Abstract
Human immunodeficiency virus (HIV)–associated neurocognitive disorder (HAND) is found in 30%–50% of individuals with HIV infection. To date, no HIV+ individual has been reported to have a positive amyloid PET scan. We report a 71-year-old HIV+ individual with HAND. Clinical and neuropsychologic evaluations confirmed a progressive mild dementia. A routine brain MRI was normal for age. [18F]Fluorodeoxyglucose–PET revealed mild hypermetabolism in bilateral basal ganglia and hypometabolism of bilateral parietal cortex including the posterior cingulate/precuneus. Resting state functional MRI revealed altered connectivity as found with individuals with mild AD. CSF examination revealed a low Aβ42/tau index but a low phospho-tau. An amyloid PET/CT with [18F]florbetaben revealed pronounced cortical radiotracer deposition. This case report suggests that progressive dementia in older HIV+ individuals may be due to HAND, AD, or both. HIV infection does not preclude CNS Aβ/amyloid deposition. Amyloid PET imaging may be of value in distinguishing HAND from AD pathologies.
Keywords: Human immunodeficiency virus, HIV, HIV-associated neurocognitive disorder, HAND, Dementia, Alzheimer's disease, Amyloid PET, Functional MRI, Biomarker
1. Introduction
More than 36.9 million individuals worldwide are infected with human immunodeficiency virus (HIV) in 2014 [1]. HIV infection has largely changed from a fatal illness to a chronic manageable condition since the introduction of combination antiretroviral treatment (cART) in 1996. HIV-infected adults older than 55 years comprise the fastest growing age group in the HIV+ population [2]. HIV-associated neurocognitive disorder (HAND) occurs in 30%–50% of HIV+ individuals treated with cART [3]. The etiology of HAND remains unclear but may be due to viral infection and inflammation accelerating CNS aging [4] and decreasing cognitive reserve. It is currently unknown whether chronic HIV infection and/or treatment are risk factors for Alzheimer's disease (AD). As an increasing fraction of the HIV+ populace advances into the geriatric age range, clinicians will be challenged to differentiate HAND from other dementias of aging, including AD.
Putative biomarkers of AD pathology, including cerebrospinal fluid (CSF) proteomics—Aβ/amyloid, tau, phospho-tau, and others, and amyloid PET neuroimaging are supportive of a clinical diagnosis of AD pathology [5] in HIV-uninfected individuals. Only one case of HAND and biomarker-supported AD has been reported—with abnormal [18F]fluorodeoxyglucose-PET and CSF proteomics [6]. However, a review of CSF AD biomarkers in subjects with HAND reveals low amyloid levels in both diagnoses, increased phospho-tau in AD, and inconsistent tau levels in HAND [7]. To date, no HIV+ individual has been reported to have a positive amyloid PET scan. In fact, Ances et al. [8] suggest that HAND is not associated with increased CNS fibrillar amyloid as detected by amyloid PET imaging because all five subjects examined were negative, but the oldest was 67 years old. Given the aging HIV+ populace, we report the sentinel case of a possible new emerging epidemic of HAND/AD.
2. Methods and results
2.1. Case study
The subject is a 71 year-old man with a 14-year history of HIV infection diagnosed after presenting with flu-like symptoms and a viral pneumonia. He was subsequently treated with cART. He and his wife noted mild short-term memory problems for 5 years with insidious onset and a more noticeable decline in the last 3 years. His symptoms manifested by comprehension difficulty, forgetting recent conversations, and difficulty with multitasking. Functionally, he stated that he took longer to complete projects and sometimes made mistakes. He could no longer work as an attorney. His spouse stated that he had trouble learning new skills such as using his cellular telephone. As calculations became more challenging, his spouse assumed household financial management. He currently shops independently but requires a list. He performs personal care and basic activities of daily living with minimal or no assistance. He describes his mood as fearful of his cognitive disorder. He remains socially active, exercises daily, and enjoys weekly religious services. He denies aggression, anxiety, agitation, hallucinations, delusions, paranoia, and suicidal ideation. He has a long-standing history of sleep problems. His spouse also reports frequent (2–3 times a week) episodes of violent movements and screaming while dreaming. The patient reports these events as acting out his dreams. His spouse also reports occasional jerks of his extremities during sleep. Review of clinical records indicates a stable HIV infection with consistent compliance with cART (most recently abacavir, lamivudine, darunavir, and ritonavir). He also takes atorvastatin for hypercholesterolemia. There is no history of CNS infection or injury, stroke, transient ischemic attack, or alcohol or drug abuse. He had one episode of loss of consciousness with a minor head injury secondary to syncope in 2002. His mother died at age 89 years with probable AD; his father died at age 71 years with parkinsonism and dementia.
