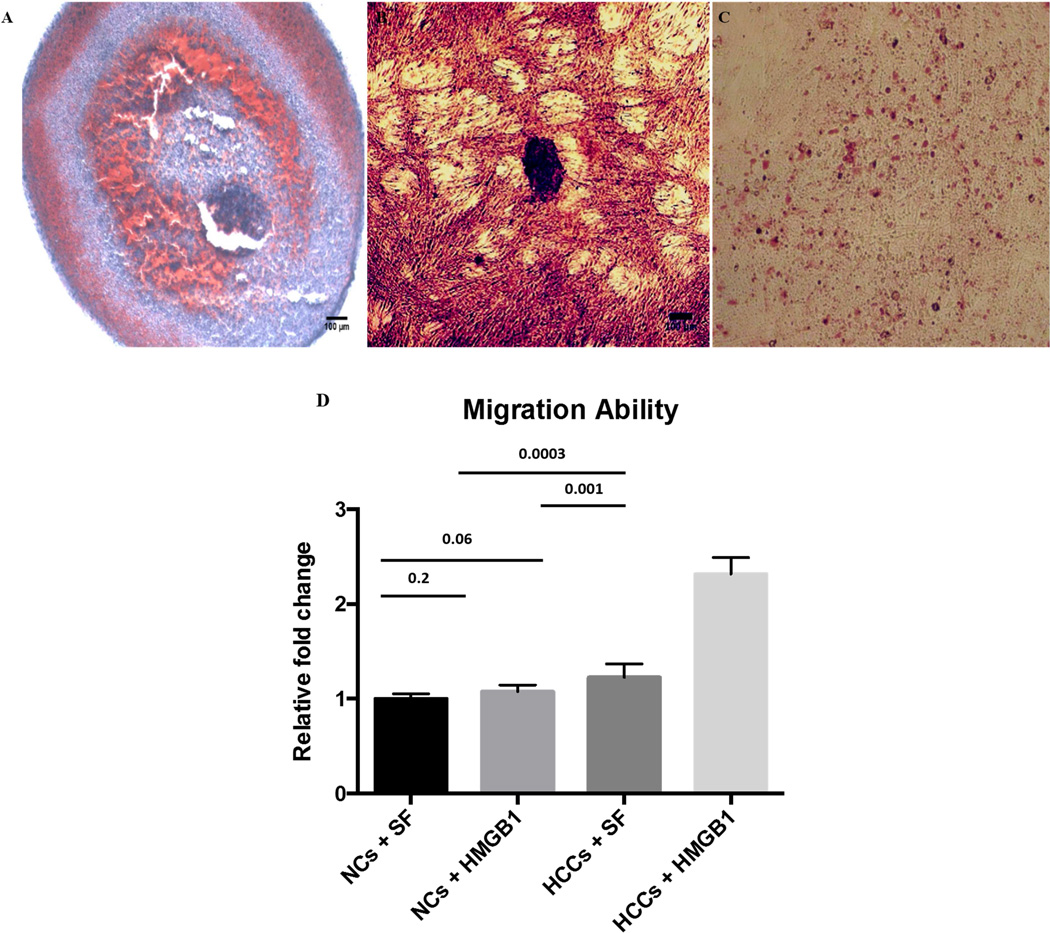
In general, HCCs were significantly more active in Transwell chemotaxis assay than normal chondrocytes with stimulation of chemotactic factor (P = 0.0003). Upon HMGB1 stimulation, HCCs showed strongly increased cell migration (P = 0.001) than untreated, while NCs did not have significant response to HMGB1 (P = 0.2). Compared with NCs, although HCCs did not show significantly more active migration ability (P = 0.06), they responded more rigorously to HMBG1 upon stimulation, with significantly increased migrating cells (P = 0.003) (Figure 4D).
Figure 4. Multi-lineage differentiation of HCCs and chemotactic migration.
