Abstract
Recent studies have reported that the ABO gene can affect circulating expression levels of soluble intercellular adhesion molecule 1 (sICAM-1) and soluble P-selectin (sP-selectin) in Caucasians. However, several factors may affect the association, including the distribution and variations of the ABO gene, ethnic diversity and the inflammatory response status. The aim of the present study was to investigate this issue in Asian subjects of various blood groups. A total of 800 blood samples were randomly selected from healthy blood donors. The ABO blood groups were examined using standard serological tests, and ABO genotypes of group A and group AB specimens were analyzed. Plasma concentrations of sICAM-1 and sP-selectin were detected by standard enzyme-linked immunosorbent assays. In healthy Chinese individuals, blood group A was detected to be significantly associated with lower circulating expression levels of sICAM-1 and sP-selectin, compared with group O. Individuals with ≥1 A1 allele had significantly lower expression levels of sICAM-1 and sP-selectin compared with all other ABO groups. The data indicate the significant association of ABO blood group antigens with sICAM-1 and sP-selectin expression levels in a healthy Chinese population, independent of the specific variations and distributions of ABO blood groups among ethnic populations. This result provides evidence for the previously unidentified role of ABO blood group antigens in the regulation of the inflammatory adhesion process. Accordingly, it can be proposed that ABO blood groups may require consideration when soluble adhesion molecules are identified as predictors for cardiovascular disease.
Keywords: ABO blood group, soluble intercellular adhesion molecule 1, soluble P-selectin, cardiovascular disease, inflammatory diseases
Introduction
Cardiovascular diseases comprise a complex pathological inflammatory process, including leukocyte migration and adhesion to vascular endothelial cells (1,2). This process is mediated by adhesion molecules expressed on leukocytes and endothelial cells in response to inflammatory stimuli (3). Numerous soluble adhesion molecules are identified as plasma predictors for the risk of CVD, important among which are soluble intercellular adhesion molecule 1 (sICAM-1) and soluble P-selectin (sP-selectin)(1,2,4,5).
ICAM-1, also called CD54, is a member of the immunoglobulin supergene family (6). Being the ligand for the lymphocyte function associated antigen-1, ICAM-1 may facilitate leukocyte migration and adhesion (7). sICAM-1 may inhibit additional leukocyte adhesion, possibly resulting in inflammation inhibition (8). Thus, elevated expression levels of sICAM-1 may serve as a marker for atherosclerosis, the development of coronary heart disease and other CVD events (9–12).
P-selectin serves an important role in facilitating leukocyte and/or endothelial cell adhesion (13), platelet aggregation and stabilizing initial gpIIb/IIIa-fibrinogen interactions (14). The soluble form, sP-selectin, is proved to originate from the surface of the platelet membrane, particularly when thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome occur (15–17). Raised sP-seletin expression levels are observed in stable peripheral artery disease and stable coronary artery disease (18). In addition, higher expression levels of sP-selectin are associated with patients with CVD (19).
A meta-analysis reported that the ABO blood group, blood group A in particular, is associated with a higher risk of myocardial infarction, peripheral vascular disease, strokes and venous thromboembolism (20), suggesting that it may have a role in inflammatory adhesion. Notably, the circulating expression levels of sICAM-1 and sP-selectin are reported to be significantly associated with ABO blood group antigens in Caucasian populations (21–23).
The distribution and variations of ABO blood group antigens differ considerably among races; however, it is uncertain whether the observed association in Caucasians is due to race specificity (21,22). Until now no reports are available in other populations. In addition, in previous studies of patients with inflammatory diseases, the data collected may be influenced by the inflammatory response itself, as both sICAM-1 and sP-selectin participate in the inflammatory process (7,13). Whether the association observed in patients is the same as in healthy individuals was not clarified (21,22). In order to address these issues, the association of ABO with sICAM-1 and sP-selectin in a healthy Chinese population was investigated in the present study.
