Abstract
Overexpression of Aurora A kinase occurs in certain types of cancer, and therefore results in chromosome instability and phosphorylation-mediated ubiquitylation and degradation of p53 for tumorigenesis. The high-risk subtype human papillomavirus (HPV)16 early oncoprotein E6 is a major contributor inducing host cell immortalization and transformation through interaction with a number of cellular factors. In the present study, co-immunoprecipitation, glutathione S-transferase pull-down and immunostaining were used to show that HPV16 E6 and Aurora A bind to each other in vivo and in vitro. Western blotting and reverse transcription-polymerase chain reaction were used to reveal that HPV16 E6 inhibited cell apoptosis by stabilizing Aurora A expression. The present study may report a new mechanism for the involvement of HPV16 E6 in carcinogenesis, as HPV16 E6 elevates Aurora A expression and the latter may be a common target for oncogenic viruses that result in cell carcinogenesis.
Keywords: human papillomavirus, E6, Aurora A, gene regulation
Introduction
Cervical cancer is the second highest cause of female cancer-associated mortality worldwide, accounting for 288,000 mortalities yearly (1). In total, ~510,000 cases of cervical cancer are reported each year, with almost 80% of cases occurring in developing countries. Persistent infection with high-risk human papillomavirus (HPV) is regarded as an etiological origin of cervical carcinogenesis (2,3). HPV16 is the most common type of high-risk HPV, and accounts for >50% of all cervical cancers. HPV16 early protein E6 and E7 are the major oncoproteins that are crucial for host cell immortalization and transformation. In particular, E6 recruits the ubiquitin protein ligase E6-associated protein, and the resulting complex targets the p53 tumor suppressor protein for proteasome-mediated degradation (4,5). HPV16 E6 also interacts with several other cellular proteins, including activating transcription factor 3 (6), E6 binding protein (7), human discs large (8), interferon regulatory factor 3 (9), B-cell lymphoma 2-antagonist/killer 1 (10), E6-targeted protein 1 (11) and human telomerase reverse transcriptase (12). There is also a switch between mouse double minute 2 homolog (Mdm2) and HPV E6-mediated degradation of p53 in cervical cancer cells (13). HPV16 E6 regulates cell differentiation, adhesion, polarity, proliferation, apoptosis, gene transcription and chromosomal stability through these interactions. The interactions are not only important for cell carcinogenesis, but also for the survival of the virus within the host.
Aurora A is a centrosomal serine-threonine kinase that is responsible for proper mitotic progression. This kinase plays an essential role in coordinating mitotic events, including centrosome separation, bipolar spindle assembly, chromosome segregation and cytokinesis (14). The expression and kinase activity of Aurora A are regulated by the cell cycle, peaking when the cells reach M phase. The Aurora A protein is localized in the centrosomes of interphase cells and the spindle of mitotic cells (15). The centrosomes maintain genomic stability through the establishment of bipolar spindles during cell division, ensuring equal segregation of replicated chromosomes to the two daughter cells. The abnormal duplication and distribution of centrosomes in segregation leads to the aneuploidy observed in numerous cancer cell types. The expression of Aurora A is upregulated in several human tumors, including colon, breast, ovarian, gastric and pancreatic cancers, and hematological malignancies (16). Aurora A is also involved in the regulation of drug resistance to several chemotherapeutic agents. The overexpression of Aurora A may inhibit the cell death induced by Taxol (17), and knock-down of Aurora A increases the sensitivity of tumor cells to cisplatin toxicity (18). Aurora A abrogates p53 DNA binding and transactivation activity through the phosphorylation of serine 215 and 315, resulting in the ubiquitylation and proteasomal degradation of p53 via the Mdm2-mediated pathway (19,20). Overexpression of Aurora A correlates with an advanced clinical stage and shortened survival period (21). Although the deregulated expression and mechanism of the Aurora family involvement in carcinogenesis have been reported (22), the association has yet to be elucidated in virus-mediated tumorigenesis. In the present study, it was demonstrate that HPV16 E6 upregulates the expression of Aurora A.
