Abstract
It has been recently proposed that α5-subunit containing GABAA receptors (α5-GABAA receptors) that mediate tonic inhibition might be involved in pain. The purpose of this study was to investigate the contribution of α5-GABAA receptors in the loss of GABAergic inhibition and in formalin-, Complete Freund’s adjuvant (CFA)- and L5/L6 spinal nerve ligation-induced long-lasting hypersensitivity. Formalin or CFA injection and L5/L6 spinal nerve ligation produced long-lasting allodynia and hyperalgesia. Moreover, formalin injection impaired the rate-dependent depression (RDD) of the Hofmann reflex. Peripheral and intrathecal pre-treatment or post-treatment with the α5-GABAA receptor antagonist, L-655,708 (0.15–15 nmol) prevented and reversed, respectively, these long-lasting behaviors. Formalin injection increased α5-GABAA receptors mRNA expression in the spinal cord and dorsal root ganglia (DRG) mainly at 3 days. α5-GABAA receptors were localized in the dorsal spinal cord and DRG co-labeling with NeuN, CGRP and IB4 suggesting their presence in peptidergic and non-peptidergic neurons. These receptors were found mainly in small- and medium-size neurons. Formalin injection enhanced α5-GABAA receptors fluorescence intensity in spinal cord and DRG at 3 and 6 days. Intrathecal administration of L-655,708 (15 nmol) prevented and reversed formalin-induced impairment of RDD. These results suggest that α5-GABAA receptors play a role in the loss of GABAergic inhibition and contribute to long-lasting secondary allodynia and hyperalgesia.
Keywords: Chronic pain, GABAA receptors, α5 subunit-containing GABAA receptors, secondary allodynia, secondary hyperalgesia, RDD, loss of inhibition
Introduction
The role of GABAA receptors in chronic pain is a matter of debate. Activation of neuronal γ-amino butyric acid (GABA) receptors leads to hyperpolarization, and thus GABA is considered the main inhibitory neurotransmitter in the mature central nervous system. Interestingly, GABAA receptors activation also leads to depolarization [38,61]. Under normal conditions, GABAA receptor agonists produce antinociception while antagonists elicit pronociception [5,65,72]. However, tissue or nerve injury appears to switch GABAA receptors function with GABAA receptors agonists promoting nociception whereas antagonists produce antinociception [3,19,55]. This switch has been attributed to a loss of GABAA receptors-mediated spinal inhibition (central disinhibition). The loss in inhibition has been explained by a depolarizing shift of GABA reversal potential (EGABA) produced by an increased expression of the Na+-K+-2Cl− co-transporter 1 (NKCC1) and/or reduced expression of K+-Cl− co-transporter 2 (KCC2) or by the participation of extrasynaptic GABAA receptors [56,70,73].
GABAA receptors are heteropentameric ligand-gated chloride channels [51]. To date, several subunits have been cloned including α (1–6), β (1–3), γ (1–3), δ, ε, θ, π and ρ (1–3) [25]. The GABAA receptors mediate both phasic and tonic inhibition. The subunit composition of the GABAA receptors determines whether the receptors are more likely to participate in the generation of transient post-synaptic or pre-synaptic inhibition or a form of tonic inhibition [25]. Tonic inhibition is generated by extrasynaptic α5 subunit-containing GABAA receptors (α5-GABAA receptors), among others, in the brain [7,25,32,62,63] and particularly in the spinal cord [14,21,41]. It has been demonstrated that tonically active α5-GABAA receptors modulate dorsal root reflexes by affecting excitability via tonic depolarization, suggesting that they might play an important role in pain [21,41,62]. Consistent with this hypothesis, we previously demonstrated that α5-GABAA receptor blockade leads to antinociception in the rat formalin test [11]. Notably, α5-GABAA receptors have been localized at the dorsal horn in mice and rats [43,53] and are also found in DRG and peripheral nerve fibers in turtles [41], making them well placed to modulate the nociceptive process. However, the role of α5-GABAA receptors in chronic inflammatory or neuropathic pain is unclear. Here we used formalin-, Complete Freund’s adjuvant (CFA)- and spinal nerve ligation-induced long-lasting hypersensitivity to assess the possible involvement of α5-GABAA receptors in central disinhibition and chronic pain.
