Abstract
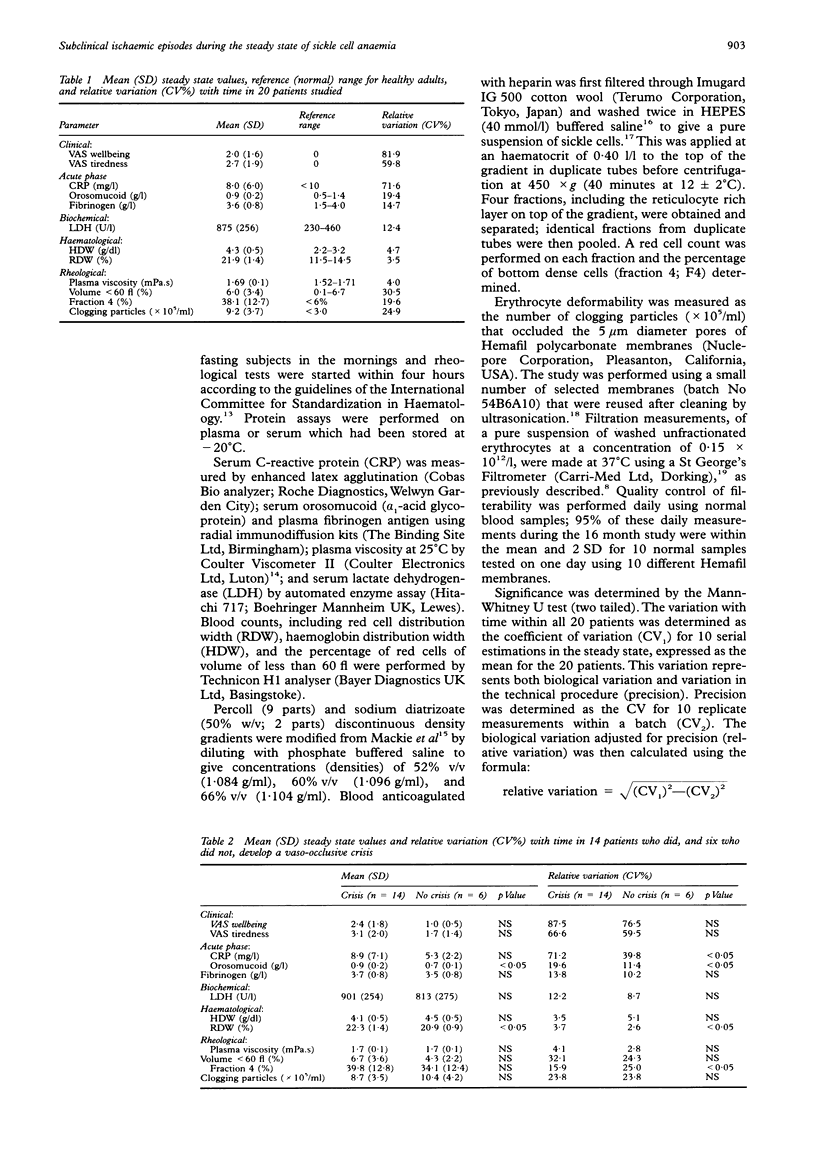
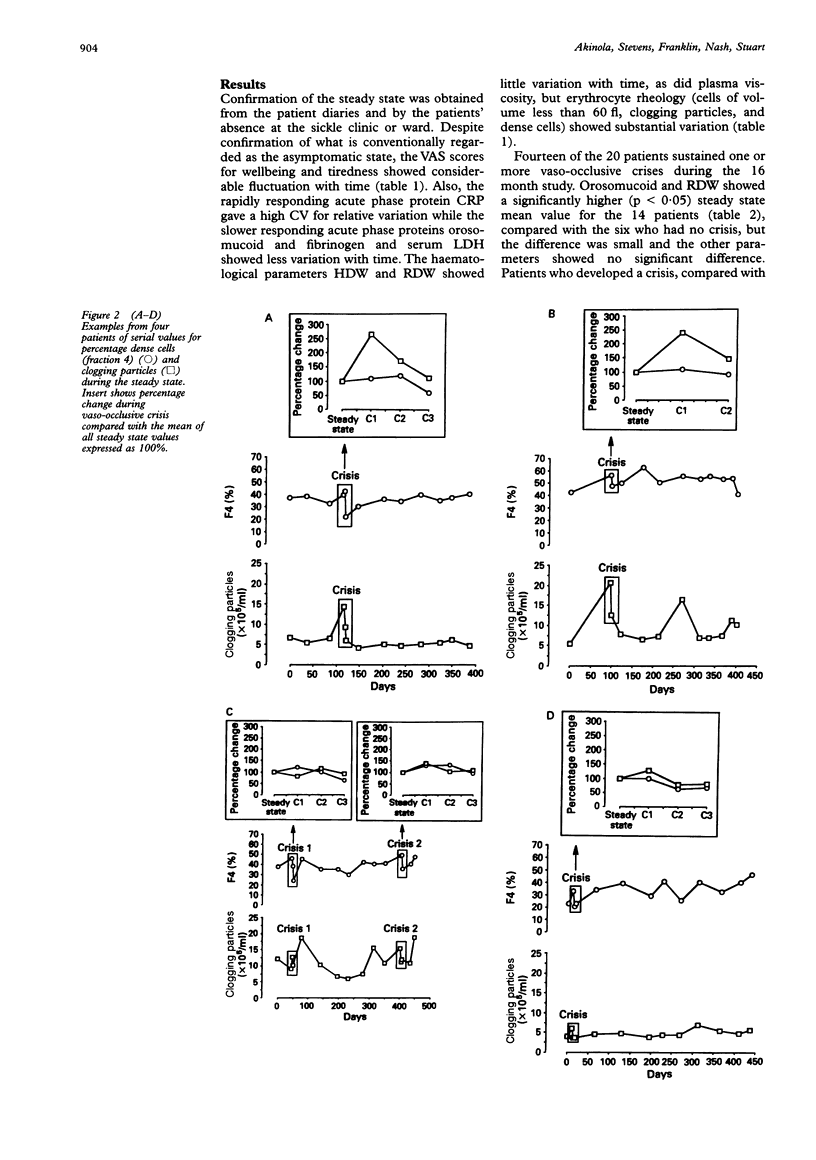
AIMS: To determine the clinical, haematological, biochemical and rheological changes that occur in the asymptomatic steady state of sickle cell anaemia. METHODS: Patient self-assessment visual analogue scores (for wellbeing and tiredness), the blood concentration of acute phase proteins (C-reactive protein, orosomucoid, and fibrinogen), and blood rheology (percentage of dense cells and the number of sickled cells that occluded pores 5 microns in diameter) were studied longitudinally on 10 occasions in each of 20 outpatients with sickle cell anaemia. RESULTS: Patients in the steady state showed fluctuation in visual analogue scores, in concentration of acute phase proteins, and in rheological parameters consistent with minor episodes of tissue injury. Significantly more variation in acute phase proteins occurred in the steady state of 14 of the 20 patients who developed one or more vaso-occlusive crises during the 16 month study period. Rheological fluctuation in the steady state simulated rheological change during crisis, namely a transient rise and then fall in the number of dense and poorly filterable cells. CONCLUSIONS: The term "steady state" is a misnomer, being characterised by biochemical and rheological fluctuation consistent with minor episodes of microvascular occlusion that are insufficient to cause the overt tissue infarction of painful crisis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akinola N. O., Stevens S. M., Franklin I. M., Nash G. B., Stuart J. Rheological changes in the prodromal and established phases of sickle cell vaso-occlusive crisis. Br J Haematol. 1992 Aug;81(4):598–602. doi: 10.1111/j.1365-2141.1992.tb02998.x. [DOI] [PubMed] [Google Scholar]
- Ballas S. K. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol. 1991 Feb;36(2):122–130. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- Billett H. H., Kim K., Fabry M. E., Nagel R. L. The percentage of dense red cells does not predict incidence of sickle cell painful crisis. Blood. 1986 Jul;68(1):301–303. [PubMed] [Google Scholar]
- Cooke B. M., Stuart J. Automated measurement of plasma viscosity by capillary viscometer. J Clin Pathol. 1988 Nov;41(11):1213–1216. doi: 10.1136/jcp.41.11.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987 Nov;70(5):1245–1266. [PubMed] [Google Scholar]