His physical and neurologic examination was remarkable only for cognitive impairment. His Mini-Mental State Examination [9] score was 22/30, and Montreal Cognitive Assessment [10] score was 20/30. He recalled zero of five words on delayed recall. He had difficulty with repetition and gave concrete answers to similarities. He named only seven words beginning with F in 1 minute. His paragraph recall was 5/25 immediately and 2/25 after 30 minutes. He underwent two neuropsychological evaluations 27 months apart—both consistent with dementia—which revealed interval decline of working memory and verbal fluency (Table 1). His laboratory workup revealed a chronic subnormal CD4 T cell count (∼300–350/μL), depressed CD4/CD8 ratio (0.75), and nondetectable plasma HIV RNA (<20 copies/μL); all other blood tests were normal. A polysomnogram revealed no significant sleep disordered breathing with minimal periodic limb movements unassociated with arousals and sleep fragmentation.
Table 1.
Neuropsychologic evaluations demonstrate progressive cognitive decline
| Task | Evaluation 1 | Evaluation 2 (27 mo later) |
|---|---|---|
| Working memory and information processing speed | ||
| Working Memory Index | Average (50th percentile) | Low average (23rd percentile) |
| Arithmetic | High average (75th percentile) | Average (50th percentile) |
| Digit span | Average (5F/4B; 25th percentile) | Borderline (5F/2B; 9th percentile) |
| Processing speed index | Borderline (8th percentile) | Impaired (5th percentile) |
| Digit symbol coding | Impaired (5th percentile) | Impaired (5th percentile) |
| Symbol search | Low average (16th percentile) | Borderline (9th percentile) |
| Executive functioning | ||
| Rey complex figure copy | Impaired: poor planning and organization; inaccurate | Impaired: poor planning and organization; inaccurate |
| WAIS-IV picture completion | Borderline (9th percentile) | Average (50th percentile) |
| WAIS-III picture arrangement | Borderline (9th percentile) | Low average (16th percentile) |
| Language | ||
| Phonemic verbal fluency (FAS) | Superior (Σ = 60, 91st percentile) | Low average (Σ = 29, 13th percentile) |
| Semantic verbal fluency (animals) | Low average (Σ = 18, 23rd percentile) | Impaired (Σ = 11, 1st percentile) |
| Boston naming test | Impaired (47/60 correct) | Impaired (40/60 correct) |
| Repeatable battery for the assessment of neurocognitive status | ||
| Total score | Not tested | Impaired (1st percentile) |
| Attention | Not tested | Impaired (1st percentile) |
| Immediate memory | Not tested | Impaired (<1st percentile) |
| Visuospatial/constructional | Not tested | Low average (14th percentile) |
| Language | Not tested | Impaired (<1st percentile) |
| Delayed memory | Not tested | Low average (14th percentile) |
| Fine motor speed and coordination | ||
| Grooved pegboard dominant (R) | Impaired (Σ = 121 s, 2nd percentile) | Impaired (Σ = 121 s, 2nd percentile) |
| Grooved pegboard nondominant | Impaired (Σ = 133 s, 3rd percentile) | Impaired (Σ = 121 s, 2nd percentile) |
WAIS, Wechsler Adult Intelligence Scale.
