Abstract
Meta-analytic evidence supports a gene-environment (G×E) interaction between life stress and the serotonin transporter polymorphism (5-HTTLPR) on depression, but few studies have examined factors that influence detection of this effect, despite years of inconsistent results. We propose that the “candidate environment” (akin to a candidate gene) is key. Theory and evidence implicate major stressful life events (SLEs)—particularly major interpersonal SLEs—as well as chronic family stress. Participants (N = 400) from the Youth Emotion Project (which began with 627 high school juniors oversampled for high neuroticism) completed up to five annual diagnostic and life stress interviews and provided DNA samples. A significant G×E effect for major SLEs and S-carrier genotype was accounted for significantly by major interpersonal SLEs but not significantly by major non-interpersonal SLEs. S-carrier genotype and chronic family stress also significantly interacted. Identifying such candidate environments may facilitate future G×E research in depression and psychopathology more broadly.
Keywords: major depressive disorder, 5-HTTLPR, stressful life events, chronic family stress, interpersonal, young adults, Cox regression, gene environment interaction
Following an initial, seminal report by Caspi and colleagues (2003), the gene by environment interaction effect between the serotonin transporter-linked polymorphic region (5-HTTLPR) and stressful life events on onsets of major depressive episodes (MDEs) has been a source of debate among researchers. The polymorphism at the center of this debate, 5-HTTLPR, refers to an insertion/deletion polymorphism in the promoter region of the serotonin transporter gene, SLC6A4, that yields a transcriptionally less-efficient short (S) allele, and a relatively more efficient long (L) allele (Heils et al., 1996). The largest and most recent meta-analysis provided support for a significant gene by environment (G×E) interaction effect where individuals with the S allele report greater depression under increasing stress relative to L/L homozygotes (Karg, Burmeister, Shedden, & Sen, 2011), although two smaller, earlier meta-analyses were negative (Munafò, Durrant, Lewis, & Flint, 2009; Risch et al., 2009).
In the context of this debate, the possibility of a significant interaction effect—and moreover, the potential for identifying factors that enhance its detection—remains intriguing for several reasons. First, there has been longstanding interest in the serotonin transporter molecule in MDE etiology (e.g., Owens & Nemeroff, 1994). Second, there is evidence that this polymorphism alters expression of the serotonin transporter (Heils, et al., 1996). Third, meta-analytic evidence suggests that this polymorphism accounts for up to 10% of the variance in amygdala activation to emotional stimuli (Munafò, Brown, & Hariri, 2008), which is implicated in risk for depression (Monk et al., 2008). Finally, additional meta-analytic evidence indicates that life stress interviews and other objective measures yield more robust G×E interaction effects compared to lower validity measures (i.e., life stress questionnaires; Karg, et al., 2011) consistent with several reviews (Monroe & Reid, 2008; Uher & McGuffin, 2010). Unfortunately, a majority of investigations of this question have used checklist measures of life stress. Thus, a goal of studies that use interview and objective measures of life stress could be to identify additional factors that influence the detection of the 5-HTTLPR G×E effect. Identifying such factors may not only contribute greater understanding of 5-HTTLPR’s role in depression, but moreover, may facilitate future G×E research on depression with novel candidate polymorphisms. We propose that an important factor may be the “candidate environment” (the specific type of stressor, analogous to candidate genes) that is examined.
Many G×E studies have examined episodic stressful life events (SLEs) in keeping with the notion that SLEs are the environmental pathogen most consistently associated with MDE onset (Brown & Harris, 1989; Monroe, 2008), but few have examined characteristics of SLEs that are particularly implicated in the etiology of MDEs. (SLEs are acute, temporary, psychologically threatening experiences, that are conceptualized as distinct from chronic stress, which refers to enduring pressures, strains, or quality of life (e.g., Hammen, 2005).) One important characteristic is SLE severity. We refer to SLEs with moderate to severe impact or threat, considering the entire context of the SLE (i.e., “long term contextual threat”) as “major” SLEs and those with less than moderate impact or threat as “minor” SLEs, terms used in other stress research (e.g., Monroe & Harkness, 2005). Major SLEs are thought to most increase risk for MDE onset (Brown & Harris, 1978; Kendler, Karkowski, & Prescott, 1998; Monroe, 2008) and may thus be more likely to reveal G×E effects than minor SLEs. In contrast to the possibility that major SLEs would not reveal G×E effects if most people became depressed following a major SLE, there is no evidence for such a ceiling effect of major SLEs on depression risk (i.e., even after a major SLE, only a minority of individuals experience an MDE onset; e.g., Kendler et al., 1995). Only one study has examined the influence of SLE severity on the G×E interaction, concluding that the effect was most robust for relatively minor SLEs, in contrast to the authors’ own predictions (Kendler, Kuhn, Vittum, Prescott, & Riley, 2005).
Among major SLEs, major interpersonal SLEs (i.e., major events which primarily impact the quality or quantity of one’s interpersonal relationships) are a particularly intriguing candidate environment. Interpersonal SLEs, often representing losses and sometimes representing targeted rejection, may be particularly likely to evoke depression (Brown & Harris, 1978; Hammen, 2005; Slavich, Thornton, Torres, Monroe, & Gotlib, 2009; Tennant, 2002). Numerous other interpersonally-relevant findings (Joiner & Timmons, 2009) and the efficacy of interpersonal therapy for treating depression (Beach, Jones, & Franklin, 2009) are consistent with an interpersonal sensitivity in depression. Further, laboratory-based social stress protocols in which stress responding is differentiated by 5-HTTLPR genotype (Gotlib, Joormann, Minor, & Hallmayer, 2008; Miller, Wankerl, Stalder, Kirschbaum, & Alexander, in press) may derive their stressful quality in part from an interpersonal evaluative component. Indeed, one study showed that only a critical evaluative audience condition (not a supportive evaluative audience condition or a no-audience condition) revealed effects of 5-HTTLPR genotype on stress reactivity (Way & Taylor, 2010a). Finally, G×E research on rhesus macaque infants reared either with peers (a stressor) or with their mothers has provided consistent findings. This consistency may, in part, be due to isolating not only a homogeneous, lab-controlled type of stress, but also a potent social stressor. Among the findings most closely related to depression, rearing condition interacted with serotonin transporter genotype to predict both cerebrospinal fluid serotonin metabolite level (Bennett et al., 2002) and higher maternal separation-induced adrenocorticotropic hormone levels (Barr et al., 2004). Despite evidence from independent lines of research for the potential importance of interpersonal stress, to our knowledge, no study has yet examined whether the G×E interaction effect might be present for major interpersonal SLEs but not for major non-interpersonal SLEs.