Materials and Methods
Animals
These experiments were carried out in female Wistar rats. We did not find significant differences between formalin- and CFA- (unpublished data) or spinal nerve injury [12]-induced responses between male and female rats. For the evaluation of formalin- and CFA-induced long-lasting hypersensitivity, rats at 8–10 weeks of age (body weight 180–200 g) were used. In the case of the L5/L6 spinal nerve ligation model, rats of 6 weeks (140–160 g) were used. Animals had free access to food and drinking water before the experiments. All experiments followed the Guidelines of Ethical Standards for Investigation of Experimental Pain in Animals [76] and were approved by our local Ethics Committee (Cinvestav, Mexico City) and by the Institutional Animal Care and Use Committees of the University of California, San Diego. Efforts were made to minimize the number of animals used.
Induction and assessment of long-lasting secondary allodynia and hyperalgesia
Rats were restrained gently in order to inject formalin (0.5% or 1%) or Complete Freund’s adjuvant (CFA) subcutaneously (s.c.) into the dorsal surface or the plantar surface on the right hind paw, respectively. Formalin-induced mechanical secondary allodynia and hyperalgesia starts 1 day after injection and lasts at least 12 days [1,15,28,30]. CFA-induced tactile allodynia starts at 6 h [44] and lasts at least 18 days [33]. Formalin-induced sensitization was assessed 3 and 6 days after injection [1,2,44]. CFA- or spinal nerve ligation-induced tactile allodynia was assessed at 3, 7 and 14 days later. At the end of the experiment, rats were sacrificed in a CO2 chamber.
In order to assess mechanical secondary allodynia and hyperalgesia or tactile allodynia, rats were placed into testing cages on a wire mesh bottom and allowed to acclimate for 30 min. Baseline measurements were recorded by applying two von Frey filaments (Stoelting Co, Wood Dale, IL, USA, bending forces of 10 mN [1 g] and 250 mN [26 g]) to the base of the third toe on the plantar surface of both paws 10 times during each testing period to determine the response frequency for each filament. Three trials were completed to determine the paw response frequency [10,27]. A force of 10 mN does not activate cutaneous nociceptors in naïve rats [40]; therefore, it does not lead to a paw withdrawal. Responses to the 10 mN filament are therefore a clear indicator of the presence of secondary allodynia. On the other hand, a force of 250 mN or more is considered a noxious stimulus [40] and hyperalgesia occurs when there is an increased response to these stimuli. Formalin-induced allodynia and hyperalgesia were considered secondary, as stimuli with the von Frey filaments were applied in a site different from that of the formalin injection.
Assessment of tactile allodynia in spinal nerve ligated or CFA treated rats was carried out using a previously reported method [17].
Intrathecal catheterization
Spinal catheterization was performed 5 days before injection of formalin, CFA or L5/L6 spinal nerve ligation by using a previously reported method [42]. Briefly, rats were anesthetized with a ketamine/xylazine mixture (45:12 mg/kg, i.p.). Then, they were placed in a stereotaxic head holder, the atlanto-occipital membrane exposed and pierced in order to insert a polyethylene catheter (PE-10, 7.5–8 cm length) and moved towards the thoracolumbar region. Rats were housed and allowed to recover for 5 days before drug administration and testing.
Drugs
L-655,708 (ethyl(S)-11,12,13,13a-tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate) and CFA were purchased from Sigma-Aldrich (St. Louis, MO). L-838,417 (3-(2,5-difluorophenyl)-7-(1,1-dimethylethyl)-6-[(1-methyl-1H-1,2,4-triazol-5-yl)methoxy]-1,2,4-triazolo[4,3-b]pyridazine) was purchased from Tocris Bioscience (Elisville, MO). All drugs were diluted in 20% dimethyl sulfoxide (DMSO). Formaldehyde (37%) was purchased from Merck Mexico, S.A. (Naucalpan, Mexico) and diluted in saline.
The drugs were chosen based on the availability, selectivity and efficacy as follows: i) L-655,708, a selective α5-GABAA receptors antagonist (pKi 9.3) [57] and ii) L-838,417, an α2-, α3- and α5-GABAA receptors partial agonist with a pKi 8.6 for α5-GABAA receptors [46].
