Abstract
The aim of the present study was to investigate the cardioprotective effect of tanshinone IIA and the underlying molecular mechanisms. An in vitro model of oxidative stress injury was established in cardiac H9c2 cells, and the effects of tanshinone IIa were investigated using cell viability, reverse transcription-quantitative polymerase chain reaction and western blotting assays. The results demonstrated that tanshinone IIA protects H9c2 cells from H2O2-induced cell death in a concentration-dependent manner, via a mechanism involving microRNA-133 (miR-133), and that treatment with TIIA alone exerted no cytotoxic effects on H9c2. In order to further elucidate the mechanisms underlying the actions of TIIA, reverse transcription-quantitative polymease chain reaction and western blot analysis were performed. Reductions in miR-133 expression levels induced by increasing concentrations of H2O2 were reversed by treatment with tanshinone IIA. In addition, the inhibition of miR-133 by transfection with an miR-133 inhibitor abolished the cardioprotective effects of tanshinone IIA against H2O2-induced cell death. Furthermore, western blot analysis demonstrated that tanshinone IIA activated Akt kinase via the phosphorylation of serine 473. Inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by pretreatment with the PI3K specific inhibitors wortmannin and LY294002 also eliminated the cardioprotective effects of tanshinone IIA against H2O2-induced cell death. Western blot analysis demonstrated that H2O2-induced reductions in B cell lymphoma 2 (Bcl-2) expression levels were reversed by tanshinone IIA. In addition, the effect of tanshinone IIA on Bcl-2 protein expression level in an oxidative environment was suppressed by a PI3K inhibitor, wortmannin, indicating that tanshinone IIA exerts cardioprotective effects against H2O2-induced cell death via the activation of the PI3K/Akt signal transduction pathway and the consequent upregulation of Bcl-2. In conclusion, the present study demonstrates that TIIA is able to protcet H9c2 cells from oxidative stress-induced cell death through signalling pathways involving miR-133 and Akt, and that tanshinone IIA is a promising natural cardioprotective agent.
Keywords: tanshinone IIA, miR-133, Akt kinase, B cell lymphoma-2, cardioprotective effect
Introduction
Danshen (root of Salviae miltiorrhizae) is widely prescribed in traditional Chinese medicine (1,2), and tanshinone IIA (C19H18O3) may be obtained from it by extraction (3,4). It has been demonstrated that tanshinone IIA exerts a variety of biological activities in the cardiovascular system, serving as an antioxidant and anticoagulant, and possesses anti-atherosclerotic, anti-apoptotic and anti-hypertrophic properties (5–9). Accumulating evidence suggests that tanshinone IIA reduces the area of ischemic infarction and improves cardiac function (7,10). Although the cardioprotective effects of tanshinone IIA have been investigated for numerous years, the underlying molecular mechanisms remain elusive.
MicroRNAs (miRNAs) are small endogenous ~22 nucleotide noncoding RNAs. They regulate post-transcriptional gene expression by complementing the 3′-untranslated regions of their target mRNAs, resulting in mRNA degradation or the inhibition of translation (11,12). Due to their involvement in the regulation of gene expression, miRNAs serve an important role in cardiac function, including the conductance of electrical signals, heart muscle contraction, development and morphogenesis; miRNAs are also involved in the proliferation and apoptosis of cardiomyocytes, cardiac hypertrophy and heart failure (13–15). In particular, a number of studies suggest that miRNA-133 (miR-133) serves an important role in the cardiovascular system (15–17). In the present study, the role of miR-133 in the myocardial protective effect of tanshinone IIA against hydrogen peroxide (H2O2)-induced induce cell death was investigated.
Akt is a vital regulator of cell survival that antagonizes apoptosis; when activated, Akt phosphorylates its downstream targets, contributing towards its inhibitory effect on the apoptosis of cardiomyocytes induced by multiple stimuli, including H2O2 (18,19). Numerous putative downstream targets have been identified that may contribute to the anti-apoptotic effects of Akt, including the transcriptional nuclear factor (NF)-κB and B-cell lymphoma-2 (Bcl-2) (18). In the current study, the role of the phosphatidylinositol 3-kinase (PI3K)/Akt/NF-κB/Bcl-2 axis in the myocardial protection effect of tanshinone IIA against oxidative stress-induced cell death was investigated.