Materials and methods
Specimen collection and serological testing of blood types
ABO blood groups are not evenly distributed in China (24). In particular, the AB blood group accounts for ~10% among the four major groups, not to mention the rare subtypes (24). As specimens were collected for the current study by the Blood Collection Department of the Blood Center (Blood Center of Shandong, Jinan, China) during the period between April 2013 and March 2014, the aim of the study was described to the blood donors for informed consent and their physical data, including age, gender, smoking status, height and weight were recorded. In order to avoid the distribution imbalance of the four blood groups, the plasma specimens were collected from EDTA blood specimens previously tested in the Clinical Laboratory of the Blood Center (Blood Center of Shandong, Jinan, China), a department responsible for the primary testing of the blood samples from the donors. In this department, each blood sample could be matched with the donor information through their serial number, and the qualified blood samples (for which the potential viruses such as syphilis, hepatitis B, hepatitis C and AIDS are examined, and the unqualified blood samples can be excluded) were grouped into four blood types (A, B, O and AB). A total of 200 samples were randomly collected from each blood group stored within 24 h in this department, ensuring that the donors were healthy. All the speciments were kept at −30°C until further use.
The participants were characterized by age, gender (according to the Blood Donation Law, the donor ages should range between 18 and 55 years old), smoking status and mean body mass index (BMI). All participants had normal blood pressure levels (90 mmHg-140 mmHg/60 mmHg-90 mmHg) and normal metabolic conditions (namely, normal values of hemoglobin and transaminase, heavy enough, good nutrition, without alcohol or any other illnesses for which blood donation is not permitted). Samples were from patients with no prior histories of CVD, diabetes or other chronic illness. Those with high blood lipid and cholesterol levels, and abnormal transaminase levels, were excluded; only eligible and clear plasma was included in the study. All blood donors provided written informed consent for this research. This study was approved by the Ethics Committee of the Blood Center of Shandong Province (Jinan, China). The blood groups were carefully detected using forward and reverse typing (Sanquin Reagents, Amsterdam, The Netherlands).
Determination of sICAM-1 and sP-selectin expression levels
The circulating sICAM-1 expression level was determined by a quantitative ELISA kit (cat no. CHE0052; 4A Biotech Co., Ltd., Beijing, China) according to the manufacturer's instructions. EDTA-anti-coagulated plasma from each sample was run in duplicate wells and the intra- and inter-assay coefficients of variation were both <10%. The minimum detectable dose was 60 pg/ml, with a standard curve ranging 0–5,000 pg/ml.
The sP-selectin expression level was examined from plasma with a quantitative ELISA kit (BMS219/4; eBioscience, Inc., Vienna, Austria) with a minimum detectable dose of 0.2 ng/ml. EDTA-anti-coagulated plasma from each sample was run in duplicate wells and the intra- and inter-assay coefficients of variation were 7.8 and 5.4%, respectively.
Standard genotyping of ABO blood groups
Genomic DNA was extracted from blood samples using a QIAamp DNA Blood Mini kit (51104; Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. ABO allele genotyping was performed with a commercially available kit (B9801B; G&T SSPTyper; G&T Biotech, Inc., Rockville, MD, USA). The DNA polymerase was commercially available (M1665S; Promega Corp., Madison, WI, USA). Polymerase chain reaction (PCR) analysis was performed in a 10 µl reaction volume, containing 1 µl template DNA (0.04–0.07 µg DNA), 1 µl Taq polymerase (0.33–0.5 µg), 0.2 mmol/l dNTPs, and the primers were previously coated inside the tube provided by the G&T kit. Next, the PCT analysis was performed in a 9600 DNA Thermal Cycler (PE Biosystems, Foster City, CA, USA) under the following thermal cycling conditions: 95°C for 5 min, followed by 30 cycles, including 95°C denaturation for 30 sec, specific annealing at 60°C for 30 sec and extending at 72°C for 90 sec. The system was then extended at 72°C for 5 min. Finally, 3% agarose gel electrophoresis was conducted to analyze the PCR results (Electrophoresis apparatus: DYY-6D type, Liuyi Instrument Factory, Beijing, China; Electrophoresis tank: ABgene, ThermoFisher Scientific Inc., Waltham, MA, USA). The results were investigated under a UV transilluminator (GeneGenius, Syngene, Frederick, MD, USA). The image was analyzed using the GeneSnap software (Product version 7.01, SynGene, Cambridge, England).