Quantitative reverse transcription PCR (RT-qPCR)
Rats were sacrificed by decapitation, the dorsal spinal cord of the lumbar section was divided by a scalpel incision to enable the ipsilateral (injured) and contralateral (uninjured) sides to be identified. The ipsilateral L4-L6 DRGs were also excised. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was quantified by spectrophotometry using an absorbance of 260 nm (ThermoSpectronic, Genesys 10 UV, Madison, WI) and the sample purity ratios were calculated (260/280 nm). For cDNA synthesis, 5 μg of total RNA was subjected to reverse transcription with random hexanucleotides and the M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions. The qPCR were carried out using the TaqMan Universal PCR Master Mix in a total volume of 25 μl, containing 900 nM of each oligonucleotide, 250 nM of the Gabra 5 predesigned probe (TaqMan®, Applied Biosystems®, Foster City, CA) and 10 μl of cDNA (dilution 1:4). Amplification was performed in the CFX96 real-time PCR detection system equipment (Bio-Rad, Hercules, CA) as follows: an initial step of 50°C for 2 min and 95°C for 10 min, followed by 60 cycles of 92°C for 15 s and a final step of 60°C for 1 min. The messenger RNA (mRNA) expression was quantified by the 2ΔΔct method normalized to r18S (Applied Biosystems® TaqMan® Ribosomal RNA Control Reagents, Foster City, CA).
Immunohistochemistry
Rats were euthanized with CO2 and perfused intra-aortically with 300 mL of ice-cold, oxygenated artificial cerebrospinal fluid (ACSF) [containing (mM): NaCl 125, KCl 2.5, CaCl2 2.5, MgCl2 2, NaHCO3 26, NaH2PO4 1.25, and glucose 25], pH 7.4, at a flow rate of 18–25 mL/min. Animals were decapitated, the spinal cord obtained by hydro-extrusion with ice-cold ACSF and the ipsilateral L5 DRGs were excised. The lumbar section and the DRGs were dropped into ice-cold freshly prepared fixative [4% paraformaldehyde dissolved in 0.15 M sodium phosphate buffer, pH 7.4] and post-fixed during 60 min, rinsed with phosphate buffer (PBS), cryoprotected by 72 h in 30% sucrose in PBS, embedded in OCT compound (Tissue-Tek; Sakura Finetek, Torrance, CA), frozen with dry ice and stored at − 80°C [49].
Sections were cut from frozen blocks with a cryostat at a thickness of 30 μm for the spinal cord and 12 μm for the DRGs. The sections were mounted onto gelatin-coated slides. Pap-pen (Super Pap-Pen, Cat. # h2802, EB Sciences, East Granby, CT) was used to draw a hydrophobic ring around the sections and the slides were allowed to air-dry overnight at room temperature. Sections were first washed 3 times with TBS + 0.2% TX-100 pH 7.4, followed by 2 washes of 5 min each with 0.5% of sodium borohydride (NaBH4, Cat. # 102894, MP Biomedicals, Santa Ana, CA), followed by a blocking step with 4% normal donkey serum in TBS + 0.2% TX-100 for 1 h. Finally, the sections were incubated in blocking solution with the primary antibodies overnight at 4°C. Immunodetection of α5-GABAA receptors in the spinal cord was performed using an antibody generously provided by Dr. Jean-Marc Fritschy (dilution 1:3000) raised against extracellular epitopes located on the N-terminal segment of proteins in guinea pig serum [26]. Specificity of this antibody has been recently reported using conditional Gabra5 knock-out mice [60].
For immunodetection in DRGs, we used an antibody for the α5-GABAA receptors (rabbit anti-α5-GABAA, Cat. # SAB2100878; dilution 1:200, Sigma-Aldrich, St. Louis, MO) [41]. In order to determine the specific cellular and subcellular distribution of the α5-GABAA receptors in the spinal cord and DRG, we performed double staining with the following markers: NeuN was used as a specific neuronal marker (chicken anti-NeuN, Cat. # ABN91, dilution 1:1000, Millipore, Bilerica, MA); CGRP was used as a specific marker of peptidergic neurons (goat anti-CGRP, Cat. # Ab36001, dilution 1:1000, Abcam, Cambridge, MA); Lectin IB4 conjugated to AlexaFluor 647 (anti-IB4-AlexaFluor 647, Cat. # I32450, dilution 1:2000, Molecular Probes, Eugene, OR) was used to label non-peptidergic neurons; Iba1 was used to identify microglia (rabbit anti-Iba1, Cat. # 019-19741, dilution 1:1000, Wako, Richmond, VA), and GFAP was used to identify astrocytes (mouse anti-GFAP, Cat. # C9205, dilution 1:2000, Sigma Aldrich, St. Louis, MO). Sections were washed in TBS + 0.2% TX-100 and incubated for 60 min at room temperature with the corresponding secondary antibodies conjugated to Cy3 (Cy™3 AffiniPure Donkey Anti-Guinea Pig IgG [H+L], Cat. # 706-165-148, dilution 1:1000, Jackson ImmunoResearch, West Grove, PA) and/or Alexa 488 (Donkey anti-Goat IgG (H+L) secondary antibody, Alexa Fluor 488 conjugate, Cat. # A-11055, dilution 1:1000, Invitrogen, Carlsbad, CA), and/or Alexa 549 (Donkey anti-Rabbit IgG [H+L] secondary antibody, Alexa Fluor 594 conjugate, Cat. # A-21207, dilution 1:1000, Invitrogen, Carlsbad, CA). Sections were washed again and cover-slipped with ProLong® Gold antifade reagent with DAPI (Life technologies, Carlsbad, CA).