Materials and methods
Reagents and antibodies
The E6 DNA fragment of HPV16 was obtained by polymerase chain reaction (PCR) from SiHa genomic DNA and ligated to a PCDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as previously described (23). The p3XFLAG-E6 expression vector was generated by PCR cloning of the HPV16 PCDNA3-E6 cDNAs, followed by HindIII and XbaI double digestion and insertion into the HindIII and XbaI sites of the pA3F vector (Sigma-Aldrich, St. Louis, MO, USA). Human Aurora A cDNA (a gift from Dr Bingyi Xiao, University of Pennsylvania, Philadelphia, PA, USA) was subcloned into the pcDNA3.1HA (Invitrogen; Thermo Fisher Scientific, Inc.) and pGEX-4T-2 vectors (GE Healthcare Life Science, Chalfont, UK) to produce hemagglutinin (HA)-tagged and glutathione S-transferase (GST)-tagged plasmids. The antibodies used in the present study were the mouse monoclonal anti-FLAG M2 (catalog no. F1804; Sigma-Aldrich), anti-HA (catalog no. H3663; Sigma-Aldrich), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-Aurora A (catalog no. 610939; BD Biosciences, San Jose, CA, USA) and anti-HPV16 E6 C1P5 (catalog no. sc460; BD Biosciences) antibodies (at 1:1,000 dilution for western blotting).
Cell culture and transfection
The SiHa, CaSki, C33A and HEK293 cell lines, purchased from teh American Type Culture Collection (Manassas, VA, USA) were grown in HyClone Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life Sciences) supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin, 50 µg/ml streptomycin and 2 mM L-glutamine. The cells were transfected by electroporation using a Bio-Rad Gene Pulser II electroporator (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemistry
Slides mounted with sections of paraffin-embedded, archival, cervical cancer tissue specimens were obtained from the Department of Pathology, the First Affiliated Hospital of China Medical University (Shenyang, Liaoning, China) between January 2012 and Dedember 2012. Slides were deparaffinized in xylene and rehydrated using a graded series of alcohol (70, 80, and 100% alcohol; 5 min each). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. Following antigen retrieval in 10 mM sodium citrate buffer (pH 6.0; Haoran Bio, Shanghai, China), samples were blocked with 10% normal rabbit/goat serum (Sangerbio, Shanghai, China) prior to incubation with primary anti-Aurora antibodies overnight at 4°C. The secondary polyclonal biotinylated anti-rabbit/goat IgG antibody (1:200 dilution; S-P kit) and streptavidin-peroxidase conjugate (S-P kit; Dako, Glostrup, Denmark) were added according to the manufacturer's instructions. The enzymatic reaction was developed in a freshly prepared solution of 3,3′-diaminobenzidine (DAB) using Dako Liquid DAB Color Solution (brown color; Dako). The sections were then counterstained with hemalum, dehydrated, washed with xylene and mounted.
Immunoprecipitation and western blotting
Transfected cells were harvested, washed with ice-cold phosphate-buffered saline (PBS; Solarbio, Beijing, China), and lysed in 0.5 ml ice-cold radioimmunoprecipitation (RIPA) buffer (Novland, Shanghai, China) containing 1% Nonidet P-40 (NP-40), 10 mM Tris (pH 7.5), 2 mM EDTA and 150 mM NaCl, supplemented with protease inhibitors, which consisted of 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin, 1 µg/ml pepstatin and 1 µg/ml leupeptin. Cell debris was removed by centrifugation at 21,000 × g (10 min; 4°C), and the supernatant was transferred to a fresh microcentrifuge tube. Lysates were then precleared by end-over-end rotation with normal mouse serum (Sigma-Aldrich) and 30 µl of a 1:1 mixture of protein A-protein G-conjugated Sepharose beads (1 h at 4°C; Yanhuibio, Shanghai, China). Beads were spun out, and the supernatant was transferred to a fresh microcentrifuge tube. In total, ~5% of the lysate was saved for input control. The protein of interest was captured by rotating the remaining lysate with 1 µg of anti-HA antibody (catalog no. H3663; Sigma-Aldrich) overnight at 4°C. Immune complexes were captured with 30 µl of a 1:1 mixture of protein A and protein G Sepharose beads, pelleted and washed five times with ice-cold RIPA buffer. For western blot assays, input lysates and immunoprecipitated complexes were boiled in Laemmli buffer (Haoran Bio), fractionated by SDS-PAGE and transferred to a 0.45 µm nitrocellulose membrane. The membranes were then probed with appropriate antibodies, followed by incubation with appropriate infrared-tagged secondary antibodies and viewed on an Odyssey imager (Li-Cor Biosciences, Lincoln, NE, USA).