Statistical analysis
One-way analysis of variance was performed to analyze the association of ABO blood group antigens and the A1 gene with circulating expression levels of sICAM-1 and sP-selectin. The Student's t-test was used for comparison between the two groups where appropriate. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
As blood donors, the physical conditions of the study participants did not vary greatly (Table I). The influence of characteristics, such as age, gender, smoking status and BMI, on the expression levels of sICAM-1 and sP-selectin were analyzed in each blood group using a Student's t-test. However, no statistically significant differences were observed among the characteristics (data not shown).
Table I.
Characteristics of participants in the various blood groups.
| Blood groups | ||||
|---|---|---|---|---|
| Characteristic | A | B | O | AB |
| Individuals, n | 200 | 200 | 200 | 200 |
| Median age, years | 33.5 | 38.2 | 35.7 | 40.3 |
| Age range, years | 22–50 | 24–53 | 21–51 | 23–52 |
| Gender | ||||
| Male | 130 | 128 | 129 | 132 |
| Female | 70 | 72 | 71 | 68 |
| Currently smoking, % | 25.7 | 27.3 | 28 | 26.8 |
| Mean body mass index, kg/m2 (SD) | 23.1 (4.7) | 22.8 (3.6) | 22.7 (3.2) | 23.2 (2.8) |
SD, standard deviation. Student's t-test was used to analyze the influence of gender and smoking status on the expression levels of sICAM-1 and sP-selectin inside each blood group. One-way analysis of variance was used to analyze the differences of age, gender, smoking status, and body mass index among the four blood groups. sICAM1, soluble intercellular adhesion molecule 1; sP-selectin, soluble P-selectin.
Blood group antigen A is associated with the lowest expression levels of sICAM-1 and sP-selectin
In previous studies, antigen A has been suggested to be associated with abnormal expression levels of sICAM-1 and sP-selectin in Caucasians (21,22). These contradictory results may have been partially affected by the method by which participants were collected, as the distribution imbalance of blood groups and the disease status of the participants may have been the influencing factors. Thus, the association of blood groups with the expression levels of sICAM-1 and sP-selectin were detected in a healthy population in the present study. Blood group A individuals were found to have significantly lower sICAM-1 expression levels (252.2 ng/ml), compared with those in group O (304.4 ng/ml; P=0.002). In addition, blood group A individuals were associated with the lowest concentration of sP-selectin (49.2 ng/ml), followed by group AB (52.9 ng/ml), both of which were significantly lower compared with group O (P<0.000 and P=0.007, respectively; Table II). These results indicate that in a Chinese healthy population, blood group antigen A is associated with the lowest expression levels of sICAM-1 and sP-selectin.
Table II.
Association of ABO blood group antigens with expression levels of sICAM1 and sP-selectin.
| Protein | Blood group | Expression (ng/ml) | P-value |
|---|---|---|---|
| sICAM1 | Oa | 304.4 (7.3) | – |
| A | 252.2 (6.7) | 0.002 | |
| B | 272.9 (9.6) | 0.08 | |
| AB | 295.8 (8.1) | 0.682 | |
| sP-selectin | Oa | 64.5 (3.1) | – |
| A | 49.2 (3.9) | <0.001 | |
| B | 59.3 (4.4) | 0.188 | |
| AB | 52.9 (4.6) | 0.007 |
One-way analysis of variance was used to analyze the expression levels of sICAM1 and sP-selectin among the four blood groups
Levels of sICAM-1 and sP-selectin in A, B and AB blood groups were compared with those in blood group O. sICAM1, soluble intercellular adhesion molecule 1; sP-selectin, soluble P-selectin.