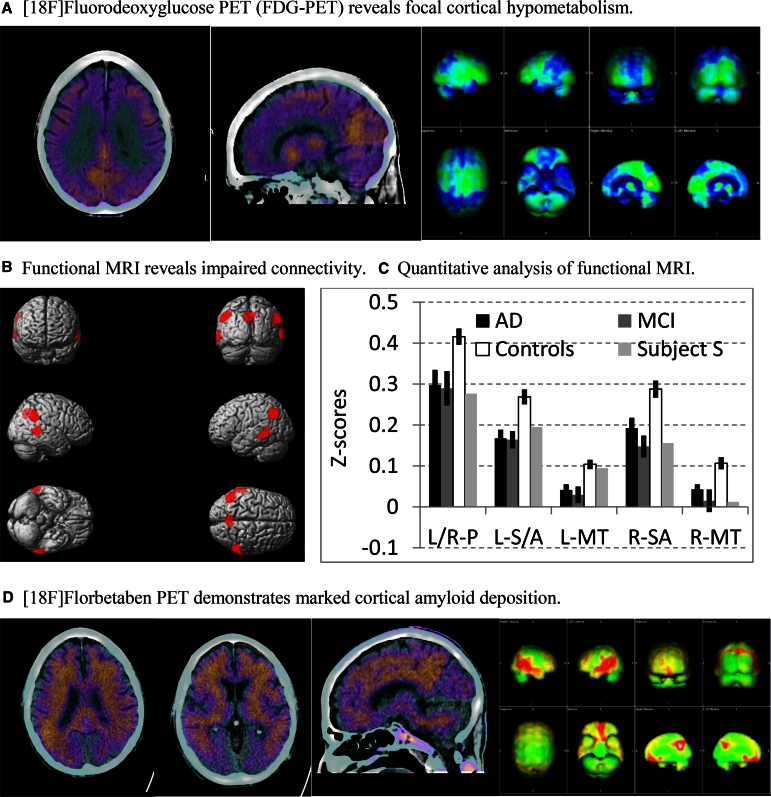
A routine brain MRI revealed atrophy and white matter changes consistent with age. [18F]fluorodeoxyglucose-PET revealed mild hypermetabolism in bilateral basal ganglia (consistent with HAND [11]) and marked hypometabolism of parietal cortex including the posterior cingulate/precuneus (consistent with AD; Fig. 1A). Likewise, resting state functional MRI (fMRI) revealed altered connectivity as found in individuals with MCI/AD using the posterior cingulate cortex as a seed region (Fig. 1B and C). CSF examination revealed 0 cells, normal glucose (59 mg/dL), elevated protein (118 mg/dL), a low Aβ42/tau index (consistent with AD), and a low phospho-tau (indeterminate for AD). More specifically, analysis of CSF proteomics revealed Aβ42 237 pg/mL, total tau 285 pg/mL, phospho-tau 49 pg/mL, and an amyloid/tau index of 0.41 (Athena Diagnostics, Worcester, MA, USA). An amyloid PET/CT with [18F]florbetaben (NEURACEQ; Piramal Imaging, Matran, Switzerland) revealed pronounced radiotracer deposition in the frontal, temporal, and parietal lobes bilaterally including the posterior cingulate/precuneus consistent with AD (Fig. 1D). His APOE genotype was not determined. He was prescribed a cholinesterase inhibitor for dementia due to AD. Enrollment in treatment trials of AD was precluded by HIV infection.
Fig. 1.
(A) The two fused FDG-PET/CT images on the left demonstrate reduced activity in the bilateral parietal cortex and posterior cingulate/precuneus. The panel on the right shows projection maps (Syngo.via software, Siemens Medical Solutions, PA, USA) with areas of reduced activity in the temporoparietal regions and precuneus bilaterally. A voxel-based analysis was conducted with comparison to a normal age-matched database. Regions at least 3 SDs below the normal reference value were considered abnormal and displayed in blue. Specifically, these brain regions (with corresponding SDs below the normal reference value) were L parietal (6.5), R parietal (4.5), L frontal (3.9), and L temporal (3.1). (B) Functional MRI (fMRI). Six minutes of resting state fMRI data were collected from 22 HIV-uninfected individuals with mild cognitive impairment (mild cognitive impairment [MCI]; n = 8) or mild AD (n = 14; age 69.4 ± 7.5, 13 males) and 42 age-matched cognitively normal controls (age 67.4 ± 5.1, 14 males). Standard fMRI preprocessing procedures were followed [12]. After regressing out nuisance factors including head movements, global signal variations, and signal in white matter and CSF, we used the posterior cingulate cortex (PPC; left and right collapsed together) as the seed region, and obtained the correlation coefficients between PPC and other brain regions. The correlation coefficients were then z-transformed and entered into second-level whole brain analysis. The contrast of controls >MCI/AD revealed decreased connectivity between PPC and left and right precuneus, left and right supramarginal/angular gyrus, and left and right middle temporal region (threshold, P < .005 uncorrected, at least 200 contiguous voxels). (C) The mean Z-scores for the connectivity between PPC and the regions of interest (ROIs) identified in (B) from HIV-uninfected individuals with mild AD or MCI, controls, and the subject (Subject S). The profile of resting state connectivity in this subject is similar to HIV-uninfected individuals with MCI or mild AD. Error bars represent SEM. L/R-P, left/right precuneus; L-SA, left supramarginal/angular gyrus; R-SA, right supramarginal/angular gyrus; L-MT, left middle temporal region; R-MT, right middle temporal region. (D) The three images on the left show areas of at least moderate amyloid deposition in the frontal, parietal, temporal regions, and posterior cingulate/precuneus. The panel on the right shows projection maps (Syngo.via software; Siemens Medical Solutions, USA) with areas of increased amyloid deposition (shown in red). A visual and ratio analysis was conducted using the cerebellar cortex as reference. The SUVr is the ratio of the individual standardized uptake value (SUV) for each of the brain regions compared with the SUV of the cerebellar cortex. Specifically, the SUVr values were as follows: posterior cingulate 1.99, parietal 1.95, temporal 1.89, frontal 1.81, anterior cingulate 1.6, occipital 1.53, average 1.8. FDG, [18F]fluorodeoxyglucose; SD, standard deviation; AD, Alzheimer's disease; SEM, standard error of the mean.
3. Discussion
This case report suggests that progressive dementia in older HIV+ individuals may be due to HAND, AD, or both. We propose that the individual described here may have a mixed dementia—HAND and probable AD, but dementia due to either diagnosis alone cannot be excluded. This case suggests that CNS HIV infection does not preclude Aβ/amyloid deposition. In fact, chronic HIV infection may be a risk factor for AD because of neuroinflammation, accelerated CNS aging, and reduced cognitive reserve—thus constituting a “double-hit.” In support of this notion, Cysique et al. [13] report that CSF biomarker profiles of HIV+ individuals suggest a 10× higher risk for AD compared with age-matched uninfected subjects. Although CNS HIV infection may be the primary etiology of HAND, a role for cART is also possible because of adverse effects of chronic treatment. Consistent with this notion, Caniglia et al. [14] report that cART regimens with a high CNS penetration effectiveness score increase the risk of HIV dementia. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons in vitro [15]. In contrast, Lan et al. [16] suggest that cART inhibits Aβ clearance in macrophages and Aβ production in neurons, but these effects do not significantly alter CNS Aβ accumulation in a mouse model. Clearly, the potential interactions of HAND and AD pathologies leading to dementia, including a possible role of cART, require further research.
Cognitive decline in HAND may be mediated in part by CNS Aβ/amyloid accumulation [17]. Although pathologic studies of brain show that HIV increases intracellular and possibly extracellular Aβ42, Ortega and Ances [18] suggest that HIV+ individuals are not at increased risk for AD. Amyloid PET neuroimaging will be useful to distinguish putative neuropathologies of HAND—either HIV associated, amyloid associated, or both. However, to date, only five individuals with HAND have undergone amyloid PET imaging—all were negative, but the oldest subject was 67 years old [8]. Longitudinal studies of older HIV-infected versus uninfected cohorts are now needed to further define the potential interactions of HIV with AD–clinically, pathologically, and effects on prognostic, diagnostic, and theragnostic biomarkers, including fMRI.
Older individuals with HAND may be included in the target population of a new study designed to determine the clinical utility of amyloid imaging in subjects with dementia of uncertain etiology (IDEAS, or Imaging Dementia-Evidence for Amyloid Scanning, clinicaltrials.gov #NCT02420756). Furthermore, the optimal cART regimen for HAND remains unclear; some investigators propose a regimen with a high CNS penetration effectiveness to improve efficacy. The safety and efficacy of Food and Drug Administration-approved medications for dementia due to AD in subjects with coexisting HAND is unclear because HIV infection is invariably exclusionary in MCI and AD trials. Antiamyloid and other therapies for MCI and AD now under development may also be effective for older HIV+ individuals. This sentinel case raises new questions regarding diagnosis, pathogenesis, and treatment of dementia in older HIV+ subjects that require further studies.