A) Chondrogenic differentiation in pellet culture. Safranin-O/fast green staining displayed strong proteoglycan deposition. B) Osteogenic differentiation in monolayer cultured HCCs. Alizarin Red (dark red) staining showed massive calcium phosphate deposition. C) Adipogenic differentiation in monolayer HCCs. Only few cells were positive for Oil Red O staining. D) Migrating cells responed to HMGB1 stimulation. HCCs were strongly chemotactic to HMGB1, with significantly higher cell migration than treated with SF; HCCs also showed significantly higher migration than NCs upon HMGB1 stimulation. Numbers above the bars indicate P values for differences between each group; Bars show the mean ± 95% CI. (Scale bar represents 200µm)
Multipotent differentiation ability of HCCs
Chondrogenic, osteogenic, or adipogenic induction was performed for HCCs in a 21-day culture period to evaluate their multi-lineage differentiation potential. Pellets from chondrogenic differentiation showed a substantial proteoglycan deposition throughout the histology section (Figure 4A). Similarly, HCCs cultured in osteogenic medium had some calcium phosphate deposition in extracellular matrix as detected by Alizarin Red staining (Figure 4B). However, very few cells were positive for Oil Red O staining after adipogenic induction (Figure 4C). Interestingly, HCCs from deep 2/3 showed higher potential for chondrogenicity, with stronger proteoglycan deposition in the pellets starting on day 7 (Figure 5A upper right panel) through day 21 (Figure 5A lower right panel) as compared to HCCs from superficial 1/3 (Figure 5A lower panel). HCCs from the deep 2/3 also showed substantially higher calcium phosphate deposition on Alizarin red staining, with multiple calcium nodule formations, while HCCs from the superficial 1/3 had barely detectable staining signal for calcium phosphate (Figure 5B), suggesting that these cells may be less differentiated.
Discussion
In this study, bovine chondrocytes are individually sorted into each well of the 96 well plates by fluorescence-activated cell sorting (FACS) for viable cells, which allows the assessment of the clonogenic potential of each individual cell, thus enable us to isolate clonal population which might represent progenitor cells with the whole population. The HCCs cloned from a single cell closely resemble the progenitor cells identified in our previous study and by other groups, with the stem cell marker expression, clonogenicity, and multipotency consistent with published descriptions of progenitor cells from cartilage and other somatic tissues [23, 27, 28]. Differences of potency for HCCs from superficial 1/3 and deep 2/3 articular cartilage illustrate the distinct functions they may carry.
Clonogenicity screening confirmed the presence of colony-forming cells (CFCs) within normal chondrocytes (NCs) isolated from full thickness articular cartilage. Based on differential colony-forming efficiency, we are able to identify a group of rapidly growing cells (HCCs) which either form big colonies or reach confluence in 96-well plates by day 10 post single cell seeding. Although nearly half of cells can form colonies, only very small portions of the cells are HCCs, whose active proliferating phenotype closely resembles transient amplifying progenitor cells.
High expression of stem cell markers like ABCG2 and TERT, strongly indicate that HCCs may represent a highly self-replicating progenitor cell population within cartilage. The high expression of a key chondrogenic transcription factor Sox-9 and an osteogenic transcription factor Runx-2, together demonstrated the possible bi-potency of HCCs for differentiation towards these two lineages, strongly supporting their progenitor cell nature. Over-expression of representative mesenchymal stem cell makers for HCCs compared with NCs further distinguish them from NCs, as a progenitor cell population, which is consistent with other work regarding characterization of progenitor cells within articular cartilage or other tissues.
In addition, chemokine involved in progenitor cell and leukocyte recruitment were significantly up-regulated in HCCs as compared to NCs. CXCL-12 showed much stronger band in agarose gel electrophoresis, which may indicate the ability of HCCs to attract more progenitor cells or inflammatory cells such as leukocytes and macrophages when activated (by isolation, injury, inflammatory stimuli, etc.). The ability of HCCs to secrete CXCL-12 may enable them to recruit more endogenous progenitor cells within articular joint upon injuries [29], and also facilitate the attraction of inflammatory regulator cells [30] to clean up necrotic cells and damaged tissue, thereby expediting the process of tissue repair.