Purification of GST fusion proteins
Escherichia coli BL21 (DE3) cells (Biovector, Beijing, China) were transformed with the plasmid constructs to obtain the GST and GST-E6 fusion protein. Single colonies were selected and grown overnight in 3 ml of Luria broth (Seebio, Shanghai, China) supplemented with 100 µg/ml ampicillin (Solarbio). In total, 1 ml of the overnight culture was used to inoculate a 500 ml culture. The larger culture was incubated until the optical density at 600 nm was ~0.6, at which point it was produced by adding 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 12 h at 30°C. The bacteria were pelleted, washed once with sodium chloride-Tris-ethylenediaminetetraacetic acid (EDTA) (STE) buffer (Shenxiangbio, Shanghai, China) consisting of 100 mM NaCl, 10 mM Tris and 1 mM EDTA (pH 7.5), resuspended in 3 ml NETN buffer (Helixgen, Guangzhou, China) consisting of 0.5% NP-40, 100 mM NaCl, 20 mM Tris and 1 mM EDTA (pH 8.0), supplemented with protease inhibitors (Haoran Bio), and incubated on ice for 15 min. A volume of 150 µl of 1 M dithiothreitol (DTT; Luanhuabio, Shanghai, China) and 1.8 ml of 10% Sarkosyl solution (Yantuobio, Shanghai, China) in STE buffer was added, and the suspension was sonicated (for 3 min on ice; Misonix Sonicator 4000; QSonica LLC, Newtown, CT, USA) to solubilize the proteins. The lysate was centrifuged (12,000 × g; 10 min; 4°C) to separate the insolubilized fraction. The clear supernatant was transferred to a fresh tube, to which 3 ml of 10% Triton X-100 in STE buffer and 200 µl of glutathione-Sepharose beads (Weijiabio, Guangzhou, China) were added. The tube was rotated overnight at 4°C, following which the purified protein bound to glutathione was collected by centrifugation (2 min; 600 × g; 4°C) and washed five times with NETN buffer supplemented with protease inhibitors. The level of purification was determined by SDS-PAGE, and purified proteins were stored at 4°C.
GST pull-down assays
In vitro translation of HA-Aurora A was performed using the T7-TNT Quick Coupled Transcription-Translation system (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. For in vitro binding experiments, GST fusion proteins were incubated with 35S-labeled in vitro-translated Aurora A protein in binding buffer (Yubobio, Shanghai, China) consisting of 1X PBS, 0.1% NP-40, 0.5 mM DTT and 10% glycerol, supplemented with protease inhibitors. The interating proteins were eluted in %X Laemmli loading buffer, boiled and separated on 12% SDS-PAGE. An autoradiography phosphorimager screen (Molecular Dynamics, San Diego, CA, USA) was used and scanning was performed by the Typhoon 9140 imaging system (Molecular Dynamics).
Reporter assay
In total, 1.2×107 cells were co-transfected with the pGL3-basic or pGL3-Aurora A reporter construct with combinations of various plasmids using a Bio-Rad Gene Pulser II electroporator. At 24 h post-transfection, the cells were harvested, washed in PBS and lysed in cell lysis buffer (BioVision, Milpitas, CA, USA). In total, 50 µl of cell lysate was used for the reporter assay, which was performed using an LMaxII384 luminometer (Molecular Devices, Sunnyvale, CA, USA). In total, 20% of the cell lysate was used for western blotting as aforementioned. The transferred proteins were detected with Odyssey infrared scanning technology (Li-Cor Biosciences, Inc.), using Alexa Fluor 680 and Alexa Fluor 800 (Molecular Probes; Thermo Fisher Scientific, Inc. All transfections were performed three times, and the results shown indicate the means of the data from three independent experiments.