Immunofluorescence and confocal microscopy
Fluorescence images were captured using an immunofluorescence microscope (Zeiss AxioImager M2 Microscope, Irvine, CA) at the same exposure settings and fluorescent lamp intensity using 10x and 20x objectives and with the use of Stereo investigator software (MBF Bioscience, Williston, VT). Mosaic images were captured from dorsal spinal cord at the desired wavelength/spectrum of light from each independent channel in order to obtain the best focal plane. Image J was used to perform the desired merge for each double-staining. Confocal images were taken using an Olympus FV1000 microscope (Olympus FluoView FV1000 Confocal Microscope, Melville, NY) with 63X oil immersion. In all cases sequential scanning with the 488, 543, and 647 nm laser lines was used to capture a random scan field (2–17 optical layers, z-separation 0.2–0.5 μM) and visualized in FV10-ASW, version 4 software (Olympus, Tokyo). Image J was used to construct images from 1 optical layer to show co-localization between the structures of interest in the respective double-staining.
To determine percentages of DRG neuron subpopulations, five randomly selected sections were counted for the number of labeled neurons as a percentage of the total (NeuN) neurons. Neuron sizes were determined manually using Image J software. For histological quantification of immunostained DRG and spinal cord sections, 5 sub-serial L4-L6 segments of spinal cord and L5 DRG sections were used. Mean fluorescence intensity and positively-stained elements were determined using Image J software [50, 69].
Rate-dependent depression of the Hoffmann reflex
The Hoffmann reflex was recorded as previously described [9,35]. Briefly, under ketamine anesthesia (100 mg/kg/h, i.m.), the right hind limb of the animal was secured and a pair of stimulating needle electrodes were inserted transcutaneously into the surroundings of the tibial nerve. For recording, a pair of silver needle electrodes was inserted into the interosseous muscles between the fourth and the fifth, or the first and the second metatarsal muscles of the right hind paw, and a grounding electrode was placed into the tail. The tibial nerve was stimulated using square pulses with increasing intensity (0.1–10 mA in 0.5 mA increments, 0.1 Hz, 0.2 ms, Isostim A320, WPI Inc., Sarasota, FL). Responses were recorded with an A/C-coupled differential amplifier (Model DB4; DPI, Sarasota, FL) and used to determine the intensity necessary to obtain a maximal M and H response. Then, we used this intensity to apply trains of 20 pulses at 0.1, 0.5, 1, 5 and 10 Hz in order to measure rate-dependent depression (RDD) of the Hoffmann reflex and changes in RDD at different stimulation frequencies. We used the 1 Hz stimulation frequency for data analysis. This frequency was chosen because it was associated with an approximately 40% decrease in the amplitude of the H-wave between the first and second stimulus in normal rats [39]. These allow detection of subsequent increase or attenuation in response to drug administration. RDD of the Hoffmann reflex was calculated as the percent change in the amplitude of the H-wave (% Baseline H reflex) evoked by the second stimulation (H2) compared to the H-wave amplitude evoked by the first stimulus (H1).
Data analysis and statistics
Behavioral data are expressed as mean ± SEM (n≥6) of the hind paw withdrawal response.
qPCR data are expressed as α5-GABAA receptors mRNA relative expression normalized with r18S. mRNA data are expressed as the mean ± SEM of 3 independent animals.
The histological data are expressed as the mean ± SEM of the α5 or NeuN positive neurons plotted as % of total DRG L5 neurons depending of the size or as the α5 mean fluorescence intensity.
RDD of the Hoffmann reflex and the effect of treatment are expressed as the mean ± SEM of 5 independent animals and plotted as % of baseline H reflex.
Statistical differences between groups were determined by one- or two-way analysis of variance (ANOVA), followed by the Student–Newman–Keuls test for post-hoc comparison. P values less than 0.05 (P<0.05) were considered significant.