A1 gene is associated with the lowest expression levels of sICAM-1 and sP-selectin
Each blood group comprises several subgroups, depending on the blood group gene it contains (25). For example, blood group A consists of subgroups including group A2, A3 and Ax and the A1 antigen is the most commonly expressed in Asians (26). It is therefore important to determine the association between specific blood group genes and the expression levels of sICAM-1 and sP-selectin. As antigen A was demonstrated to be associated with the lowest sICAM-1 and sP-selectin expression levels in the present study, a standard genotyping assay in groups A and AB was performed to further characterize the differences between subgroups (Table III). Among group A participants, individuals with genotypes A1/O and A1/A1 numbered 176 and 22, respectively. As there were only two A2/O individuals, they were not included in this analysis. The sICAM-1 expression levels in the genotype O/O were compared to the expression levels in genotypes the A1/O and A1/A1, and the A1/A1 subgroup was found to have a lower circulating expression level (P<0.001) than the A1/O genotype (P=0.002). In the AB group, there were 182 A1/B and 18 A2/B individuals. sP-selectin expression levels in the O/O genotype were compared with the A1/B and the A2/B genotypes, and the A1/B genotype was found to have a significantly expression lower level (P<0.001) than the A2/B genotype (P=0.01) (Fig. 1). These results indicate that the A1 gene is associated with the lowest expression levels of sICAM-1 and sP-selectin.
Table III.
Association between ABO genotypes, and sICAM1 and sP-selectin expression levels.
| Genotype | Subjects (n) | sICAM-1 (ng/ml) | P-value | sP-selectin (ng/ml) | P-value |
|---|---|---|---|---|---|
| A1/O | 176 | 253.2 (6.2) | 0.002 | 49.5 (3.8) | 0.001 |
| A1/A1 | 22 | 244.6 (3.7) | <0.001 | 46.8 (4.1) | <0.001 |
| A1/B | 182 | 295.3 (8.5) | 0.679 | 52.5 (4.7) | <0.001 |
| A2/B | 18 | 300.9 (7.6) | 0.735 | 57.5 (3.2) | 0.01 |
| O/O | 200 | 304.4 (7.3)a | – | 64.5 (3.1)b | – |
One-way analysis of variance was used to analyze the results.
sICAM-1 expression levels in A1/O and A1/A1 groups were compared with that in the O/O group.
sP-selectin expression levels in A1/B and A2/B groups were compared with that in the O/O group. sICAM1, soluble intercellular adhesion molecule 1; sP-selectin, soluble P-selectin.
Figure 1.
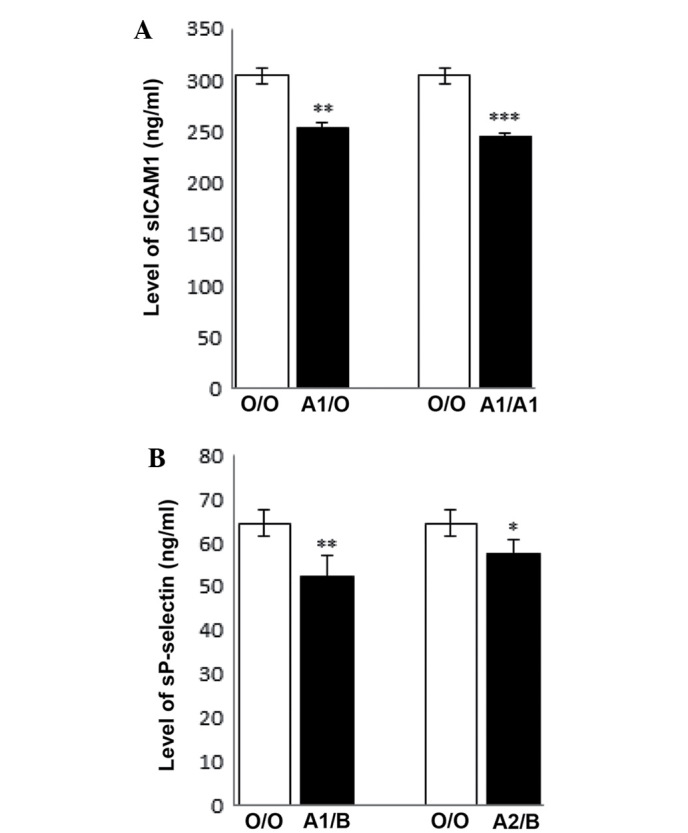
Expression levels of sICAM1 and sP-selectin in different genotypes. (A) sICAM1 expression levels in A1/O and A1/A1 groups compared with the O/O group. (B) Expression levels of sP-selectin in A1/B and A2/B groups compared with the O/O group. One-way analysis of variance was used to analyze the results. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01, ***P<0.001 vs. O/O. sICAM1, soluble intercellular adhesion molecule 1; sP-selectin, soluble P-selectin.