Materials and methods
Materials
Tanshinone IIA was purchased from Dasherb Corp. (Shengyang, China). All the chemicals of reagent grade were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-Ser473 phospho-Akt (cat. no. 9271; 1:1,000) and rabbit polyclonal anti-Akt antibodies (cat. no. 9272; 1:1,000) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal anti-Bcl-2 (cat. no. sc-492; 1:2,000) and rabbit polyclonal anti-β-actin antibodies (cat. no. sc-7210; 1:2,000), and goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies (cat no. sc-2030; 1:10,000) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture and treatment protocol
Cardiac H9c2 cells are cloned heart muscle cells derived from embryonic rat hearts that retain a number of cardiomyocyte phenotypes (20). The cells used in the present study were derived from a CRL-1446 cell culture obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (both Gibco-BRL; Thermo Fisher Scientific, Inc.,Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich). Cells were placed in a 95% humidified incubator containing 95% air and 5% CO2 at 37°C with replenishment of the medium every 3 days. Prior to the experiments, cells were starved of serum for 24 h in DMEM supplemented with 1% fetal bovine serum. Next, the starved cells were treated with H2O2 (50, 100, 200, 400 or 800 µM) and tanshinone IIA (0.01, 0.1, 0.3, 1, 3 or 10 µM) alone, or in combination for 24 h at 37°C. For transfection experiments, cells were transfected with 50 nM miR-133 mimic or miR-133 inhibitor (Guangzhou RiboBio Co., Ltd., Guangzhou, China); after 8 h, transfected cells were treated for 24 h at 37°C with various combinations of H2O2 and tanshinone IIA. For inhibitor experiments, cells were pre-incubated with a selective PI3K inhibitor (100 nM wortmannin or 10 nM LY294002; both Beyotime Institute of Biotechnology, Haimen, China) for 30 min and then treated with H2O2 and/or tanshinone IIA as described above.
Cell viability assay
Cell viability was determined using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, cells were cultured in 96-well plates and after 24 h, CCK-8 reagent was added to each well according to the manufacturer's protocol. After a further 2 h of incubation, cell viability was determined by measuring the absorbance at 450 nm using a VICTOR X multi-label reader (PerkinElmer, Inc., Waltham, MA, USA). Data were presented as a percentage of the control value. The percentage cell viability was calculated as: Adrug / Acontrol × 100.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA samples were extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.) from cultured cells. miR-133 expression levels were quantified using the Bulge-Loop miRNA wRT-PCR Primer Set (Guangzhou RiboBio Co., Ltd.) in conjunction with RT-qPCR using SYBR Green I dye (Beijing TransGen Biotech Co., Ltd., Beijing, China). U6 (included in the Bulge-Loop miRNA qRT-PCR Primer Set) was used as an internal control. The relative expression of miR-133 was calculated and normalized to U6 using the comparative Cq method. Relative expression intensity values were calculated as 2−ΔΔCq (16). The RT-qPCR was performed using the SYBR green method with an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.).
Cell transfection with miR-133 mimic or miR-133 inhibitor
Transfection was performed using Effectene Transfection Reagent, according to the protocol recommended by the manufacturer (Qiagen GmbH, Hilden, Germany). In brief, cells were seeded into 96-well plates 1 day prior to transfection. Next, the cells were transfected with an miR-133 mimic or an miR-133 inhibitor at a final concentration of 50 nM using Effectene Transfection Reagent, in accordance with the manufacturer's instructions.