Lentiviral small hairpin (sh)RNA vector constructs
For the lentivirus-mediated stable knockdown of HPV16 E6, the E6 shRNA sequence (sequence, 5′-GGACAGAGCCCATTACAATAT-3′; LC-Bio, Hangzhou, China) was inserted into the pGIPZ vector according to the manufacture's protocol (Open Biosystems; GE Dharmacon, Lafayette, CO, USA), resulting in the HPV16 E6 shRNA-expressing vector (sh-E6). In addition, a 21-mer oligonucleotide (sequence, 5′-TCTCGCTTGGGCGAGAGTAAG-3′) that had no significant homology to any known human messenger (m)RNA in the databases was cloned in the same vector and used as control shRNA (sh-C).
Virus production and transduction of CaSki cells
Lentivirus was produced by transient transfection of the aforementioned plasmids into HEK293T cells. A total of 2×106 HEK293T cells were seeded in 10-cm dishes containing DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic and cultured in a 5% CO2 incubator for 24 h prior to transfection. A total of 20 µg plasmid DNA was added to each dish for transfection, including 1.5 µg envelope plasmid pCMV-VSV-G (catalog no. 8454; Addgene, Inc., Cambridge, MA, USA), 3 µg packaging plasmid pRSV-REV (catalog no. 12251 Addgene, Inc.), 5 µg packaging plasmid pMDLg/Prre (catalog no. 12251; Addgene, Inc.) and 10.5 µg lentiviral vector plasmid. The precipitation was formed by adding the plasmids to a final volume of 438 µl H2O and 62 µl 2 M CaCl2, and mixing well. A total of 500 µl of 2X 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered saline (Yeasenbio, Shanghai, China) was then added and the solution was incubated at room temperature for 30 min. Chloroquine (Haoran Bio) was added to the 10-ml plated media 5 min prior to transfection at a final concentration of 25 µM. Subsequent to 12 h of chloroquine treatment, the medium was replaced with DMEM supplemented with 10% FBS, 10 mM HEPES and 10 mM sodium butyrate (Sangonbio, Shanghai, China). The medium was replaced again 10 h later by DMEM supplemented with 10% FBS and 10 mM HEPES. The conditioned medium was collected four times at 12 h intervals, filtered through 0.45 µm pore-size cellulose acetate filters, and stored on ice. The virus was concentrated by centrifugation at 70,000 × g for 2.5 h. The concentrated virus was resuspended in RPMI-1640 (Qcbio, Shanghai, China) then used to infect 106 cells in the presence of 20 µm/ml Polybrene (Haoran Bio). Following 72 h, puromycin was added to a final concentration of 2 µg/ml for the selection of transfected cells. GFP immunofluorescence was assessed using an Olympus IX71 microscope (Olympus, Tokyo, Japan) filtered with 560 nm excitation and 645 nm emission filters. The cells were grown to 80% confluency in the presence of 2 µg/ml puromycin prior to western blot analysis as aforementioned.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from cells was extracted using TRIzol reagent and cDNA was generated using a Superscript II Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.). The primers for RT-qPCR were as follows: Aurora A sense, 5′-GGAGAGCTTAAAATTGCAGATTTTG-3′ and antisense, 5′-GGCAAACACATACCAAGAGACCT-3′; HPV E6 sense, 5′-GACCCAGAAAGTTACCACAG-3′ and antisense, 5′-CACAACGGTTTGTTGTATTG-3′; and GAPDH sense, 5′-CTCCTCTGACTTCAACAGCG-3′ and antisense, 5′-GCCAAATTCGTTGTCATACCAG-3′. cDNA was amplified using 10 µl Master Mix from the DyNAmo SYBR green RT-qPCR kit (MJ Research; Bio-Rad Laboratories, Inc.), 1 mM of each primer, and 2 µl of the cDNA product in a 20 µl total volume. Thirty cycles of 1 min at 94°C, 30 sec at 55°C, and 40 sec at 72°C were followed by 10 min at 72°C in an MJ Research Opticon II thermocycler (Bio-Rad Laboratories, Inc.). A melting curve analysis was performed to verify the specificity of the amplified products. The values for the relative levels of change were calculated using the 2−ΔΔCq method (24), and each sample was tested in triplicates.