Additionally, in contrast to the focus on SLEs to-date, some have suggested that it is also important to consider the cumulative nature of long-standing environmental pathogens such as chronic stress (Moffitt, Caspi, & Rutter, 2005). Among the various forms of chronic stress that can be assessed, several studies have reported specifically that chronic family stress significantly interacts with 5-HTTLPR genotype to predict depressive symptoms in youth and young adults. One report on 346 youth showed a significant G×E interaction, where greater chronic family stress at age 15 predicted higher levels of depressive symptoms at age 20 among S-carriers, but not among their L/L counterparts (Hammen, Brennan, Keenan-Miller, Hazel, & Najman, 2010). Similarly, in a community sample of 200 youth, baseline chronic family stress and 5-HTTLPR genotype produced a significant G×E effect on increases in depressive symptoms over a 6-month prospective period, such that greater chronic family stress predicted increasing depressive symptoms in S-carriers but not in L/L youth (Jenness, Hankin, Abela, Young, & Smolen, 2011). However, no one has yet examined whether such an interaction with chronic family stress might predict clinically significant MDE onsets. As such, in addition to episodic SLEs, the present study also considers chronic family stress.
We hypothesized that an interaction would occur between 5-HTTLPR genotype and major SLEs but not minor SLEs, despite one finding to the contrary (Kendler, et al., 2005). Among major SLEs, we hypothesized that interpersonal SLEs but not non-interpersonal SLEs would produce a significant G×E effect. Finally, we hypothesized that chronic family stress would also interact with 5-HTTLPR genotype.
Method
Participants
Detailed information regarding recruitment and demographics of the larger Youth Emotion Project (YEP) sample is reported elsewhere (e.g., Zinbarg et al., 2010). In summary, high school juniors were screened for neuroticism using the Revised Eysenck Personality Questionnaire (EPQ-R-N) (Eysenck, Eysenck, & Barrett, 1985). Those scoring in approximately the top third on this measure were oversampled into the longitudinal sample to increase the number of prospective onsets of emotional disorders. Participants (N = 627) provided informed consent for the longitudinal study and completed the baseline diagnostic and life stress interviews described below. Participants were asked to repeat these interviews annually; five annual interviews (the baseline plus four follow-ups) are reported here. Beginning in the sixth year of the larger YEP study, participants who were still in contact with the study were invited to provide a DNA sample; 410 participants consented and provided a sample. Ten who had completed at least the baseline interviews and provided a DNA sample were excluded from analyses due to a diagnosis of either bipolar disorder (I or II; n = 8) or psychotic symptoms (n = 3), or both. Remaining were 400 individuals; those who were included did not differ on demographic variables or EPQ-R-N scores from those who were excluded (see Table 1 for details).
Table 1.
Demographic and genotype characteristics of included and excluded participants
| Variable | Included (N = 400) | Excluded (N = 227) |
|---|---|---|
| Female | 69.35% | 68.28% |
| Race and ethnicity | ||
| Asian | 4.50% | 3.96% |
| Black | 13.50% | 12.33% |
| Caucasian | 48.25% | 48.02% |
| Hispanic / Latino | 14.25% | 17.18% |
| Pacific Islander | 0.75% | 0.44% |
| Other | 5.50% | 5.29% |
| Multiple | 13.25% | 12.78% |
| Screener risk level | ||
| Highest tertile | 57.75% | 60.35% |
| Middle tertile | 24.25% | 21.15% |
| Lowest tertile | 18.00% | 18.50% |
| 5-HTTLPR genotype | ||
| S/S | 19.50% (N = 78) | N/A |
| S/L | 49.50% (N = 198) | N/A |
| L/L | 31.00% (N = 124) | N/A |
| Mean (SD) | ||
| Included (N = 400) | Excluded (N = 227) | |
| Age in years at baseline | 16.91 (0.38) | 16.88 (0.41) |
| Baseline EPQ-R Neuroticism score | 11.82 (4.39) | 12.01 (4.76) |
| Hollingshead SES Score | 48.33 (12.52) | 47.62 (13.69) |
Note: Individuals from the original Youth Emotion Project sample of 627 who were included in the present analyses (N = 400) did not differ from those who were excluded (N = 227) in gender (χ2(1) = .06, p = .80, minority racial or ethnic status (χ2(1) = .00, p = .99), SES (F(1,611) = .42, p = .52) (Hollingshead, 1975), age at baseline (F(1,625) = .63, p = .43), or EPQ-R neuroticism score (F(1,625) = .27, p = .60).
Materials and Procedure
Assessment of Psychopathology
A baseline Structured Clinical Interview for DSM-IV, non-patient edition (SCID; First, Spitzer, Gibbon, & Williams, 2001) assessed lifetime diagnoses of mental disorders. Four subsequent annual follow-up SCIDs assessed diagnoses of psychopathology occurring during the period of time since the participant’s previous assessment. All interviewers possessed at least a bachelor’s degree and had completed an intensive SCID administration and scoring training program, including demonstrating reliability of diagnoses compared to a set of “gold standard” ratings. Interviewers were blind to the results of previous assessments and presented cases to a doctoral level supervisor. MDEs reported here represent clinically significant manifestations. Inter-rater reliability was assessed for approximately 10% of SCIDs in the larger study. Across the five assessments, kappa values adjusted due to departure from equiprobable distributions (i.e., low base rates of diagnoses) ranged from .82–.94 (M = .89, SD = .05).
Life Stress Assessment
Chronic and episodic stress over the past year were assessed at the baseline interview using the UCLA Life Stress Interview (LSI; Hammen, 1991; Hammen et al., 1987). The LSI administered at each follow-up interview assessed chronic family stress and SLEs occurring in the interim since the previous interview, unless an interview had been missed, in which case only the previous 12 months were assessed. Person-months without LSI information were excluded from the present analyses. In the LSI, chronic family stress was measured as one of 10 life domains: best friend relationship, social circle, romantic relationship, family relationships, academics, work, finances, neighborhood conditions, physical health, and family’s health. Ratings for chronic family stress were assigned by the interviewer for each domain on a scale from 1 (best circumstances) to 5 (worst circumstances) in half-point increments. To the extent possible, episodic stressors were excluded from consideration in the evaluation of chronic family stress. Average inter-rater reliability (ICCs) for the five time points studied for chronic family stress was M = .77 (SD = .07) within site and M = .80 cross-site (SD = .08).