Western blot analysis
Following treatment, H9c2 cells were harvested and lysed in radioimmunoprecipitation assay buffer (Applygen Technologies, Inc., Beijing, China). The whole cell lysates were then resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a 0.4 µm-polyvinylidene difluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). After blocking in 5% non-fat milk for 2 h at room temperature, the PVDF membranes were probed with primary antibody overnight. Following a 30-min wash with Tris-buffered saline containing 0.1% Tween-20 (TBST), the membranes were incubated with HRP-conjugated secondary antibody (1:10,000) for 1 h at room temperature. The membranes were then washed with TBST for 30 min and visualized using an Enhanced Chemiluminescence detection kit (Merck Millipore, Darmstadt, Germany). β-actin served used as an internal control.
Statistical analysis
Data were analyzed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA) and presented as the mean ± standard deviation. Numeric variables were compared using one-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of tanshinone IIA on H2O2 cytotoxity in H9c2 cells
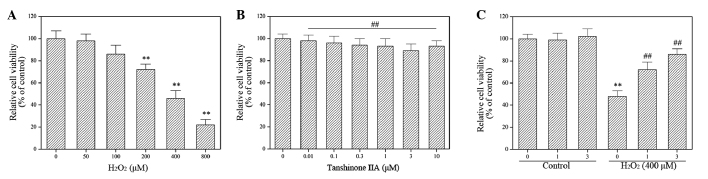
The molecular mechanisms underlying the effect of tanshinone IIA on H2O2 cytotoxicity in H9c2 cells were investigated using an H9c2 cell line. The results demonstrated that H2O2 induced H9c2 cell death in a concentration-dependent manner (Fig. 1A), while tanshinone IIA displayed no cytotoxic effect on H9c2 cells at any concentration studied (Fig. 1B). In addition, the exposure of H9c2 cells to tanshinone IIA significantly suppressed the cytotoxic effect of H2O2 in dose-dependent manner (P<0.05; Fig. 1C), confirming the previously reported cardioprotective effects of tanshinone IIA (21–24).
Figure 1.
Effect of H2O2 and/or tanshinone IIA on H9c2 cell viability. (A and B) Cells were incubated with increasing concentrations of H2O2 (0–800 µM) or tanshinone IIA (0–10 µM) for 24 h. Cell viability was determined using a Cell Counting Kit-8 (CCK-8) assay. (A) H2O2 decreased H9c2 cell viability in dose-dependent manner. **P<0.05 vs. control. (B) Tanshinone IIA demonstrated no cytotoxic effect on H9c2 cells. ##P>0.05 vs. control. (C) Tanshinone IIA protected H9c2 cells from oxidative stress-induced death. Cells were treated with different concentration of tanshinone IIA in the presence or absence of 400 µM H2O2 for 24 h. Cell viability was measured using a CCK-8 assay. **P<0.05 vs. control; ##P<0.05 vs. H2O2 alone. Data are presented as the mean ± standard deviation of three experiments.
Involvement of miR-133 in the mechanism of action of tanshinone IIA