In vitro chemotaxis assays by HMGB-1, a nuclear protein, previously identified as core chemoattractant post-traumatic cartilage injury [24], revealed that HCCS were more active in migration than NCs upon stimulation. This was consistent with previous study that chondrogenic progenitor cells have migratory capability, and respond rigorously towards focal injury after cartilage impact. This might also indicate that HCCs can be attracted by damage associated chemotactic factors for tissue repair and regeneration.
Multilineage differentiation, along with colony-forming ability and surface marker expression, is one of the defining characteristics of mesenchymal stem cell populations. Therefore, our study evaluated HCCs multipotency in classic differentiation culture system. We found that HCCs can be readily induced toward chondrogenic and osteogenic differentiation. However, they have very limited adipogenic ability. This result is consistent with published data for cartilage progenitor cells, as well as chondrogenic progenitor cells (CPCs) in our previous study [24]. Therefore, HCCs are not like mesenchymal stem cells, which are multi-potent and are able to differentiate towards three-lineages. Instead HCCs are more likely to be progenitor cells, which have lost some of the “stemness” and appear later along the lineage commitment path. They appear to reside quietly in the articular cartilage matrix until they are activated by conditions, such as trauma and inflammation, to restore damaged cartilage tissue.
Although previous studies have pointed out that cartilage progenitor cells mainly reside on the superficial layer of immature bovine articular cartilage [18], our study demonstrates that HCCs can be isolated from both the superficial 1/3 and the deep 2/3 of articular cartilage, albeit the superficial 1/3 has significantly more progenitor cells than the deep 2/3. This difference may result from the distinct environment of the superficial vs. the deep zone of articular cartilage. The superficial zone cells are subject to more shear stress and undergo rapid turnover [31], requiring more progenitor cells to replenish tissue loss [32], while the deep zone cells are subject to more compressive stress [33], and thus may quietly reside in the surrounding ECM with minimal tissue remodeling required. Progenitors from both layers show their bi-lineage differentiation potential for chondrogenesis and osteogenensis. Deep 2/3 progenitors showed stronger chondrogenic and osteogenic ability than superficial 1/3 progenitors, as well as higher progenitor cell transcription factors gene expression, which may indicate that these cells represent a more primitive population with less a differentiated phenotype and more plasticity to give rise to multiple cell types in osteochondral tissues, or it may indicate that during the process of tissue maturation early progenitors have differentiated again to produce distinct sub-populations specific for the different zones of articular cartilage. Their primary function could involve formation of neo-cartilage, as well bone tissue during the skeletal development. Moreover, upon injury or degradation of articular cartilage and/or sub chondral bone, they could be called upon for tissue repair.
Our previous study showed that there exists a highly proliferative cell population (chondrogenic progenitor cells), which responds to cartilage superficial focal damage, and secretes lubricin, a protein for joint lubrication and cartilage surface maintenance [24]. These cells might come from the proliferation of progenitors identified in the superficial 1/3 in this study since we also showed in this study that progenitor cells from the superficial 1/3 has higher lubricin expression compared with those from deep 1/3. Such cells may normally proliferate only slowly and reside in their niche of cartilage extra cellular matrix (ECM). When they are activated by traumatic injuries or other biochemical and/or mechanical alteration, they may actively proliferate and migrate towards injury sites for tissue repair. In addition, in the late stage osteoarthritis, they might also present in the repair tissue. Here we also reveal that less differentiated population of cells resides in the deep 2/3 of the articular cartilage. Thus, the progenitors from the superficial 1/3 may actually originate from these deep 2/3 progenitors during the growth and development of articular cartilage, gradually gaining their more committed phenotype with specified functions like joint lubrication.
In conclusion, although articular cartilage is notorious for its poor healing ability posttraumatic injuries, our discovery of high-efficiency colony-forming cells (HCCs) from a single cell cloned progenitor cell population, demonstrates the cartilage’s intrinsic self-repairing potential. Our single cell sorting and clonogenecity screening successfully identify HCCs from both the superficial 1/3 and the deep 2/3. Further, they have different lineage specific gene expressions and distinct differentiation potential, which may represent their distinct function in regard to cartilage repair.