SLEs were assessed throughout the LSI in each of the 10 life domains, with additional SLEs queried at the interview’s conclusion. Interviewers gathered information regarding the context, impact, and date of each SLE, then later presented this information to a team of two or more raters who were blind to the participant’s diagnoses and reported subjective responses to SLEs. Context-based SLE severity ratings were assigned by the consensus of the independent rating team, ranging from 1 (a non-event, no significant threat or negative implications) to 5 (a very severe event, maximal negative impact or threat) in half-point increments. Each SLE was assigned a code from a modified list of 77 numeric codes (Paykel & Mangen, 1980), describing the nature of each event (e.g., traffic accident, end of a friendship). Inter-rater reliability (ICCs) for SLE severity cross-site for the five interview periods ranged from .69 to .76 (M = .72, SD = .03); due to team rating of SLE severity no within-site reliabilities are available. Based on an a priori, contextually-based decision applied to all previous published LSI analyses in the present sample, events were classified as major SLEs if assigned a severity rating of 2.5 or greater, reflecting events with moderate to severe levels of contextual impact or threat (Adam et al., 2010; Uliaszek et al., 2012; Vrshek-Schallhorn et al., 2013). Events with a severity rating of 1.5–2.0 were classified as minor SLEs. In order to classify SLEs as interpersonal or non-interpersonal, two raters with LSI experience (SVS and KWT) assigned a category to each of the 77 Paykel codes. Interpersonal SLEs were defined as those events that, in the majority of instances, primarily affect the quality or quantity of the participant's relationships. Agreement was 96% (κ = .92); three discrepant ratings were resolved by consensus.
To address temporal precedence for SLEs and MDEs (i.e., whether the SLE preceded and potentially triggered the MDE, or vice versa), in all instances when an MDE and an SLE were dated to the same person-month, trained staff examined records to determine the order of occurrence. When the MDE preceded the SLE, or when the order was indeterminate, the SLE (but not the MDE or the participant) was excluded from analyses. To determine the influence of excluding SLEs when the order was indeterminate, follow-up analyses re-including these SLEs were conducted. The pattern of results did not change (results not presented).
Genotyping
After agreeing by phone to provide a DNA sample, participants provided saliva samples using Oragene kits (DNA Genotek, Ontario, Canada) in their homes and mailed them to study offices. Extraction was performed by Kbioscience (United Kingdom). Genotyping of 5-HTTLPR was conducted by the UCLA Core Genetics Lab based on a previously published protocol (Lesch et al., 1996) using modifications described in detail elsewhere (Taylor et al., 2006). Only traditional 5-HTTLPR genotypes are reported here due to of a lack of consensus regarding the single nucleotide polymorphism (SNP) rs25531 (Uher & McGuffin, 2008). This SNP has been suggested to modify the function of a subset of L alleles (Hu et al., 2005; Wendland, Martin, Kruse, Lesch, & Murphy, 2006). However, several reports have not supported this notion (Martin, Cleak, Willis-Owen, Flint, & Shifman, 2007; Philibert et al., 2008).
Statistical Approach
Cox regressions (Cox, 1972) were conducted using person-month datasets.1 MDE onset and offset dates, as well as SLEs, were assigned to the nearest month, with the start of LSI data gathering (one year prior to the baseline interview date) for each individual marking the beginning of the study period. Consistent with other studies examining dated MDE onsets and SLEs (e.g., Kendler, Karkowski, & Prescott, 1999; Kendler, et al., 2005; Kendler, Thornton, & Gardner, 2000), individuals experiencing an ongoing MDE at the beginning of the study period were excluded from analyses until the MDE ended. Similarly, after the month in which an individual experienced a new MDE onset, they were excluded from analyses until the MDE ended, when the individual re-entered analyses. Multiple MDEs with fewer than two months of recovery separating episodes were combined into a single, longer episode per DSM-IV-TR (American Psychiatric Association, 2000). The MDE onset variable was coded as 1 (present) in months in which an MDE onset occurred, and 0 (absent) in months in which onsets did not occur.
The primary analyses examined dichotomous occurrences of various types of SLEs rather than dimensional SLE severity for several reasons. Only major (and not minor) SLEs are thought to be significantly associated with MDE onsets, and major SLEs occur infrequently (e.g., in the present data, they occur in less than 5% of months). Our dichotomous but time-specific approach is consistent with a substantial body of previous SLE and depression research (e.g., Kendler, et al., 1998; Kendler, et al., 1995; Kendler, et al., 2000; Kendler, Thornton, & Gardner, 2001). The occurrence of each type of SLE (all major, all minor, etc.) was coded as 0 (absent) or 1 (present) for each person-month. The presence of SLEs was lagged to two months (e.g., if one occurred in month number 10, it was treated as present for months 10 and 11), consistent with a previous G×E interaction study that used time-specific analysis (Kendler, et al., 2005). This was because some evidence suggests most MDE onsets triggered by an SLE occur within a month of the SLE (Kendler, et al., 1995), whereas other evidence suggests a somewhat longer period is possible (Kendler, et al., 1998; Surtees & Wainwright, 1999). To support the primary dichotomous SLE analyses, dimensional SLE severity was also examined secondarily. For these variables, the maximum SLE severity score (1.5 to 5.0) for each person-month was used, with SLEs lagged to two months. Months with no SLEs were coded as 1.0, corresponding to a rating of no threat or impact.
Next, because chronic family stress scores were assigned for each interview period (i.e., ratings did not vary by month), these scores were applied uniformly to all person-months covered by an interview. Dimensional variables (chronic family stress and SLE severity variables) were centered. Genotype was coded as 1 for S-carriers (S/S + S/L) and 0 for L/L homozygotes. In all models testing a gene-environment interaction, gender (male=1, female=0) was entered as a covariate in a first block (Caspi, et al., 2003), genotype and life stress variable(s) were entered in a second block, followed by the G×E interaction effect in a final block. Hazard ratios (HRs) reported throughout refer to the difference in likelihood of MDE onset associated with a one unit increase in the predictor (Singer & Willett, 2003). For all analyses, p-values ≤ .05 were considered statistically significant. Power to detect a significant G×E interaction effect was estimated to be .775 for a G×E effect size of 4.0, and .589 for a G×E effect size of 3.0 (Demidenko, 2007, 2008).2 In addition to specifying a priori hypotheses, to address multiple testing, we applied false discovery rate adjustments separately to the hypothesized and non-hypothesized primary G×E tests (Benjamini & Hochberg, 1995; Benjamini & Yekutieli, 2001).