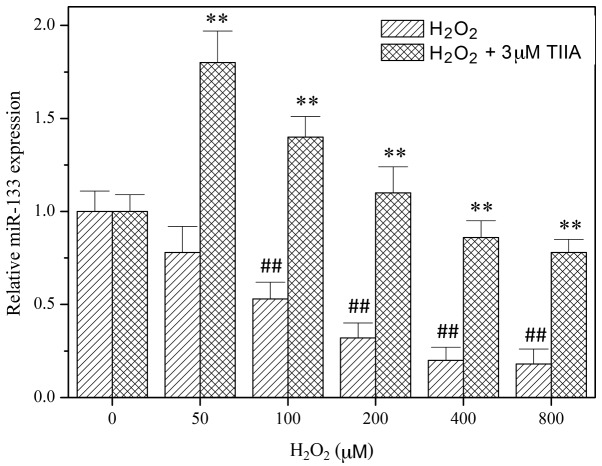
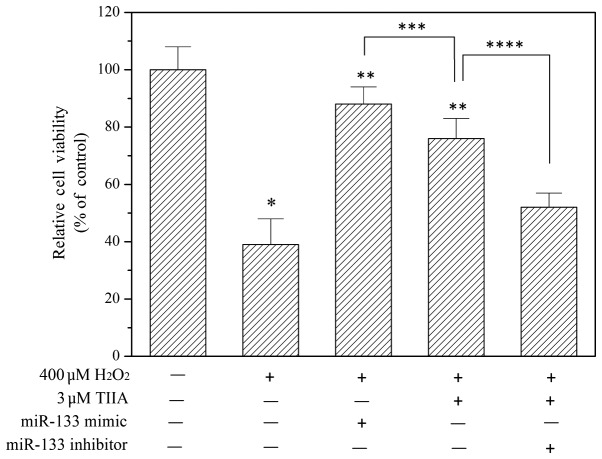
In order to study the role of miR-133 in the function of tanshinone IIA, miR-133 expression levels in H9c2 cells were determined using RT-qPCR following treatment with tanshinone IIA and/or H2O2 (Fig. 2). The results demonstrated that H2O2 decreases the expression of miR-133 in a dose-dependent manner. However, the downregulation of miR-133 by H2O2 was reversed by treatment with tanshinone IIA (Fig. 2), indicating that miR-133 is involved in the myocardial protective effect of tanshinone IIA. To verify this, an miR-133 mimic and miR-133 inhibitor were transfected into H9c2 cells to potentiate and suppress miR-133, respectively. As presented in Fig. 3, the miR-133 mimic alleviated oxidative injury in H9c2 cells. Furthermore, the myocardial protective effect of tanshinone IIA was inhibited by the miR-133 inhibitor (Fig. 3).
Figure 2.
Fold change of miR-133 expression in H2O2- and/or TIIA-treated H9c2 cells. Cells were incubated with increasing concentrations of H2O2 in the absence or presence of 3 µM TIIA for 24 h. miR-133 expression levels were relatively quantified using reverse transcription-quantitative polymerase chain reaction. H2O2 concentration-dependently decreased miR-133 expression, while TIIA reversed this decline. ##P<0.05 vs. control (0 µM); **P<0.05 vs. H2O2. Data are presented as the mean ± standard deviation of three experiments. TIIA, tanshinone IIA; miR-133, microRNA-133.
Figure 3.
Role of miR-133 in the protective effect of tanshinone IIA against H2O2-induced H9c2 cell death. Cells with or without transfection with miR-133 mimic or inhibitor were incubated with 400 µM H2O2 in the presence or absence of tanshinone IIA (TIIA) for 24 h. Cell viability was determined using a Cell Counting Kit-8 assay. miR-133 mimic transfection protected against H2O2-induced cell death and miR-133 inhibitor transfection suppressed the protective effect of TIIA against H2O2-stimulated cell death. Data are presented as the mean ± standard deviation of three experiments. *P<0.05 vs. control; **P<0.05 vs. H2O2 only; ***P>0.05; ****P<0.05.
Role of Akt activation in the mechanism of action of tanshinone IIA
The results of western blot analysis demonstrated that treatment with tanshinone IIA promoted Akt phosphorylation at the amino acid serine 473 in a concentration-dependent manner (Fig. 4A), indicating that the PI3K/Akt signaling pathway was activated. Blocking the signaling pathway using the PI3K-specific inhibitors wortmannin and LY294002 eliminated the ability of tanshinone IIA to protect H9c2 cells from oxidative injury (Fig. 4B).
Figure 4.