Discussion
Instead of encoding ABO blood group antigens directly, the ABO gene encodes glycosyltransferases that transfer distinct monosaccharides to the precursor H antigen to form ABO blood group antigens (27,28). The gene A encodes stronger transferase activity compared with the other alleles, and the A1 allele encodes the strongest (29). In the present study, the lower expression levels of sICAM-1 and sP-selectin found in donors with the A1 allele compared with the A2 allele reflects the A1 allele having 30–50 fold more A transferase activity than the A2 allele (18). The frequency distributions of the ABO blood group antigens vary considerably among races. In the European population, the frequency of the A antigen (25–55%) is much higher than in other parts of the world, while in aboriginal Americans and the majority of native Australians, the B antigen has a very low frequency (30). Conversely, the B antigen is very common in Asians (20–30%). There are also variations between the ABO alleles within races (31).
Previous studies have shown the association of ABO blood groups with sICAM-1 and sP-selectin expression levels (21,22 and 32). In a European ancestry population, it was observed that the A1 blood group allele was associated with the lowest expression levels of these proteins (32). In a study of a group of Caucasian women without chronic disease history, the A1 allele was associated with the lowest sICAM-1 expression level, while the A2 allele was associated with a slightly higher expression level, intermediate between the A1 and O alleles (21). The data in the current study were compatible with the above two studies. However, in a case-control study within a Caucasian population, contradictory results were found, associating blood group A with the highest sICAM-1 expression level (22). This discordant result may be due to the specific selection of participants, namely whether the individuals were healthy or had a history of CVD, or how they were grouped when the data were collected. There may also be an inflammatory response of adhesion molecules in patients, and genetic variations in patients with a history of chronic illness may affect the inflammatory adhesion process. The observation of significantly increased expression levels of sICAM-1 in completely healthy individuals compared with patients with CVD remained unexplained. Furthermore, variations in population and subpopulation, and the varied distribution of ABO blood groups within races, may also contribute towards the difference. In the present study, the significantly reduced expression levels of sICAM-1 and sP-selectin were observed in completely healthy participants. Furthermore, the possible distribution imbalance of blood groups was also excluded. In addition to previous studies (9–12,19,20), the authors of the current study speculate that the risk of suffering from CVD may be higher if sICAM-1 or sP-selectin expression levels are elevated in group A individuals. However, this hypothesis requires further investigation.
It is important to note the role of ABO blood group antigens in the inflammatory adhesion process. Aside from its association with CVD (20), the presence of the A antigen is understood to be closely associated with severe malaria infection (33). In China, pancreatic cancer was detected more frequently in group A patients compared with group O patients, and the Tumor Node Metastasis staging was significantly increased in the non-O group (34). In Japanese and Taiwanese populations, individuals with blood group A were detected to have a higher susceptibility to inflammation and cancer (35,36). This may be due to the low level of soluble forms of adhesion molecules in group A individuals, with a conversely high concentration of membrane forms, which could mediate leukocyte migration and adhesion in response to inflammatory stimuli (7,13).
The detailed mechanism underlying the observed association of sICAM-1 and sP-selectin with the ABO blood group remains elusive. The glycosylation process of ABO antigens may affect the shedding or clearance of soluble adhesion molecules, as soluble and cellular forms of ICAM-1 are known to be glycosylated (37). Additionally, glycosylation is crucial to the binding activity of the P-selectin receptor, P-selectin glycoprotein ligand-1 (38). In the present study, the lower expression levels of sICAM-1 and sP-selectin found in donors with the A1 allele compared with the A2 allele may reflect the stronger shedding or clearance ability of the A1 allele, as the A1 allele has 30–50 fold more A transferase activity than the A2 allele (23). The pursuit of further investigation into this topic is encouraged.