Population stratification refers to higher rates of an allele and coincidentally higher rates of disorder in a racial or ethnic subgroup creating a spurious main effect of genotype on risk for disorder. Thus, when significant interactions emerged, we applied a correction similar to those used in other genetic association work (e.g., Wu, DeWan, Hoh, & Wang, 2011). In lieu of a SNP panel to identify ancient geographic ancestry (which is then typically covaried in regressions) we used several self-reported race and ethnicity variables, which others have shown account for nearly all variance identified by such SNP panels (Tang et al., 2005). Specifically, we covaried membership in the sample’s largest two racial and ethnic minority groups (black race and Hispanic ethnicity). Further, to partial out the influence of group membership from an interaction effect (and not only from a main effect of a gene, which is sufficient for case-control genetic association studies), we also covaried each of the two-way interactions of black race and Hispanic ethnicity with S-carrier status and the stressor variable.
Results
Sample demographics and genotype frequencies are presented in Table 1. Zero-order associations of predictors are presented in Table 2. The 400 participants completed a mean of 4.40 (SD=0.88) of five possible annual diagnostic and life stress assessments, providing 21,340 person-months, containing 149 MDE onsets in 100 individuals. These comprised 56 first onsets, 52 second onsets, and 41 onsets of a third or higher episode number. Genotypes did not depart from Hardy-Weinberg equilibrium (χ2(1) = 2.95, NS).
Table 2.
Zero-order associations as Pearson’s r’s and logistic regression odds ratios
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Pearson r | |||||||||
| 1. Number of major SLEs (all types) | -- | ||||||||
| 2. Number of major interpersonal SLEs | .89* | -- | |||||||
| 3. Number of major non-interpersonal SLEs | .74* | .35* | -- | ||||||
| 4. Number of minor SLEs (all types) | .31* | .29* | .29* | -- | |||||
| 5. Chronic family stress | .39* | .34* | .29* | .13* | -- | ||||
| 6. Number of MDEs in study period | .29* | .28* | .18* | .10* | .22* | -- | |||
| Odds Ratios | |||||||||
| 7. Gender (male = 1, female = 0) | 0.92 | 0.75* | 1.14 | 0.97 | 0.80 | 0.71* | -- | ||
| 8. Black race (yes = 1, no = 0) | 1.21* | 1.26* | 1.33* | 0.98 | 2.24* | 1.29 | 1.13 | -- | |
| 9. Hispanic ethnicity (yes = 1, no = 0) | 1.15* | 1.18* | 1.23* | 1.03 | 1.59* | 0.95 | 0.52* | 1.13 | -- |
| 10. S-carrier 5-HTTLPR genotype | 0.97 | 0.95 | 1.00 | 1.05* | 0.86 | 1.10 | 1.06 | 0.41* | 1.93* |
Note: Pearson correlations are presented for associations of continuous variables with other continuous variables. Odds ratios derived from logistic regression are presented for association of any variable (IV) with a dichotomous variable (DV).
= Significant one-way associations (p ≤ .05).
Linear regressions with S-carrier status as a predictor of each life stress variable examined gene-environment (G-E) correlations. For these correlations, the number of occurrences of each type of SLE was totaled across the five annual Life Stress Interviews. Chronic family stress was examined as an average calculated within-person across the five years; in contrast, chronic family stress was permitted to vary as a function of interview timepoint in the G×E analyses. Black race and Hispanic ethnicity were covaried in G-E correlation analyses to prevent population stratification. S-carrier status did not significantly predict any of the stress variables examined (all t between -.652 and .334, all p ≥ .515), with the exception of the total number of minor SLEs, for which the relationship approached significance (t = 1.945, p=.052). S-carriers had somewhat more minor SLEs than their L/L counterparts.
Moderation of SLE Effect by 5-HTTLPR
Selected regression results are presented in Table 3; additional results are presented throughout the text. There was a significant interaction of major SLEs and 5-HTTLPR, such that S-carriers were at significantly greater risk for MDE onset than L/L homozygous individuals, given the occurrence of any major SLE. Minor SLEs did not significantly interact with genotype. When all major SLEs were separated into interpersonal and non-interpersonal major SLEs and these were entered into a single model with their respective G×E interaction effects, interpersonal SLEs interacted significantly with S-carrier status, but non-interpersonal SLEs did not interact significantly (Figure 1a).3