Results
Peripheral and spinal α5-GABAA receptors are involved in formalin-induced secondary allodynia and hyperalgesia
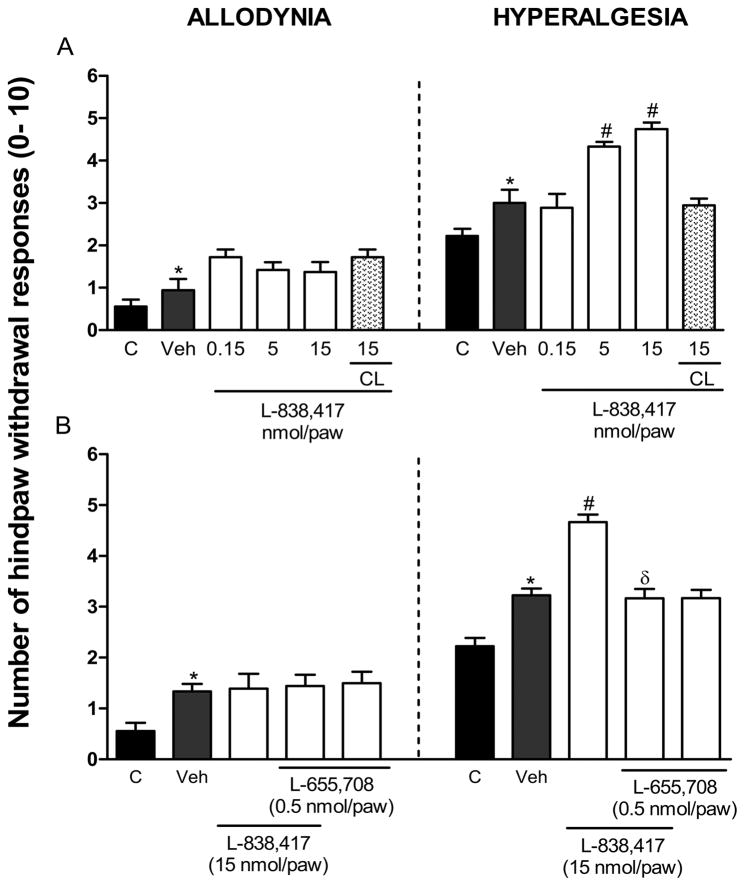
Local peripheral ipsilateral, but not contralateral, pre-treatment with the α5-GABAA receptors partial agonist L-838,417 increased 0.5% formalin-induced secondary hyperalgesia (F7,44=17.323, P=0.001) (Fig. 1A). In contrast, the same treatment did not modify secondary allodynia. In order to investigate if the peripheral pronociceptive effect of L-838,417 was due to activation of α5-GABAA receptors, we studied the effects of this agonist in presence of the α5-GABAA receptors antagonist L-655,708. L-655,708 (0.5 nmol/paw) completely prevented L-838,417-induced increase in secondary hyperalgesia (F4,28=27.433, P=0.001) (Fig. 1B). The above dose of antagonist, which did not affect formalin-induced secondary allodynia and hyperalgesia per se (Fig. 1B), is supposed to completely block α5-GABAA receptors [66].
Figure 1.
Peripheral α5-GABAA receptors are involved in the development of formalin-induced long-lasting secondary hyperalgesia. Effect of pre-treatment (10 min) with the α2-, α3- and α5-GABAA receptors partial agonist L-838,417 (panel A) and effect of L-655,708 (0.5 nmol/paw) on the pronociceptive effect produced by L-838,417 (15 nmol/paw) in 0.5% formalin-induced mechanical secondary allodynia and hyperalgesia (panel B). Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the applications of von Frey filaments (10 and 250 mN) to the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus control group, #P<0.05 versus Veh group, and &P<0.05 versus L-838,417 group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test.