In conclusion, by using phenotype and genotype assay methods, it is confirmed that the ABO blood group and the A allele are significantly associated with the lowest circulating sICAM-1 and sP-selectin expression levels in a healthy Chinese population, independent of variations and distributions of ABO blood groups among races. As blood group A is detected to be associated with the lowest sICAM-1 and sP-selectin expression levels in healthy Asian individuals, it is suggested that ABO blood groups be considered when abnormal elevated sICAM-1 and sP-selectin expression levels are perceived as predictors for risk the of CVD. In addition, the present data provide evidence for the previously unknown role of ABO blood group antigens in the inflammatory adhesion process, and contribute towards the understanding of the underlying mechanism of the pathological inflammatory adhesion process.
Acknowledgements
The present study was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2010HM093). The authors thank Dr Elizabeth Furlong, [MT (ASCP) SBB, Department of Transfusion Medicine, Clinical Center, National Institutes of Health, USA], for critically reading and improving the English manuscript.
References
- 1.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 2.Pober JS, Gimbrone MA, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 3.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/S0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 4.O'Malley T, Ludlam CA, Riemermsa RA, Fox KA. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1); potential risk factor for the acute coronary syndromes. Eur Heart J. 2001;22:1226–1234. doi: 10.1053/euhj.2000.2480. [DOI] [PubMed] [Google Scholar]
- 5.Barbaux SC, Blankenberg S, Rupprecht HJ, Francomme C, Bickel C, Hafner G, Nicaud V, Meyer J, Cambien F, Tiret L. Association Between P-Selectin Gene Polymorphisms and Soluble P-Selectin Levels and Their Relation to Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2001;21:1668–1673. doi: 10.1161/hq1001.097022. [DOI] [PubMed] [Google Scholar]
- 6.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 7.Van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl) 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 8.Rieckmann P, Michel U, Albrecht M, Brück W, Wöckel L, Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995;60:9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1 and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.CIR.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 10.O'malley T, Ludlam CA, Riemermsa RA, Fox KA. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1); potential risk factor for the acute coronary syndromes. Eur Heart J. 2001;22:1226–1234. doi: 10.1053/euhj.2000.2480. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1 and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106:820–825. doi: 10.1161/01.CIR.0000025636.03561.EE. [DOI] [PubMed] [Google Scholar]
- 12.Albert MA, Glynn RJ, Buring JE, Ridker PM. Differential effect of soluble intercellular adhesion molecule-1 on the progression of atherosclerosis as compared to arterial thrombosis: A prospective analysis of the Women's Health Study. Atherosclerosis. 2008;197:297–302. doi: 10.1016/j.atherosclerosis.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 14.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Vianelli N, Catani L, Gugliotta L, Nocentini F, Baravelli S, Lancellotti G, Tura S. Increased P-selectin plasma levels in patients with thrombotic thrombocytopenic purpura. Haematologica. 1996;81:3–7. [PubMed] [Google Scholar]
- 16.Chong BH, Murray B, Bemdt MC, Dunlop LC, Brighton T, Chesterman CN. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 1994;83:1535–1541. [PubMed] [Google Scholar]
- 17.Katayama M, Handa M, Araki Y, Ambo H, Kawai Y, Watanabe K, Ikeda Y. Soluble P-selectin is present in normal circulation and its plasma level is elevated in patients with thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome. Br J Haematol. 1993;84:702–710. doi: 10.1111/j.1365-2141.1993.tb03149.x. [DOI] [PubMed] [Google Scholar]