- Fabry M. E., Benjamin L., Lawrence C., Nagel R. L. An objective sign in painful crisis in sickle cell anemia: the concomitant reduction of high density red cells. Blood. 1984 Aug;64(2):559–563. [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Windisch P., Baez S., Nagel R. L. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983 Jul;72(1):22–31. doi: 10.1172/JCI110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidan A. J., Noguchi C. T., Player M., Chalder S. M., Stuart J. Erythrocyte heterogeneity in sickle cell disease: effect of deoxygenation on intracellular polymer formation and rheology of sub-populations. Br J Haematol. 1989 Jun;72(2):254–259. doi: 10.1111/j.1365-2141.1989.tb07691.x. [DOI] [PubMed] [Google Scholar]
- Keidan A. J., Sowter M. C., Johnson C. S., Noguchi C. T., Girling A. J., Stevens S. M., Stuart J. Effect of polymerization tendency on haematological, rheological and clinical parameters in sickle cell anaemia. Br J Haematol. 1989 Apr;71(4):551–557. doi: 10.1111/j.1365-2141.1989.tb06316.x. [DOI] [PubMed] [Google Scholar]
- Lucas G. S., Caldwell N. M., Stuart J. Fluctuating deformability of oxygenated sickle erythrocytes in the asymptomatic state and in painful crisis. Br J Haematol. 1985 Feb;59(2):363–368. doi: 10.1111/j.1365-2141.1985.tb03001.x. [DOI] [PubMed] [Google Scholar]
- Mackie L. H., Frank R. S., Hochmuth R. M. Erythrocyte density separation on discontinuous "Percoll" gradients. Biorheology. 1987;24(2):227–230. doi: 10.3233/bir-1987-24214. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Boghossian S., Parmar J., Dormandy J. A., Bevan D. Alteration of the mechanical properties of sickle cells by repetitive deoxygenation: role of calcium and the effects of calcium blockers. Br J Haematol. 1989 Jun;72(2):260–264. doi: 10.1111/j.1365-2141.1989.tb07692.x. [DOI] [PubMed] [Google Scholar]
- Neely C. L., Wajima T., Kraus A. P., Diggs L. W., Barreras L. Lactic acid dehydrogenase activity and plasma hemoglobin elevations in sickle cell disease. Am J Clin Pathol. 1969 Aug;52(2):167–169. doi: 10.1093/ajcp/52.2.167. [DOI] [PubMed] [Google Scholar]
- Rieber E. E., Veliz G., Pollack S. Red cells in sickle cell crisis: observations on the pathophysiology of crisis. Blood. 1977 Jun;49(6):967–979. [PubMed] [Google Scholar]
- Thame M., Grandison Y., Mason K., Thompson M., Higgs D., Morris J., Serjeant B., Serjeant G. The red cell distribution width in sickle cell disease--is it of clinical value? Clin Lab Haematol. 1991;13(3):229–237. doi: 10.1111/j.1365-2257.1991.tb00277.x. [DOI] [PubMed] [Google Scholar]