Table 3.
Select Cox Regression Results
| Model/Variable | Model -2 log likelihood |
χ2
(df) Change from previous step |
β | SE (β) | HR | 95% Lower CI |
95% Upper CI |
p | FDR adjusted p |
|---|---|---|---|---|---|---|---|---|---|
| All major SLEs × 5-HTTLPR | |||||||||
| Main effects step | 2594.40 | 27.17(2)*** | |||||||
| Major SLEs | 1.059 | .190 | 2.884 | 1.988 | 4.186 | .000*** | |||
| 5-HTTLPR S-carrier | .261 | .187 | 1.299 | .901 | 1.872 | .161 | |||
| Interaction step | 2589.96 | 4.44(1)* | |||||||
| Major SLEs† | .312 | .444 | 1.367 | .572 | 3.265 | .482 | |||
| 5-HTTLPR S-carrier† | .045 | .207 | 1.046 | .697 | 1.570 | .828 | |||
| Major SLEs × S-carrier | .974 | .492 | 2.649 | 1.009 | 6.950 | .048* | |||
| Interpersonal vs. non-interpersonal major SLEs (Combined Dichotomous SLE model) | |||||||||
| Main effects step | 2595.19 | 26.37(3)*** | |||||||
| Non-interpersonal major SLEs | .837 | .268 | 2.309 | 1.366 | 3.903 | .002** | |||
| Interpersonal major SLEs | .960 | .226 | 2.611 | 1.677 | 4.063 | .000*** | |||
| 5-HTTLPR S-carrier | .264 | .187 | 1.302 | .903 | 1.877 | .157 | |||
| Interaction step | 2581.11 | 14.08(2)*** | |||||||
| Non-interpersonal major SLEs† | 1.219 | .480 | 3.383 | 1.319 | 8.676 | .011* | |||
| Interpersonal major SLEs† | −1.318 | 1.016 | .268 | .037 | 1.962 | .195 | |||
| 5-HTTLPR S-carrier† | .051 | .205 | 1.052 | .704 | 1.573 | .803 | |||
| Non-interpersonal major SLEs × S-carrier | −.518 | .578 | .596 | .192 | 1.849 | .370 | .733 | ||
| Interpersonal major SLEs × S-carrier | 2.704 | 1.044 | 14.946 | 1.933 | 115.573 | .010** | .020* | ||
| Interpersonal vs. non-interpersonal dimensional maximum monthly SLE severity | |||||||||
| Main effects step | 2584.36 | 37.11 (3)*** | |||||||
| Non-interpersonal SLE severity | .410 | .133 | 1.506 | 1.161 | 1.953 | .002** | |||
| Interpersonal SLE severity | .610 | .112 | 1.840 | 1.477 | 2.292 | .000*** | |||
| 5-HTTLPR S-carrier | .276 | .187 | 1.318 | .913 | 1.901 | .140 | |||
| Interaction step | 2571.97 | 12.39 (2)** | |||||||
| Non-interpersonal SLE severity† | .719 | .187 | 2.052 | 1.421 | 2.963 | .000*** | |||
| Interpersonal SLE severity† | −.107 | .294 | .899 | .506 | 1.598 | .716 | |||
| 5-HTTLPR S-carrier† | .173 | .201 | 1.189 | .802 | 1.763 | .390 | |||
| Non-interpersonal SLE severity × S-carrier | −.475 | .258 | .622 | .375 | 1.032 | .066 | |||
| Interpersonal SLEs severity × S-carrier | .931 | .318 | 2.538 | 1.360 | 4.736 | .003** | |||
| All minor SLEs × 5-HTTLPR | |||||||||
| Main effects step | 2619.97 | 1.59(2) | |||||||
| Minor SLEs | −.006 | .174 | .994 | .707 | 1.397 | .971 | |||
| 5-HTTLPR S-carrier | .231 | .186 | 1.260 | .874 | 1.816 | .215 | |||
| Interaction step | 2619.85 | 0.12(1) | |||||||
| Minor SLEs† | .094 | .340 | 1.098 | .564 | 2.138 | .783 | |||
| 5-HTTLPR S-carrier† | .275 | .228 | 1.317 | .842 | 2.061 | .228 | |||
| Minor SLEs × S-carrier | −.135 | .395 | .874 | .403 | 1.896 | .733 | .733 | ||
| Chronic Family Stress × 5-HTTLPR | |||||||||
| Main effects step | 2551.62 | 69.37(2)*** | |||||||
| Chronic family stress | .881 | .103 | 2.414 | 1.972 | 2.955 | .000*** | |||
| 5-HTTLPR S-carrier | .327 | .187 | 1.387 | .961 | 2.003 | .081 | |||
| Interaction step | 2546.88 | 4.74(1)* | |||||||
| Chronic family stress† | .536 | .195 | 1.710 | 1.167 | 2.505 | .006** | |||
| 5-HTTLPR S-carrier† | .122 | .203 | 1.130 | .759 | 1.683 | .547 | |||
| Chronic family stress × S-carrier | .497 | .232 | 1.643 | 1.044 | 2.587 | .032* | .032* | ||
Note:
= Effect sizes with p-values ≤ .05 are considered significant.
p ≤ .01,
p ≤ .001.
SLE = stressful life event.
Gender (not shown) was added as a covariate in a first step of all models. Main effects of stress and genotype were added in the second step, presented here (“Main effects step”). As such, in the third step (“Interaction step”) presented here, stress variables and genotype characterize simple main effects denoted with † (i.e., for reference groups—stress variables refers to stress only for L/L, and genotype refers to S-carriers not under stress for models on SLEs, or in dimensional analyses with centered stress variables, at the mean of stress). False discovery rate (FDR) adjustment was conducted separately for hypothesized (i.e., major interpersonal SLEs, chronic family stress) and non-hypothesized (i.e., major non-interpersonal SLEs, minor SLEs) G×E effects.
Figure 1.
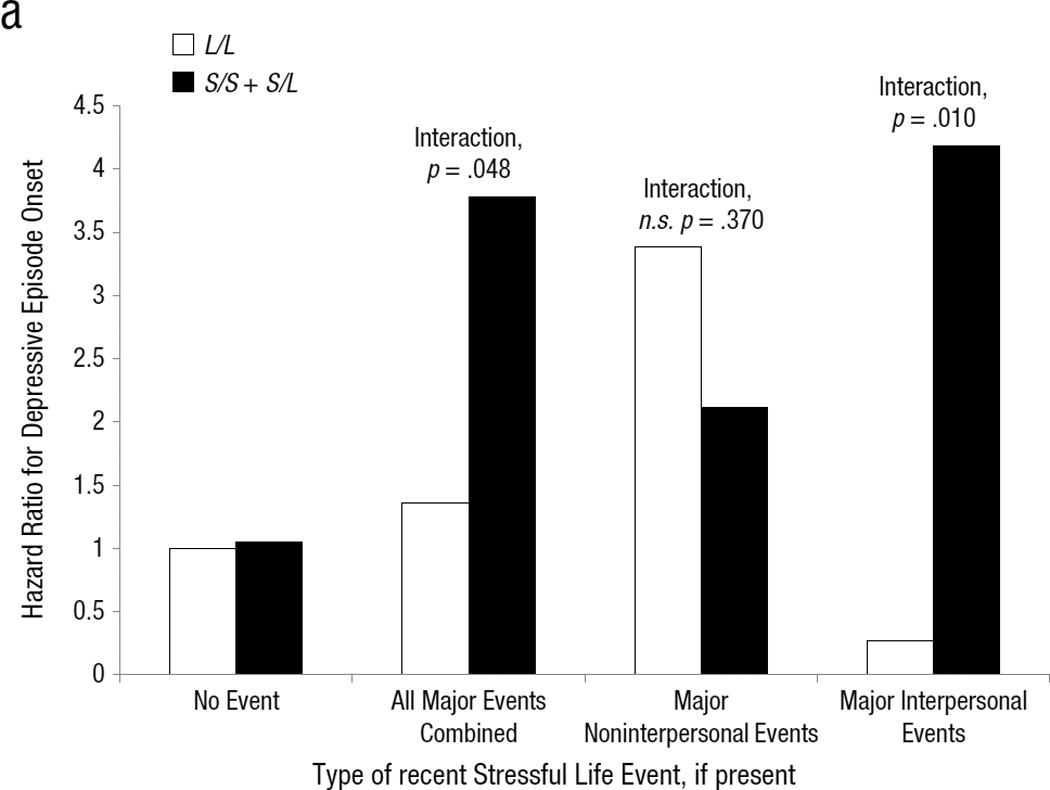
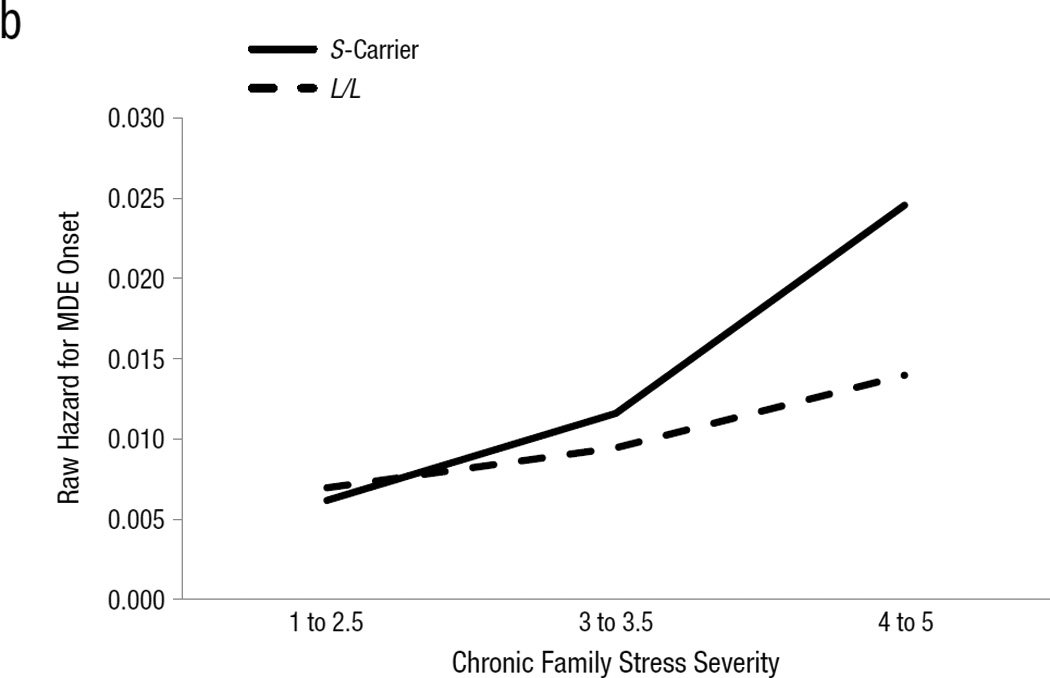