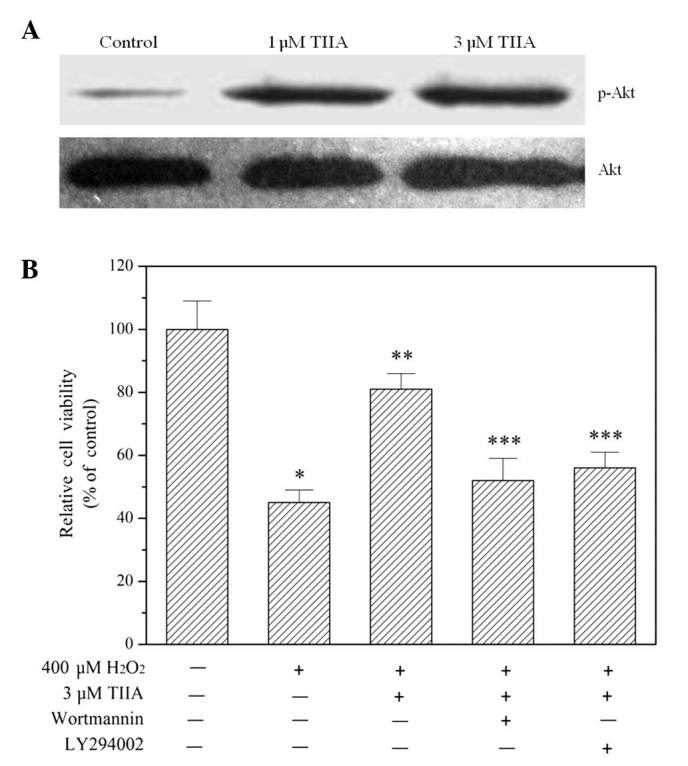
Effect of TIIA on Akt phosphorylation, and of PI3K inhibition on the protective effects of TIIA against H2O2-induced cell death. (A) Cells were incubated with various concentrations of TIIA for 24 h, then harvested and lysed. Cell lysates were analyzed using a western blot assay to examine Akt phosphorylation. TIIA increased Akt phosphorylation at serine 473. (B) Effects of PI3K inhibitors wortmannin and LY294002 on the protective effects of TIIA against H2O2-stimulated cell death. H9c2 cells were pre-incubated with wortmannin (100 nM) or LY294002 (10 nM) for 30 min and then treated with 400 µM H2O2 in the presence or absence of 3 µM TIIA for 24 h. Wortmannin and LY294002 reversed the increase in cell viability induced by TIIA in H2O2-stimulated H9c2 cells. Data presented are the mean ± standard deviation of three experiments. *P<0.05 vs. control; **P<0.05 vs. H2O2 along; ***P<0.05 vs. H2O2 + TIIA. TIIA, tanshinone IIA; PI3K, phosphoinositide 3-kinase.
Role of Bcl-2 expression in the mechanism of action of tanshinone IIA
Expression of Bcl-2 is regulated by the PI3K/Akt/NF-κB signaling pathway (25). Since tanshinone IIA was observed to activate PI3K/Akt signaling in H9c2 cells (Fig. 4A) and protect H9c2 cells from oxidative stress-induced death (Fig. 4B), activation of the PI3K/Akt signaling pathway by tanshinone IIA appears to be instrumental to the survival of H9c2 cells. This was further investigated by evaluating the expression of Bcl-2. Western blotting results demonstrated that H2O2 decreased the expression of Bcl-2 in H9c2 cells in a time-dependent manner, whereas tanshinone IIA increased Bcl-2 expression in H9c2 cells (Fig. 5A and B). Furthermore, the results demonstrated that the downregulation of Bcl-2 expression by H2O2 in H9c2 cells is reversed by treatment with tanshinone IIA (Fig. 5C). However, this reversion process is inhibited by the PI3K-specific inhibitor wortmannin (Fig. 5C), indicating that tanshinone IIA upregulates Bcl-2 expression by activating the PI3K/Akt signaling pathway.
Figure 5.