Results
HPV16 E6 combines with Aurora A
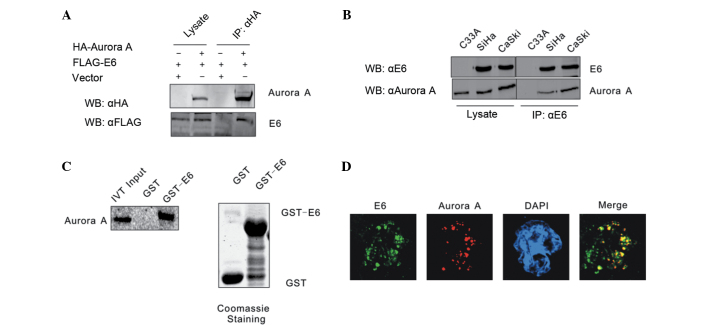
It has been shown that Aurora A accumulates and functions as an inhibitor of p53 in the majority of cancer cells. In order to determine the association between HPV16 E6 and Aurora A, these two molecules were first confirmed to form a complex by co-immunoprecipitation assays. HEK293 cells were co-transfected with expression constructs for Flag-tagged HPV16 E6 and HA-tagged Aurora A, with empty vectors acting as negative controls. Whole-cell extracts of the transfected HEK293 cells were precipitated with anti-HA antibody, and the precipitates were analyzed by western blot analysis with anti-Flag antibody. The transfected Flag-HPV16 E6 and HA-Aurora A were found to associate with each other, but not empty vectors, indicating a specific interaction between ectopically expressed HPV16 E6 and Aurora A (Fig. 1A). Whether HPV16 E6 combines with Aurora A in cervical carcinoma cell lines was then investigated. Endogenously expressed HPV16 E6 was immunoprecipitated from HPV16 positive cervical carcinoma CaSki and SiHa cells. The co-immunoprecipitation of Aurora A was monitored by the polyclonal antibody reactive to Aurora A. The HPV-negative cervical carcinoma C33A cell line was used as a negative control. The results revealed that HPV16 E6 formed a stable complex with Aurora A (Fig. 1B). An in vitro binding assay was also performed to determine whether HPV16 E6 directly interacts with Aurora A. The GST-E6 and GST expression constructs were bacterially expressed and incubated with in vitro translated 35S-labeled Aurora A, and the GST pull-down assay result showed that GST-E6 beads, but not GST alone, precipitated a significant amount of Aurora A protein with radioactivity. The result showed there is a direct association between HPV16 E6 and Aurora A (Fig. 1C). This interaction between HPV16 E6 and Aurora A was also shown by an immunostaining assay. Endogenous HPV16 E6 accumulated in the nucleus of CaSki cells co-localized with Aurora A (Fig. 1D).
Figure 1.
HPV16 E6 combines with Aurora A. (A) HEK293 cells were co-transfected with Flag-E6 and HA-Aurora A, balanced with an empty vector. The cell lysates were subjected to IP with a HA-specific antibody and protein expression was detected by WB. (B) Lysates from the HPV-negative cervical carcinoma C33A cell line and the two HPV16-positive SiHa and CaSki cell lines were subjected to IP with a HPV16 E6 specific antibody C1P5. The expression of the indicated proteins was detected by WB. (C) Either GST control or GST-E6 beads were incubated with Aurora A in vitro-translated 35S-radiolabeled protein. In addition, 5% of in vitro translated protein input was used as a comparison. Precipitated proteins were resolved by SDS-PAGE, exposed to phosphorimager screen and scanned by the Typhoon 9410 imaging system. Coomassie blue staining of SDS-PAGE-resolved purified GST and GST-E6 proteins was shown in the right panel. (D) Colocalization of endogenous HPV16 E6 and Aurora A in CaSki cells. HPV, human papillomavirus; IP, immunoprecipitation; HA, hemagglutinin; WB, western blotting; GST, glutathione S-transferase; SDS-PAGE sodium dodecyl sulfate-polyacrylamide gel electrophoresis; DAPI, 4′,6-diamidino-2-phenylindole; IVT, in vitro translation.