- 18.Blann AD, Dobrotova M, Kubisz P, McCollum CN. Von Willebrand factor, soluble P-seletin, tissue plasminogen activator and plasminogen activator inhibitor in atherosclerosis. Thromb Haemost. 1995;74:626–630. [PubMed] [Google Scholar]
- 19.Carter AM, Anagnostopoulou K, Mansfield MW, Grant PJ. Soluble P-selectin levels, P-selectin polymorphisms and cardiovascular disease. J Thromb Haemost. 2003;1:1718–1723. doi: 10.1046/j.1538-7836.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu O, Bayoumi N, Vickers MA, Clark P. ABO (H) blood groups and vascular disease: A systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 21.Paré G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S, Miletich JP, Ridker PM. Novel association of ABO histo-blood group antigen with soluble ICAM-1: Results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4:e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q, Girman CJ, Laurie CC, Mirel DB, Hunter DJ, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto F, Hakomori S. Sugar-nucleotide donor specificity of histo-blood group A and B transferases is based on amino acid substitutions. J Biol Chem. 1990;265:19257–19262. [PubMed] [Google Scholar]
- 24.Tongmao Z. Human blood group genetics. Beijing: Science Press; 1987. pp. 51–57. (In Chinese) [Google Scholar]
- 25.Reid ME, Lomas-Francis C. The Blood Group Antigen FactsbBook (Second Edition) ISBN: 978-0-12-586585-2. [Google Scholar]
- 26.Tongmao Z. Human blood group genetics. Beijing: Science Press; 1987. pp. 32–35. (In Chinese) [Google Scholar]
- 27.Kobata A, Grollman EF, Ginsburg V. An enzymic basis for blood type A in humans. Arch Biochem Biophys. 1968;124:609–612. doi: 10.1016/0003-9861(68)90373-1. [DOI] [PubMed] [Google Scholar]
- 28.Race C, Ziderman D, Watkins WM. An K-D-galactosyltransferase associated with the blood group B character. Biochem J. 1968;107:733–735. doi: 10.1042/bj1070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schachter H, Michaels MA, Crookston MC, Tilley CA, Crookston JH. A quantitative di¡erence in the activity of blood group A-specific N-acetylgalactosylaminyltransferase in serum from A1 and A2 human subjects. Biochem Biophys Res Commun. 1971;45:1011–1018. doi: 10.1016/0006-291X(71)90438-4. [DOI] [PubMed] [Google Scholar]
- 30.Daniels G. Human Blood Groups. Second. Blackwell Science Ltd.□; 2002. ISBN 0-632-056460. [DOI] [Google Scholar]
- 31.Mollison PL, Engelfriet CP, Contreras M. Blood Transfusion in Medicine. Blackwell Science Oxford; 1997. [Google Scholar]
- 32.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathirana SL, Alles HK, Bandara S, Phone-Kyaw M, Perera MK, Wickremasinghe AR, Mendis KN, Handunnetti SM. ABO-blood-group types and protection against severe, Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2005;99:119–124. doi: 10.1179/136485905X19946. [DOI] [PubMed] [Google Scholar]
- 34.Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: A case-control study. Int J Cancer. 2011;128:1179–1186. doi: 10.1002/ijc.25426. [DOI] [PubMed] [Google Scholar]
- 35.Nakao M, Matsuo K, Hosono S, Ogata S, Ito H, Watanabe M, Mizuno N, Iida S, Sato S, Yatabe Y, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102:1076–1080. doi: 10.1111/j.1349-7006.2011.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng MS, Hsu LA, Wu S, Chou HH, Chang CJ, Sun YZ, Juan SH, Ko YL. Mediation analysis reveals a sex-dependent association between ABO gene variants and TG/HDL-C ratio that is suppressed by sE-selectin level. Atherosclerossis. 2013;228:406–412. doi: 10.1016/j.atherosclerosis.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Otto VI, Damoc E, Cueni LN, Schürpf T, Frei R, Ali S, Callewaert N, Moise A, Leary JA, Folkers G, Przybylski M. N-glycan structures and N-glycosylation sites of mouse soluble intercellular adhesion molecule-1 revealed by MALDI-TOF and FTICR mass spectrometry. Glycobiology. 2006;16:1033–1044. doi: 10.1093/glycob/cwl032. [DOI] [PubMed] [Google Scholar]
- 38.Martinez M, Joffraud M, Giraud S, Baïsse B, Bernimoulin MP, Schapira M, Spertini O. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: Role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280:5378–5390. doi: 10.1074/jbc.M410899200. [DOI] [PubMed] [Google Scholar]