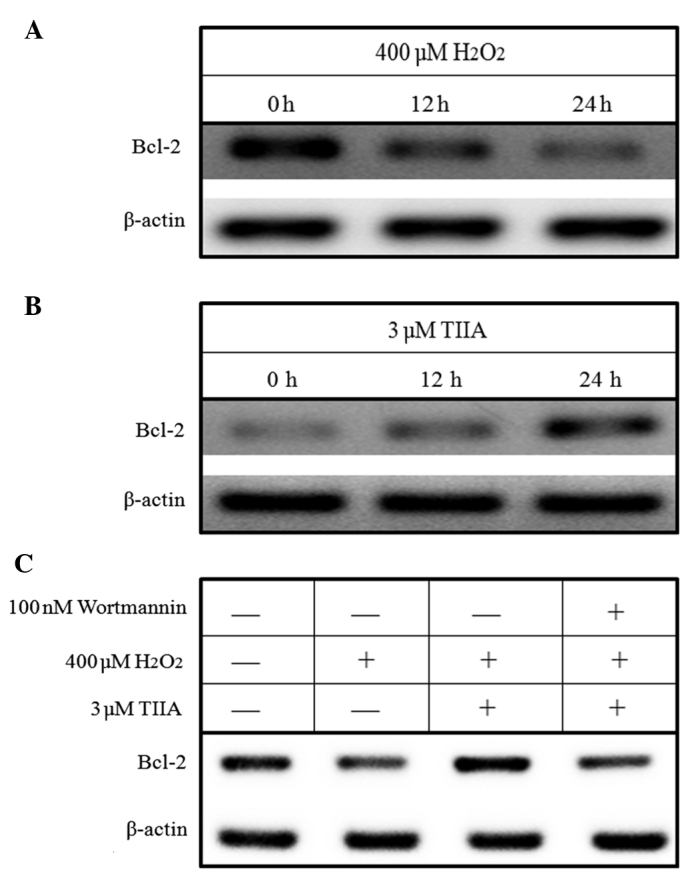
Regulation of Bcl-2 expression by TIIA. (A and B) H9c2 cells were incubated with400 µM H2O2 or 3 µM TIIA, then harvested and lysed. A western blot was performed to examine Bcl-2 protein expression. β-actin was used as a protein loading control. (A) A reduction in Bcl-2 expression was observed in response to 400 µM H2O2 treatment, while (B) TIIA increased Bcl-2 expression in a time-dependent manner. (C) H9c2 cells were pre-incubated with wortmannin (100 nM) for 30 min then treated with 400 µM H2O2 in the presence or absence of 3 µM TIIA for 24 h. Wortmannin treatment decreased the Bcl-2 protein expression induced by TIIA. TIIA, tanshinone IIA; Bcl-2, B cell lymphoma-2.
Discussion
Tanshinone IIA, one of the most abundant tanshinones, exhibits a variety of cardiovascular activities, including vasorelaxation, and protection against ischemia-reperfusion injury and the effects of antiarrhythmia (4,7,26). A number of studies have demonstrated that tanshinone IIA increases coronary blood flow and protects the heart against cardiac injury (7,10,22,23,27–31). The molecular mechanisms underlying the cardioprotective effects of tanshinone IIA were investigated in the present study. The results demonstrated that tanshinone IIA protects H9c2 cells from H2O2-induced cell death, which confirms the cardioprotective effect of tanshinone IIA in the oxidative stress injury model in vitro. In addition, the current study provides evidence that miR-133, Akt activation and Bcl-2 are involved in the cardioprotective effects of tanshinone IIA against oxidative stress-induced cell death.
miRNAs serve an important role in the proliferation and apoptosis of cardiomyocytes, cardiac hypertrophy and heart failure (13–15). Previous studies have demonstrated that muscle-specific miRNAs (miR-1 and miR-133) are important regulators of myogenesis (32). In addition, their upregulation or downregulation may influence the pathogenesis of cardiac diseases (33–35). He et al reported that miR-133 provides protection in myocardial ischemic post-conditioning via the regulation of the initiator caspase, caspase-9 (36).
In the present study, changes in the expression of miR-133 that were associated with the cardioprotectve effects of tanshinone IIA against H2O2-induced cell death were investigated. The results demonstrated that tanshinone IIA reversed the reduction in miR-133 expression levels induced by H2O2 in H9c2 cells. In addition, transfection with an miR-133 inhibitor attenuated the cardioprotective effects of tanshinone IIA against H2O2-induced cell death in H9c2 cells, indicating that miR-133 serves a vital role in the cardioprotective action of tanshinone IIA.