(a) Model-estimated hazard ratio for major depressive episode onset, in the presence or absence of several types of major severity stressful life events (SLEs) within the previous two months, separated by 5-HTTLPR genotype. Hazard ratios for major non-interpersonal and major interpersonal SLEs are derived from the combined dichotomous SLE model for these variables reported in Table 3. SE(β)s and 95% CIs for each term in the models are presented in Table 3. Raw hazard for major depressive episode onset (number of onsets divided by number of person months available under a given set of conditions) by severity of chronic family stress and 5-HTTLPR genotype.
Several follow-up tests were conducted on the G×E interaction with major interpersonal SLEs. First, to reduce the possibility of population stratification, black race, Hispanic ethnicity, and their two-way interactions with S-carrier status and major interpersonal SLEs were covaried. The interaction remained significant (b =2.552, SE(β)=1.053, HR=12.837, 95% CI=[1.629, 101.171], p =.015). Thus, this G×E effect cannot be due to population stratification arising from either of the two largest minority groups in the sample. Next, a secondary model examining dimensional SLE severity was conducted (Table 3). Supporting the dichotomous results, a significant G×E interaction of interpersonal SLE severity and S-carrier status emerged (HR=2.538, p=.003) such that those with the S-allele were at significantly enhanced risk for MDE onsets as SLE severity increased, compared to their similarly-stressed L/L counterparts. In addition, the interaction for non-interpersonal SLE severity approached significance in the opposite direction (HR=.622, p=.066). This suggests that, when interpersonal SLE severity is accounted for, S-carriers experiencing increasing non-interpersonal SLE severity are slightly (but not significantly) more resilient against MDE onsets than their L/L counterparts under similar stress. Furthermore, the simple main effects of stress were revealing: L/L individuals were at significant risk for MDE onsets in the context of increasing non-interpersonal SLE severity (HR=2.052, p<.001) but not in the context of increasing interpersonal SLE severity (HR=0.899, p=.716). Taken together, this pattern of results suggests that when both types of SLEs are accounted for, genotype may differentiate the type of SLE to which an individual is most sensitive.
Moderation of Chronic Family Stress by 5-HTTLPR
Chronic family stress produced the hypothesized G×E interaction with 5-HTTLPR genotype (Table 3, Figure 1b). Specifically, although chronic family stress significantly predicted MDE onsets in L/L individuals (simple main effect of family stress in L/Ls: HR=1.710, p=.006), it predicted MDE onsets in S-carriers with significantly greater strength (G×E effect: HR=1.643, p=.032). This interaction remained significant after covarying black race, Hispanic ethnicity, and their 2-way interactions with chronic family stress and S-carrier status (β = .559, SE(β)=.248, HR=1.749, 95% CI=[1.077, 2.842], p=.024).
False Discovery Rate Adjustment
False discovery rate adjustment (Benjamini & Hochberg, 1995) for multiple tests was applied to p-values obtained for G×E interactions separately for hypothesized interactions (i.e., major interpersonal SLEs and chronic family stress) and non-hypothesized interactions (i.e., major non-interpersonal SLEs and minor SLEs) (Table 3). After adjustment, the p-values for both the major interpersonal SLE G×E (p=.040) and the chronic family stress G×E (p=.032) remained significant.
Discussion
The present study investigated an interaction effect of the serotonin transporter-linked polymorphic region, 5-HTTLPR, and several forms of stress on MDE onsets in 400 individuals during late adolescence to young adulthood, using clinical diagnostic interviews and objectively-rated life stress interviews. Some have suggested that such stress interviews will produce more valid G×E research (Monroe & Reid, 2008) and others have indeed shown that interview and other objective measures have produced more robust G×E findings than have questionnaire measures (Karg, et al., 2011; Uher & McGuffin, 2010). Although several studies have previously examined an interpersonal form of chronic stress, specifically chronic family stress, the current study is the first to examine major interpersonal and non-interpersonal episodic SLEs separately, and to show that among major SLEs, only major interpersonal SLEs significantly contributed to a G×E interaction with 5-HTTLPR.