HPV16 E6 enhances Aurora A expression
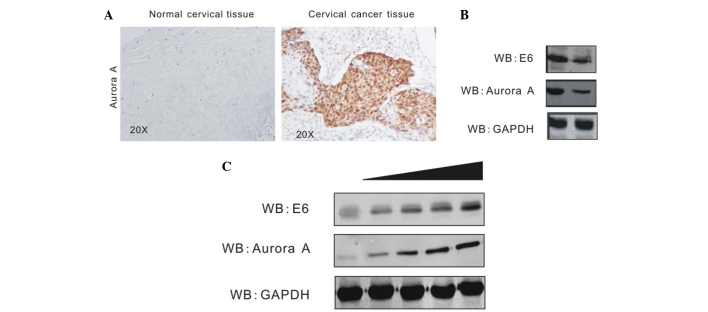
Aurora A has been shown to aberrantly accumulate in numerous types of cancer cells (25,26). In order to investigate Aurora A expression and its association with HPV in cervical cancer, the present study assessed the protein level of Aurora A in HPV-positive cervical cancer tissue and HPV-negative normal cervical tissue by immunohistochemistry assays. The result revealed that Aurora A is highly expressed in cervical cancer tissue, but barely expressed in normal cervical tissue (Fig. 2A). To determine that the elevated Aurora A level is due to HPV16 E6, stable CaSki cell lines carrying HPV16 E6 knockdown (Sh-E6) or control (Sh-Cr) were created by transduction of the cells with shRNA-containing lentivirus followed by selection of lentivirus-expressing cells using puromycin (23). The Aurora A level was determined by western blot analysis. The protein level of Aurora A in E6-knockdown cells was evidently decreased compared with the control cells (Fig. 2B). In addition, the present study confirmed the effect of HPV16 E6 on Aurora A expression by transient transfection. In total, 1.5×107 HEK293 cells were transiently transfected either with the empty vector or increasing amounts of Flag-E6. At 36 h post-transfection, the cells were harvested, lysed and individually subjected to western blot analysis with antibodies against Aurora A, HPV16 E6 and GAPDH. HPV16 E6 induces Aurora A expression in a dose-dependent manner (Fig. 2C).
Figure 2.