The PI3K signaling pathway controls cardiomyocytes survival and function (37). The downstream effects of the PI3K signaling pathway are primarily mediated by Akt, a serine/threonine kinase, to coordinate a variety of intracellular signals, and thereby regulate cell proliferation and survival (38). Activation of the PI3K/Akt signaling pathway has been shown to protect the myocardium from myocardial injury and prevent the apoptosis of cardiomyocytes (38). In order to explore whether the protective effects of tanshinone IIA against H2O2-induced cell death are associated with the PI3K/Akt signaling pathway, the current study investigated the effects of tanshinone IIA on the phosphorylation of Akt and the effects of PI3K inhibition on the cardioprotective effects of tanshinone IIA against H2O2-induced cell death. The results demonstrated that tanshinone IIA increased the phosphorylation of Akt at serine 473 in a dose-dependent manner. In addition, the blockade of Akt phosphorylation with a PI3K inhibitor (wortmannin or LY294002) eliminated the cardioprotective effects of tanshinone IIA against H2O2-induced cell death, suggesting that tanshinone IIA exerts cardioprotective effects against oxidative stress-induced cell death via the activation of the PI3K/Akt signaling pathway.
The PI3K/Akt signaling pathway can upregulate the expression of anti-apoptotic genes (18,19). For example, Akt activates inhibitor of κB (IκB) kinases (IKKs), resulting in the activation of NF-κB (18,19), its translocation to the nucleus and the transcription of anti-apoptotic genes, such as BCL-2 (39,40). In the present study it was observed that H2O2 decreased the expression of Bcl-2 in a time-dependent manner, while tanshinone IIA increased Bcl-2 expression in a time-dependent manner. In addition, the reduction of Bcl-2 expression induced by H2O2 was attenuated by tanshinone IIA, and this effect was suppressed by pre-treatment with the PI3K inhibitor, wortmannin.
In conclusion, the results from the present study suggest that tanshinone IIA exerts its cardioprotective effects against H2O2-induced cell death by upregulating the expression of Bcl-2 via the activation of the PI3K/Akt signaling pathway. In addition, tanshinone IIA is able to protect H9c2 cells from oxidative stress-induced cell death. Tanshinone IIA may potentially be used to treat heart diseases involving oxidative stress; however, further studies are required in order to define and clarify the rationale for its clinical use.
References
- 1.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang PN, Mao JC, Huang SH, Ning L, Wang ZJ, On T, Duan W, Zhu YZ. Analysis of cardioprotective effects using purified Salvia miltiorrhiza extract on isolated rat hearts. J Pharmacol Sci. 2006;101:245–249. doi: 10.1254/jphs.FPJ05034X. [DOI] [PubMed] [Google Scholar]
- 3.Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD, Chen XG. Separation and determination of active components in Radix Salviae miltiorrhizae and its medicinal preparations by nonaqueous capillary electrophoresis. J Sep Sci. 2004;27:569–575. doi: 10.1002/jssc.200301710. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 5.Adams JD, Wang R, Yang J, Lien EJ. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med. 2006;1:3. doi: 10.1186/1749-8546-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Yang G, Pi R, Li R, Wang P, Zhang H, Le K, Chen S, Liu P. Tanshinone IIA protects neonatal rat cardiomyocytes from adriamycin-induced apoptosis. Transl Res. 2008;151:79–87. doi: 10.1016/j.trsl.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Zou X, Liang Q, Chen H, Feng J, Yan L, Wang Z, Zhou D, Li S, Yao S, Zheng Z. Sodium tanshinone IIA sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med. 2007;39:65–73. doi: 10.1038/emm.2007.8. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Liu A, Ma X, Li L, Su D, Liu J. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol. 2008;51:396–401. doi: 10.1097/FJC.0b013e3181671439. [DOI] [PubMed] [Google Scholar]
- 10.Shanghai Cooperative Group for the Study of Tanshinone IIA: Therapeutic effect of sodium tanshinone IIA sulfonate in patients with coronary heart disease. A double blind study. J Tradit Chin Med. 1984;4:20–24. [PubMed] [Google Scholar]