Acknowledgement
This work was supported by the Department of Defense (W81XWH-10-1-0702a) and by a grant from the World Arthrosis Organization. The authors would like to thank Abigail D. Smith, Barbara J. Laughlin and John F. Bierman for collecting specimens, and Gail L. Kurriger for her technical support in histology study. The author also would like to thank Dr. Jackie, Bickenbach for providing critical suggestion in editing and revising the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Conception and Design: Yu, Zheng, Martin
Collection and assembly of data: Yu
Analysis and interpretation of the data: Yu, Zheng, Martin
Drafting and revising of the article: Yu, Martin, Buckwalter
Conflict of Interests The authors have no financial or personal relationships with entities that could have influenced this work.
Contributor Information
Yin Yu, Email: yin-yu@uiowa.edu.
Hongjun Zheng, Email: Hongjun-zheng@uiowa.edu.
Joseph A. Buckwalter, Email: joseph-buckwalter@uiowa.edu.
James A. Martin, Email: james-martin@uiowa.edu.
References
- 1.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004 Jun;:7–16. [PubMed] [Google Scholar]
- 3.Hunziker EB. The elusive path to cartilage regeneration. Adv Mater. 2009 Sep 4;21:3419–3424. doi: 10.1002/adma.200801957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanamoto T, Nakamura N, Nakata K, Yoshikawa H. [Articular cartilage regeneration using stem cells] Clin Calcium. 2008 Dec;18:1744–1749. [PubMed] [Google Scholar]
- 5.Ogawa R, Mizuno S. Cartilage regeneration using adipose-derived stem cells. Curr Stem Cell Res Ther. 2010 Jun;5:129–132. doi: 10.2174/157488810791268627. [DOI] [PubMed] [Google Scholar]
- 6.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012 Aug;18:301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 7.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012 Dec;19:902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007 Mar;25:689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 9.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006 Jul;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 10.Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011 Apr;396:489–497. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009 Jun;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quesenberry PJ, Colvin G, Dooner G, Dooner M, Aliotta JM, Johnson K. The stem cell continuum: cell cycle, injury, and phenotype lability. Ann N Y Acad Sci. 2007 Jun;1106:20–29. doi: 10.1196/annals.1392.016. [DOI] [PubMed] [Google Scholar]
- 14.Dupuis J, Prefontaine A, Villeneuve L, Ruel N, Lefebvre F, Calderone A. Bone marrow-derived progenitor cells contribute to lung remodelling after myocardial infarction. Cardiovasc Pathol. 2007 Nov-Dec;16:321–328. doi: 10.1016/j.carpath.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004 Feb 29;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 16.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009 Apr 3;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004 May;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 18.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007 Jun 22;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012 Jul 6; doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005 Jun;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003 May;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 22.Janebodin K, Horst OV, Ieronimakis N, Balasundaram G, Reesukumal K, Pratumvinit B, et al. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6:e27526. doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012 Nov;64:3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seol D, Choe H, Zheng H, Jang K, Ramakrishnan PS, Lim TH, et al. Selection of reference genes for normalization of quantitative real-time PCR in organ culture of the rat and rabbit intervertebral disc. BMC Res Notes. 2011;4:162. doi: 10.1186/1756-0500-4-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seol D, Yu Y, Choe H, Jang K, Brouillette MJ, Zheng H, et al. Effect of Short-Term Enzymatic Treatment on Cell Migration and Cartilage Regeneration; In Vitro Organ Culture of Bovine Articular Cartilage. Tissue Eng Part A. 2014 Jan 15; doi: 10.1089/ten.tea.2013.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007 Nov;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 28.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013 Jul 23; doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 29.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011 Jan;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Shimokado K, Yoshida M. SDF-1-induced adhesion of monocytes to vascular endothelium is modulated by azelnidipine via protein kinase C inhibition. Eur J Pharmacol. 2006 Dec 15;552:162–169. doi: 10.1016/j.ejphar.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Wong BL, Bae WC, Gratz KR, Sah RL. Shear deformation kinematics during cartilage articulation: effect of lubrication, degeneration, and stress relaxation. Mol Cell Biomech. 2008 Sep;5:197–206. [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001 Jun;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995 May;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]