Aurora A expression is upregulated by HPV16 E6. (A) The protein levels of Aurora A were enhanced in cervical cancer tissue. Cervical cancer tissue specimens from 10 patients were analyzed for Aurora A expression by immunohistochemistry. A representative sample of cervical cancer tissue (right panel) and the adjacent normal tissue (left panel) are shown. (B) WB showing expression of Aurora A in the E6-knockdown CaSki cell line and the short hairpin RNA control cell line. The protein level of Aurora A was evidently decreased in the E6-knockdown cells compared with the control cells. (C) HEK293 cells were transfected with the PA3F vector or increasing amounts of PA3F E6. Cells were harvested at 36 h post-transfection, and Aurora A protein levels were assessed by WB. This showed that the Aurora A level was upregulated as the transfection of E6 increased. WB, western blot; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
HPV16 E6 affects Aurora A expression at transcription level
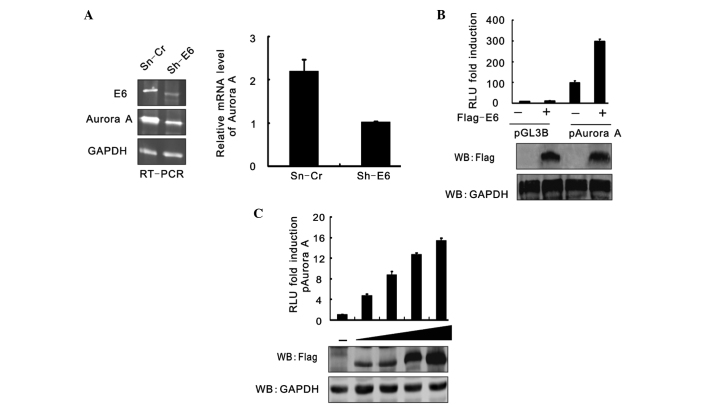
RT-PCR analysis was used to assess the effect of HPV16 E6 on Aurora A at the transcription level. The results showed that the Aurora A mRNA level was also significantly reduced when HPV16 E6 expression was inhibited (Fig. 3A). To confirm this result, the luciferase reporter gene driven by the Aurora A promoter was generated and assessed using a reporter assay. HEK293 cells were cotransfected with pGL3-basic or pGL3-Aurora A and either Flag-E6 or the empty vector, and the cells were harvested at 24 h post-transfection. The cell lysates were subjected to a luciferase reporter assay. The results were presented as the relative luciferase unit (RLU) compared with pGL3-basic and vector alone-cotransfected cells. Data is expressed as the means ± standard deviation of three independent experiments. The immunoblotting results of Flag-tagged E6 and GAPDH are shown in Fig. 3B. A reporter assay was performed of pGL3-Aurora A promoter cotransfection with an increasing amount (0, 5, 10, 15, 20 mg) of Flag-E6 (Fig. 3C). At 24 h post-transfection, the cells were harvested and subjected to a luciferase reporter assay. The results were presented as the RLU fold compared with the pGL3-Aurora A and empty vector alone-cotransfected cells. HPV16 E6 raised the RLU of pGL3-Aurora A in a dose-dependent manner.
Figure 3.
Aurora A transcript is enhanced in a E6-dependent manner. (A) RT-PCR showed that Aurora A was decreased at a trancription level in CaSki cells with E6 knockdown. (B) HEK293 cells cotransfected with pGL3-basic or pGL3-Aurora A and either pA3F-E6 or an empty vector were harvested at 24 h post-transfection. The cell lysate were subjected to luciferase reporter assay. The results are expressed as the fold RLU change compared with the RLU of cells transfected with pGL3-basic and vector alone. HPV E16 increased the pGL3-Aurora A level. Data is expressed as the mean ± standard deviation of three independent experiments. The WB results of Flag-tagged E6 and GAPDH are shown in the bottom panel. (C) The reporter assay of pGL3-Aurora A promoter cotransfection with an increasing amount (0, 5, 10, 15 and 20 mg) of Flag-tagged E6. At 24 h post-transfection, the cells were harvested and subjected to luciferase reporter assay. The results are expressed as the fold RLU change compared with cells transfected with pGL3-Aurora A and the empty vector alone. RT-PCR, reverse transcription-polymerase chain reaction; RLU, relative luciferase unit; WB, western blot; mRNA, messenger RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
In the present study, it was demonstrated that HPV16 E6 combines with Aurora A and enhances its expression. The results are consistent with other studies that have shown that Aurora A is frequently overexpressed in a variety of human tumors and cancer-derived cell lines (27–29).
Aurora A, B and C are highly conserved serine/threonine kinases that play vital and distinct roles in the process of chromosomal segregation, such as chromosome condensation, alignment, control of spindle checkpoints, chromosome segregation and cytokinesis, and these kinases have been identified as oncogenes (14,30). Specifically, Aurora A localizes to centrosomes and spindle microtubules proximal to centrosomes during mitosis, as this kinase is required for the assembly of the mitotic spindle, where Aurora A accumulates on centrosomes at the spindle poles during prophase until the cell reaches metaphase. The expression and activity of Aurora A kinase varies as the cell cycle progresses. The levels are low in the G1/S phase, upregulated during the G2/M phase and rapidly reduced subsequent to mitosis (31). Aberrant expression of Aurora A induces oncogenic transformation (32) and inhibition of Aurora kinase activity leads to the failure of multiple events in mitosis, such as incorrect separation of centriole pairs, misalignment of chromosomes on the metaphase plate and incomplete cytokinesis (33). Thus, it is reasonable to conclude that the Aurora kinases may be good molecular therapeutic targets for cancer.