Importance of Interpersonal SLEs
There are several potential implications of the interpersonal SLE G×E finding, pending replication by other studies. First, within the context of G×E interaction research on depression specifically, this finding suggests that distinguishing between major interpersonal and non-interpersonal SLEs may lead to enhanced effect sizes and more consistent results, particularly if interview measures of life stress are employed. More broadly, the finding highlights the potential benefit of examining empirically or theoretically indicated candidate stressors in G×E interaction research. Second, the apparent importance of interpersonal SLEs for detecting a significant G×E interaction effect raises a question of whether the serotonin system is preferentially sensitive to social compared to non-social stimuli. There is indeed evidence that the serotonin system mediates the effects of social or interpersonal experiences on a range of health outcomes (for a review see Way & Taylor, 2010b). However, it is also implicated in an array of other functions, leading some to conclude that its overarching function is to mediate constraint (e.g., Spoont, 1992). Thus, preferential sensitivity of the serotonin system to social conditions may be due to its associations with other neural systems that are particularly sensitive to social threat. Such systems might include the HPA axis (Dickerson & Kemeny, 2004; Doane & Adam, 2010) and the oxytocin system (e.g., Jørgensen, Riis, Knigge, Kjaer, & Warberg, 2003).
Influence of SLE Severity
Major SLEs, particularly interpersonal ones, but not minor SLEs produced a significant G×E interaction. In this regard, our results regarding the level of SLE severity that produces a significant interaction diverged from that of Kendler and colleagues (2005), possibly due to important differences between the two samples. The present sample is younger and would be expected to have a higher proportion of first onset cases of depression than the sample of Kendler et al. There is evidence that with successive episodes of depression, as individuals putatively become more sensitive to stress, they are paradoxically less often observed to succumb to recurrences due to major SLEs, which occur relatively infrequently (Kendler, et al., 2000, 2001). Instead, they may succumb to recurrences after minor severity SLEs (Stroud, Davila, Hammen, & Vrshek-Schallhorn, 2011), supporting a stress sensitization model (Monroe & Harkness, 2005) of Post’s kindling hypothesis (Post, 1992). It may be that in relatively older samples such as Kendler and colleagues’ with a higher proportion of recurrences, the peak of the G×E interaction “shifts” from more major SLEs toward minor SLEs.
Findings for Chronic Family Stress
We also report a significant association of chronic family stress and 5-HTTLPR, which was consistent with two prior reports that obtained significant chronic family stress G×Es with 5-HTTLPR genotype on depressive symptoms (Hammen, et al., 2010; Jenness, et al., 2011). We extend these earlier findings to the prediction of clinically significant MDEs. Few G×E reports have examined objectively rated enduring stressors such as chronic family stress, and the present results suggest that examining chronic family stress may be a promising approach for future work.
Limitations
Although this study has several notable strengths including a prospective design utilizing repeated measures, clinical diagnostic interviews, objective stress interviews, and a time-specific statistical approach, it also has limitations. First, the present sample size of 400 participants is modest relative to certain other genetic studies; however, this sample is larger than the median sample size of 345 participants per study from 103 G×E publications reported in one review (Duncan & Keller, 2011). Further, unlike most such studies, the present study included repeated measures assessments of both stress and depression. Second, the sample is also racially and ethnically diverse, rather than homogeneous, which might have resulted in population stratification. Beyond what we were able to address in follow-up analyses, population stratification could potentially arise from latent ancient geographic ancestry groups. Further, the G×E interaction may be stronger in certain racial or ethnic groups than others, just as it may be stronger in males or females. Unfortunately, the present sample is underpowered to compare G×E effect magnitude between subgroups (i.e., to test three-way interactions). Finally, in contrast to our hypothesis that interpersonal SLEs are important to the G×E interaction because depression is characterized by interpersonal sensitivity, it is possible that interpersonal SLEs only appear important for obtaining the G×E interaction due to certain characteristics of this sample. These could include the predominance of female participants, a large proportion of participants with high levels of neuroticism, and the developmental stage of participants (i.e., the transition to adulthood). However, regarding the larger YEP recruitment strategy of oversampling participants with high levels of neuroticism, a simulation study demonstrated that such oversampling (and corresponding higher rate of prospective disorder onsets) does not bias regression effect size estimates, but does ameliorate other problems that occur when predicting to a low number of disorder onsets (Hauner, Zinbarg, & Revelle, under revision).
Conclusions
The present study found a significant interaction between major stressful life events and having one or two S alleles of the serotonin transporter-linked polymorphic region, 5-HTTLPR, on risk for major depressive episode onset. This was accounted for by the interaction of 5-HTTLPR and major interpersonal events. Evidence also supported a G×E interaction of chronic family stress with 5-HTTLPR genotype, consistent with previous reports. These results emphasize the importance of the careful measurement and selection of “candidate environments” (analogous to candidate genes) first for gene-environment research on depression, and perhaps also for other forms of psychopathology.
Acknowledgements
This research was supported by a two-site grant from the National Institute of Mental Health to SM and REZ (R01-MH065652) and to MGC (R01-MH065651), by a William T. Grant Foundation Scholars Award to EKA, and a Postdoctoral NRSA from the National Institute of Mental Health to SVS (F32-MH091955). A portion of these data were presented by the first author at the 2012 annual meetings of the Society of Biological Psychiatry, the Society for Research in Psychopathology, and the Association for Behavioral and Cognitive Therapies. The content is the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
The authors declare no conflicts of interest.
Although person-months are technically non-independent of one another, Allison (1982) demonstrates that an assumption of independence is reasonable, as the estimated standard errors closely approximate the true standard errors.
For both power estimations, alpha was set to .05, sample size was set to 400, and the option to examine two binary variables,×and z, with their interaction, x*z, was selected. The likelihood that x, genotype, was 1 (Prx = Pr(x = 1)) was set to .7, with a main effect of genotype (x) on depression (y) of OR = 1.1. The likelihood that z, the environment or stress variable, was 1 (Prz = Pr(z = 1)) was set to .5 for the likelihood of exposure to major SLEs over a duration of several years, with a main effect of stress (z) on depression (y) of OR = 4.0. The gene-environment correlation, ORxz, was set to 1.0 (none), and disease prevalence rate was set to .20.
This simultaneous test of the G×E effect for major non-interpersonal SLEs and major interpersonal SLEs demonstrates that the major interpersonal SLE G×E possesses significant unique variance beyond the major non-interpersonal SLE G×E, which neither possesses significant unique variance in this model, nor reaches significance on its own (model not reported). That is, the major interpersonal SLE G×E significantly predicts over and above the non-interpersonal SLE G×E.