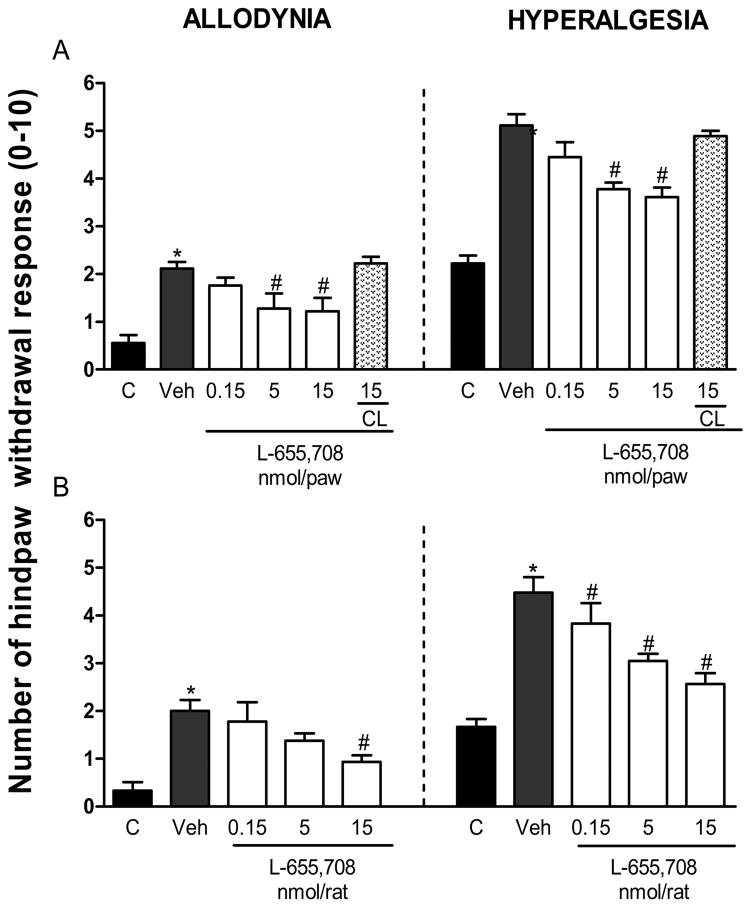
In order to investigate whether the α5-GABAA receptors are involved in development of formalin-induced mechanical secondary allodynia and hyperalgesia, we assessed the effect of pre-treatment with L-655,708. Ipsilateral, but not contralateral, local peripheral pre-treatment with L-655,708 (0.15–15 nmol/paw) prevented 1% formalin-induced secondary allodynia (F5,30=6.553, P=0.001) and hyperalgesia (F5,30=10.733, P=0.001) at 6 days post-formalin injection (Fig. 2A). Moreover, intrathecal (10 μl, into the subarachnoid space) pre-treatment with L-655,708 (0.15–15 nmol/rat), but not vehicle (20% DMSO), prevented secondary allodynia (F4,31=8.388, P=0.001) and hyperalgesia (F4,31=14.906, P=0.001) at the days post-formalin injection (Fig. 2B). Interestingly, ipsilateral, but not contralateral, peripheral pre-treatment with the greatest dose of L-655,708 (15 nmol/paw) also prevented formalin-induced secondary allodynia (F2,16=23.969, P=0.001) and hyperalgesia (F2,16=34.592, P=0.001) at 3 days post-formalin injection (Supplementary Fig. 1A). Likewise, intrathecal pre-treatment with L-655,708 (15 nmol/rat) avoided formalin-induced secondary allodynia, (F2,16=14.039, P=0.001) and hyperalgesia (F2,15=11.575, P=0.001) at 3 days post-formalin injection (Supplementary Fig. 1B).
Figure 2.
Local peripheral and spinal α5-GABAA receptors are involved in the development of formalin-induced long-lasting secondary allodynia and hyperalgesia. Effect of local peripheral (panel A) and intrathecal (panel B) pre-treatment (10 min) with the selective α5-GABAA receptors antagonist L-655,708 (0.15–15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the application of von Frey filaments (10 and 250 mN) to the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
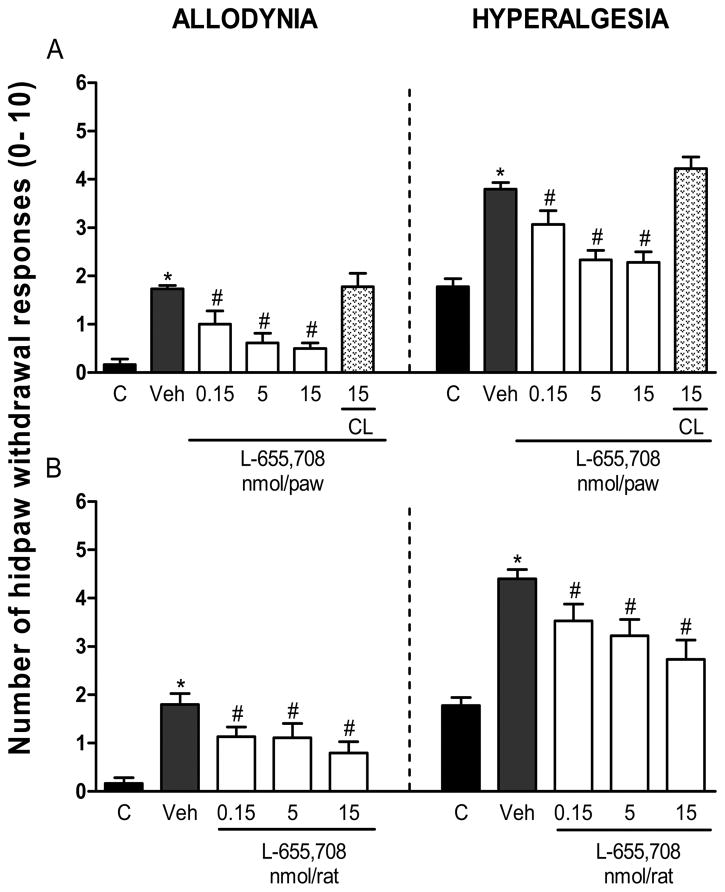