In the present study, a novel mechanism of HPV oncogene E6 in carcinogenesis is shown. However, further elucidation is required. In the next step, we will further analyze the role of Aurora A in the cell cycle and apoptosis of cervical cancer cells.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant nos., 81171649 and 81572054).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Hausen Zur H. Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 5.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Mo P, Ren S, Yan C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J Biol Chem. 2010;285:13201–13210. doi: 10.1074/jbc.M109.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JJ, Reid CE, Band V, Androphy EJ. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Underbrink MP, Howie HL, Galloway DA, Bedard KM, Koop JI. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008;82:10408–10417. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Srinivasan S, Boyer SN, Wazer DE, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1 and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/MCB.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci USA. 2009;106:18780–18785. doi: 10.1073/pnas.0906357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci USA. 2001;98:1218–1223. doi: 10.1073/pnas.98.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff JR, Plowman GD. The Aurora/Ipl1p kinase family: Regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 1999;9:454–459. doi: 10.1016/S0962-8924(99)01658-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 16.Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 17.Giovinazzi S, Morozov VM, Summers MK, Reinhold WC, Ishov AM. USP7 and Daxx regulate mitosis progression and taxane sensitivity by affecting stability of Aurora-A kinase. Cell Death Differ. 2013;20:721–731. doi: 10.1038/cdd.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–2312. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279:52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 20.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 21.Chien CY, Tsai HT, Su LJ, Chuang HC, Shiu LY, Huang CC, Fang FM, Yu CC, Su HT, Chen CH. Aurora-A signaling is activated in advanced stage of squamous cell carcinoma of head and neck cancer and requires osteopontin to stimulate invasive behavior. Oncotarget. 2014;5:2243–2262. doi: 10.18632/oncotarget.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Meng XK, Wang Q, Wang Y, Shang H. The ING4 binding with p53 and induced p53 acetylation were attenuated by human papillomavirus 16 E6. PLoS One. 2013;8:e71453. doi: 10.1371/journal.pone.0071453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCq method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Li Z, Song Y, Wang R, Han L, Wang Q, Jiang K, Kang C, Zhang Q. AURKA induces EMT by regulating histone modification through Wnt/β-catenin and PI3K/Akt signaling pathway in gastric cancer. Oncotarget. 2016 Apr 21;21 doi: 10.18632/oncotarget.8888. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Sun Y, Chen X, Squires J, Nowroozizadeh B, Liang C, Huang J. p53 mutation directs AURKA overexpression via miR-25 and FBXW7 in prostatic small cell neuroendocrine carcinoma. Mol Cancer Res. 2015;13:584–591. doi: 10.1158/1541-7786.MCR-14-0277-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Ueda A, Kanamori H, Ideguchi H, Yang J, Kitajima S, Ishigatsubo Y. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J Biol Chem. 2002;277:10719–10726. doi: 10.1074/jbc.M108252200. [DOI] [PubMed] [Google Scholar]
- 28.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 29.Warner SL, Bearss DJ, Han H, Von Hoff DD. Targeting Aurora-2 kinase in cancer. Mol Cancer Ther. 2003;2:589–595. [PubMed] [Google Scholar]
- 30.Carmena M, Earnshaw WC. The cellular geography of Aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human Aurora/IpI1-related protein kinase, AIK3. J Biol Chem. 1999;274:7334–7340. doi: 10.1074/jbc.274.11.7334. [DOI] [PubMed] [Google Scholar]
- 32.Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, Okano Y. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to aurora of Drosophila and yeast Ipl1. J Biol Chem. 1997;272:13766–13771. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- 33.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-a kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]