- 11.Bartel DP. Micrornas: Genomics, biogenesis, mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE. 2007;2007:re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 13.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: Mir-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Wu Y, Li Y, Xu C, Li X, Zhu D, Zhang Y, Xing S, Wang H, Zhang Z, Shan H. Tanshinone IIa improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell Physiol Biochem. 2012;30:843–852. doi: 10.1159/000341462. [DOI] [PubMed] [Google Scholar]
- 17.Takaya T, Ono K, Kawamura T, Takanabe R, Kaichi S, Morimoto T, Wada H, Kita T, Shimatsu A, Hasegawa K. MicroRNA-1 and microRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ J. 2009;73:1492–1497. doi: 10.1253/circj.CJ-08-1032. [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: The role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.RES.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 21.Hong HJ, Liu JC, Cheng TH, Chan P. Tanshinone IIA attenuates angiotensin II-induced apoptosis via Akt pathway in neonatal rat cardiomyocytes. Acta Pharmacol Sin. 2010;31:1569–1575. doi: 10.1038/aps.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y, Guo JP, Yang XH, Yuan SR, Chen LZ, Chai JJ, et al. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin. 2013;34:1386–1396. doi: 10.1038/aps.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis. 2014;235:318–327. doi: 10.1016/j.atherosclerosis.2014.05.924. [DOI] [PubMed] [Google Scholar]
- 24.Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin YL, Su M, Chen T, Wang YP. Sodium tanshinone IIA silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-κb/TNF-α pathway. Br J Pharmacol. 2013;169:1058–1071. doi: 10.1111/bph.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun. 2009;389:105–111. doi: 10.1016/j.bbrc.2009.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun DD, Wang HC, Wang XB, Luo Y, Jin ZX, Li ZC, Li GR, Dong MQ. Tanshinone IIA: A new activator of human cardiac KCNQ1/KCNE1 (I(Ks)) potassium channels. Eur J Pharmacol. 2008;590:317–321. doi: 10.1016/j.ejphar.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Shang Q, Xu H, Huang L. Tanshinone IIA: A promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012;2012:716459. doi: 10.1155/2012/716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 29.Tong Y, Xu W, Han H, Chen Y, Yang J, Qiao H, Hong D, Wu Y, Zhou C. Tanshinone IIA increases recruitment of bone marrow mesenchymal stem cells to infarct region via up-regulating stromal cell-derived factor-1/CXC chemokine receptor 4 axis in a myocardial ischemia model. Phytomedicine. 2011;18:443–450. doi: 10.1016/j.phymed.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Yuan X, Jing S, Wu L, Chen L, Fang J. Pharmacological postconditioning with tanshinone IIA attenuates myocardial ischemia-reperfusion injury in rats by activating the phosphatidylinositol 3-kinase pathway. Exp Ther Med. 2014;8:973–977. doi: 10.3892/etm.2014.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song W, Pu J, He B. Tanshinol protects human umbilical vein endothelial cells against hydrogen peroxide-induced apoptosis. Mol Med Rep. 2014;10:2764–2770. doi: 10.3892/mmr.2014.2541. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 34.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 35.Dong DL, Chen C, Huo R, Wang N, Li Z, Tu YJ, Hu JT, Chu X, Huang W, Yang BF. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: A novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55:946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]
- 36.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: Taking a risk for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 38.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.CIR.104.3.330. [DOI] [PubMed] [Google Scholar]
- 39.Gustin JA, Korgaonkar CK, Pincheira R, Li Q, Donner DB. Akt regulates basal and induced processing of NF-kappaB2 (p100) to p52. J Biol Chem. 2006;281:16473–16481. doi: 10.1074/jbc.M507373200. [DOI] [PubMed] [Google Scholar]
- 40.Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol. 2005;25:541–550. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]