In order to investigate whether the α5-GABAA receptors are involved in maintenance of formalin-induced mechanical secondary allodynia and hyperalgesia, we studied the effect of L-655,708 (0.15–15 nmol) as post-treatment (6 days after formalin injection). The effect was assessed 1 h after drug injection. In these conditions, local peripheral (Fig. 3A, allodynia: F5,28=11.933, P=0.001; hyperalgesia: F5,28=21.018, P=0.001) or intrathecal (Fig. 3B, allodynia: F4,22=7.283, P=0.001; hyperalgesia: F4,22=10.698, P=0.001) administration of L-655,708 reversed formalin-induced long-lasting secondary allodynia and hyperalgesia. Furthermore, local peripheral (Supplementary Fig 2A, allodynia, F3,20=13.375, P=0.001; hyperalgesia, F3,20=17.863, P=0.001) or intrathecal (Supplementary Fig 2B, allodynia, F2,15=41.155, P=0.001; hyperalgesia, F2,15=54.076, P=0.001) post-treatment, at 3 days after formalin injection, with the greatest dose of L-655,708 (15 nmol) reversed formalin-induced secondary allodynia and hyperalgesia. Neither vehicle nor contralateral treatment, at the greatest dose used, modified formalin-induced long-lasting nociceptive behaviors, suggesting a drug-specific effect as well as a local peripheral effect, respectively. Also, intrathecal pre-treatment (Supplementary Fig. 3A) or post-treatment (Supplementary Fig. 3B) with L-655,708 (15 nmol) did not have any effect in naïve rats.
Figure 3.
Peripheral and spinal α5-GABAA receptors are involved in the maintenance of formalin-induced long-lasting secondary allodynia and hyperalgesia. Effect of peripheral (panel A) and intrathecal (panel B) post-treatment (6 days after 1% formalin injection) with the selective α5-GABAA receptors antagonist L-655,708 (0.15–15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the applications of von Frey filaments (10 and 250 mN) on the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
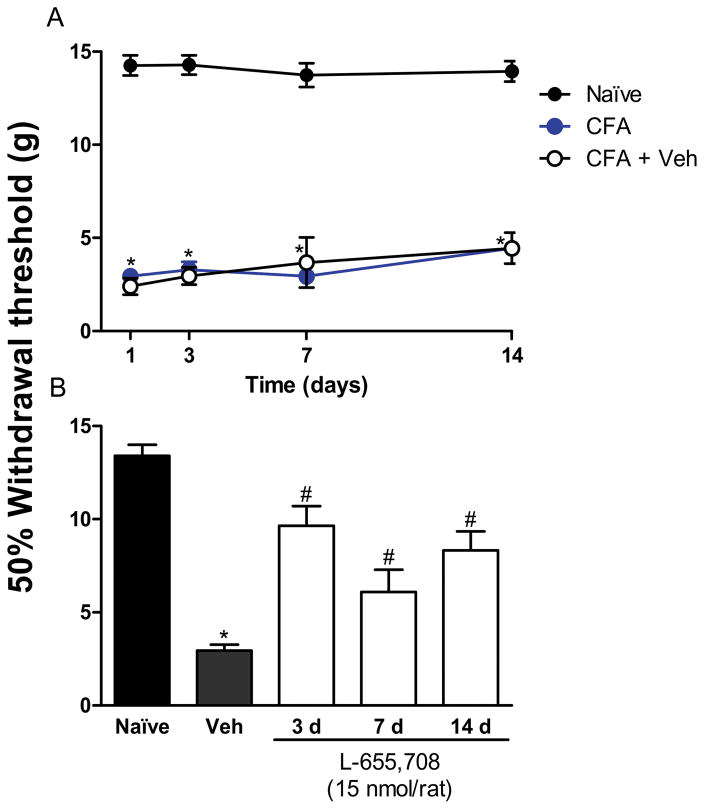
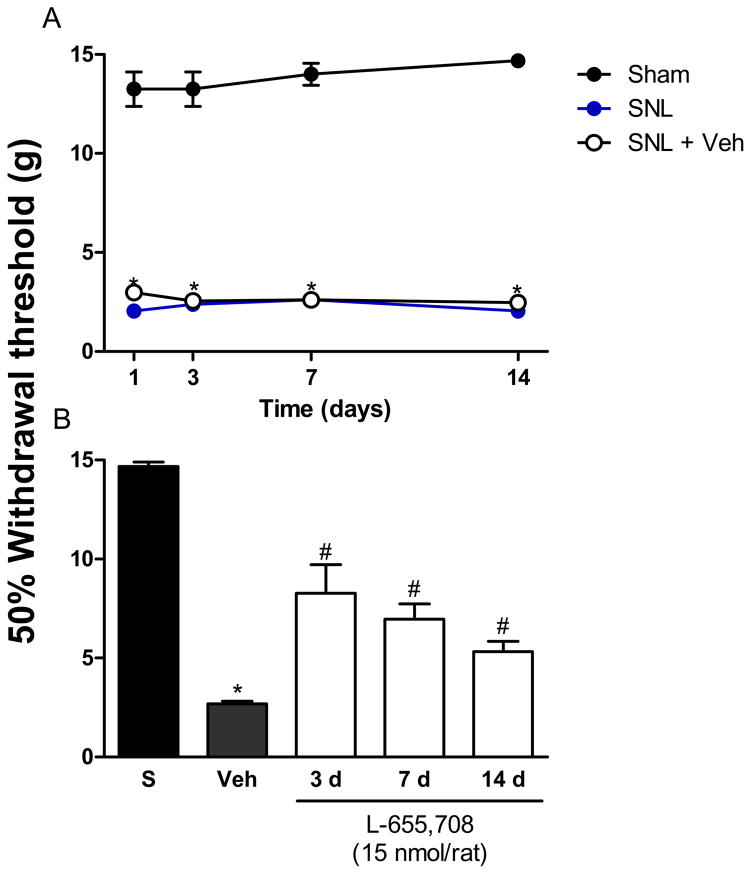