However, this is not the same as testing for a significant difference between the two G×E effects. Unfortunately, the conclusive test for this difference, given by a Gene × Major SLE × Event Type (interpersonal vs. non-interpersonal) three-way interaction, is impossible to conduct in a person-month model. The “Event Type” variable can only be specified for months in which an event of interest (a major SLE) occurred. All months without such events would be missing this specifier, and Cox regression would omit those months from the analysis, leaving in the model only months in which a major SLEs occurred. The closest approximation is to isolate months in which a major SLE occurred and test for a two-way Gene × Event Type (major interpersonal SLE vs. major non-interpersonal SLE) interaction. This test provided a significant result (β = 2.524, SE(β)=1.161, HR=12.484, 95% CI=[1.283, 121.443], p=.030), consistent with the notion that the G×E effect for major interpersonal SLEs is indeed significantly larger than the G×E effect for major non-interpersonal SLEs. However, this is still not a strict test of whether one G×E is significantly larger than the other because months without any major events are necessarily omitted.
Authorship Note
SM, REZ, and MGC developed the study concept and design for the larger Youth Emotion Project with EER, CH, and EKA contributing; SVS developed the study concept and design for this paper. Data collection was performed by SVS, JWG, JS, and KWT under the supervision of SM, REZ, and MGC. SVS performed the data analysis and interpretation with REZ and EKA contributing. SVS drafted the paper and all authors provided critical revisions.
All authors approved the final version of the paper for submission.
References
- Adam E, Doane L, Zinbarg R, Mineka S, Craske M, Griffith J. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison P. Discrete-time methods for the analysis of event histories. Sociological Methodology. 1982;13:61–98. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Beach S, Jones D, Franklin K. Marital, family, and interpersonal therapies for depression in adults. In: Gotlib IH, Hammen C, editors. Handbook of Depression. 2nd ed. New York: The Guilford Press; 2009. pp. 624–641. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Bennett AJ, Lesch K-P, Heils A, Long JC, Lorenz JG, Shoaf SE, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Brown G, Harris T. Social origins of depression: A study of psychiatric disorder in women: Thomson Learning (EMEA) Ltd. 1978 [Google Scholar]
- Brown G, Harris T. Life events and illness. London: Unwin Hyman; 1989. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression models and life-tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(2):187–220. [Google Scholar]
- Demidenko E. Sample size determination for logistic regression revisited. Statistics in Medicine. 2007;26(18):3385–3397. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
- Demidenko E. Sample size and optimal design for logistic regression with binary interaction. Statistics in Medicine. 2008;27(1):36–46. doi: 10.1002/sim.2980. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35(3):430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. The American Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–29. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I disorders-non-patient edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 2001. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, Hiroto D. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96(3):190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan P, Keenan-Miller D, Hazel N, Najman J. Chronic and acute stress, gender, and serotonin transporter gene–environment interactions predicting depression symptoms in youth. Journal of Child Psychology and Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner K, Zinbarg RE, Revelle W. The effects of oversampling on partial regression coefficients and their standard errors. (under revision). [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Jenness J, Hankin B, Abela J, Young J, Smolen A. Chronic family stress interacts with 5-HTTLPR to predict prospective depressive symptoms among youth. Depression and Anxiety. 2011;28(12):1074–1080. doi: 10.1002/da.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner T, Timmons KA. Depression in its interpersonal context. In: Gotlib IH, Hammen C, editors. Handbook of Depression. 2nd ed. New York: The Guilford Press; 2009. pp. 322–339. [Google Scholar]
- Jørgensen H, Riis M, Knigge U, Kjaer A, Warberg J. Serotonin receptors involved in vasopressin and oxytocin secretion. Journal of Neuroendocrinology. 2003;15(3):242–249. doi: 10.1046/j.1365-2826.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Karkowski L, Prescott C. Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. Journal of Nervous and Mental Disease. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler K, Karkowski L, Prescott C. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler K, Kessler R, Walters E, MacLean C, Neale M, Heath A, Eaves L. Stressful life events, genetic liability, and onset of an episode of major depression in women. American Journal of Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler K, Kuhn J, Vittum J, Prescott C, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Archives of General Psychiatry. 2005;62(5):529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kendler K, Thornton L, Gardner C. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the "kindling" hypothesis. American Journal of Psychiatry. 2000;157(8):1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kendler K, Thornton L, Gardner C. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. American Journal of Psychiatry. 2001;158(4):582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen S, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Molecular Psychiatry. 2007;12(5):421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: A meta-analysis. Molecular Psychiatry. doi: 10.1038/mp.2012.124. (in press). [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Monk C, Klein R, Telzer E, Schroth E, Mannuzza S, Moulton J, Fromm S. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monroe S. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Monroe S, Harkness K. Life stress, the "kindling" hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112(2):417–444. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe S, Reid M. Gene-environment interactions in depression research: Genetic polymorphisms and life-stress polyprocedures. Psychological Science. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene×Environment Interactions at the Serotonin Transporter Locus. Biological Psychiatry. 2009;65(3):211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clinical Chemistry. 1994;40(2):288–295. [PubMed] [Google Scholar]
- Paykel E, Mangen S. Interview for recent life events. London: St. George’s Hospital Medical School; 1980. [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post R. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149(8):999. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. USA: Oxford University Press; 2003. [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology. 2009;28(2):223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoont M. Modulatory role of serotonin in neural information processing: Implications for human psychopathology. Psychological Bulletin. 1992;112(2):330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S. Severe and nonsevere events in first onsets versus recurrences of depression: Evidence for stress sensitization. Journal of Abnormal Psychology. 2011;120(1):142–154. doi: 10.1037/a0021659. [DOI] [PubMed] [Google Scholar]
- Surtees P, Wainwright N. Surviving adversity: Event decay, vulnerability and the onset of anxiety and depressive disorder. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(2):86–95. doi: 10.1007/s004060050071. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76(2):268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: A review of recent findings. Australian and New Zealand Journal of Psychiatry. 2002;36(2):173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2008;13(2):131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton JM, Hammen C. A longitudinal examination of stress generation in depressive and anxiety disorders. Journal of Abnormal Psychology. 2012;121(1):4–15. doi: 10.1037/a0025835. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg R, Craske M, Adam EK. The Cortisol Awakening Response predicts major depression: Predictive stability over a four year follow-up and effect of depression history. Psychological Medicine. 2013;43(3):483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010a;67(5):487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE. Social influences on health: Is serotonin a critical mediator? Psychosomatic Medicine. 2010b;72(2):107–112. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- Wendland J, Martin B, Kruse M, Lesch K, Murphy D. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wu C, DeWan A, Hoh J, Wang Z. A Comparison of Association Methods Correcting for Population Stratification in Case–Control Studies. Annals of Human Genetics. 2011;75(3):418–427. doi: 10.1111/j.1469-1809.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg R, Mineka S, Craske M, Griffith J, Sutton J, Rose R, Waters A. The Northwestern-UCLA Youth Emotion Project: Associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48(5):347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