Research in context.
-
1.
Systematic review: Since the introduction of combination antiretroviral therapy, human immunodeficiency virus (HIV) has become a manageable chronic disorder. As a result, an increasing fraction of the HIV+ populace is reaching the geriatric age range and thus at risk for Alzheimer's disease (AD). About 30%–50% of HIV+ individuals will develop HIV-associated neurocognitive disorders (HANDs). Amyloid PET imaging has not yet been systematically examined in older individuals with HAND.
-
2.
Interpretation: We report the first HIV+ individual with a positive amyloid positron emission tomography (PET) scan. This case report suggests that chronic HIV infection does not preclude central nervous system (CNS) amyloid deposition. Individuals with HIV may develop cognitive decline because of HAND, AD, or both.
-
3.
Future directions: Amyloid PET imaging may be useful to distinguish HIV-associated pathology and/or CNS amyloid deposition in demented individuals. Functional magnetic resonance imaging (MRI) may also be a useful biomarker. Antiamyloid treatments now under development for mild cognitive impairment, and AD may be effective for some individuals with HAND.
Acknowledgments
The authors express their gratitude to the patient and his spouse and to Carolyn Ward, Victoria Starbuck, PhD, and Susan DeSanti, PhD. This study was supported in part by grant 20130805 from the Alzheimer's Drug Discovery Foundation, New York, NY.
References
- 1.Fact Sheet 2015. UNAIDS; Geneva, Switzerland: 2015. Available at: www.unaids.org. Accessed April 15, 2016. [Google Scholar]
- 2.Hall H.I., Song R., Rhodes P., Prejean J., An Q., Lee L.M., HIV Incidence Surveillance Group Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S., CHARTER Group. HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocchetti I., Bachis A., Esposito G., Turner R.S., Taraballi F., Tasciotti E. Human immunodeficiency virus-associated dementia: a link between accumulation of viral proteins and neuronal degeneration. Curr Trends Neurol. 2014;8:71–85. [PMC free article] [PubMed] [Google Scholar]
- 5.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mäkitalo S., Mellgren Å., Borgh E., Kilander L., Skillbäck T., Zetterberg H. The cerebrospinal fluid biomarker profile in an HIV-infected subject with Alzheimer's disease. AIDS Res Ther. 2015;12:23. doi: 10.1186/s12981-015-0063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson J., Gisslen M., Zetterberg H., Fuchs D., Shacklett B.L., Hagberg L. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One. 2014;9:e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ances B.M., Benzinger T.L., Christensen J.J., Thomas J., Venkat R., Teshome M. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–77. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11.Sathekge M., McFarren A., Dadachova E. Role of nuclear medicine in neuroHIV: PET, SPECT, and beyond. Nucl Med Commun. 2014;35:792–796. doi: 10.1097/MNM.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C., Wang C., Leclair M., Young M., Jiang X. Reduced neural specificity in middle-aged HIV+ women in the absence of behavioral deficits. Neuroimage Clin. 2014;8:667–675. doi: 10.1016/j.nicl.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cysique L.A., Hewitt T., Croitoru-Lamoury J., Taddei K., Martins R.N., Chew C.S. APOE ε4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals—a cross-sectional observational study. BMC Neurol. 2015;15:51. doi: 10.1186/s12883-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caniglia E.C., Cain L.E., Justice A., Tate J., Logan R., Sabin C., HIV-CAUSAL Collaboration Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83:134–141. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giunta B., Ehrhart J., Obregon D.F., Lam L., Le L., Jin J. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain. 2011;4:23. doi: 10.1186/1756-6606-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan X., Kiyota T., Hanamsagar R., Huang Y., Andrews S., Peng H. The effect of HIV protease inhibitors on amyloid-β peptide degradation and synthesis in human cells and Alzheimer's disease animal model. J Neuroimmune Pharmacol. 2012;7:412–423. doi: 10.1007/s11481-011-9304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.András I.E., Toborek M. Amyloid beta accumulation in HIV-1-infected brain: the role of the blood brain barrier. IUBMB Life. 2013;65:43–49. doi: 10.1002/iub.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega M., Ances B.M. Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol. 2014;9:483–491. doi: 10.1007/s11481-014-9546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]