To further demonstrate that α5-GABAA receptors participate in chronic nociception, we determined the effects of post-treatment with L-655,708 in rats injected with CFA or subjected to spinal nerve ligation at different times. As in the formalin model, the effect was assessed 1 h after drug injection. Intraplantar injection of CFA or L5/L6 spinal nerve ligation diminished withdrawal threshold from ~14 to ~3 g, which was interpreted as tactile allodynia. Intrathecal injection of L-655,708 (15 nmol) at 3, 7 and 14 days after CFA injection reversed tactile allodynia (Fig. 4, F3,25=47.81, p=0.0001). This intrathecal dose was also able to reverse spinal nerve ligation-induced tactile allodynia at the same time points after nerve injury (Fig. 5, F4,24=222.6, p=0.001).
Figure 4.
α5-GABAA receptors are involved in the development and maintenance of CFA-induced tactile allodynia. Time-course of the 50% withdrawal threshold in naïve and CFA-treated rats (panel A). Effect of intrathecal administration of the selective α5-GABAA receptors antagonist L-655,708 on CFA-induced tactile allodynia (panel B). Withdrawal threshold was assessed 1 h post-drug administration at 3, 7 and 14 days after CFA injection. Data are presented as the 50% withdrawal threshold for 6 animals ± SEM. *P<0.05 versus sham group, #P<0.05 versus Veh group, by one- or two-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CFA: Complete Freund’s adjuvant); Veh: Vehicle.
Figure 5.
α5-GABAA receptors are involved in the development and maintenance of neuropathic pain in rats. Time-course of the 50% withdrawal threshold in sham and spinal nerve ligated rats (panel A). Effect of intrathecal administration of the selective α5-GABAA receptors antagonist L-655,708 on spinal nerve ligated-induced tactile allodynia (panel B). Withdrawal threshold was assessed 1 h post-drug administration at 3, 7 and 14 days after spinal nerve ligation. Data are presented as the mean withdrawal threshold for 6 animals ± SEM. *P<0.05 versus sham group, #P<0.05 versus Veh group, by one- or two-way ANOVA followed by the Student–Newman–Keuls post-hoc test. SNL: L5/L6 spinal nerve injury (SNL); S: Sham (S); Veh: Vehicle.
Expression of α5-GABAA receptors in spinal cord and DRG
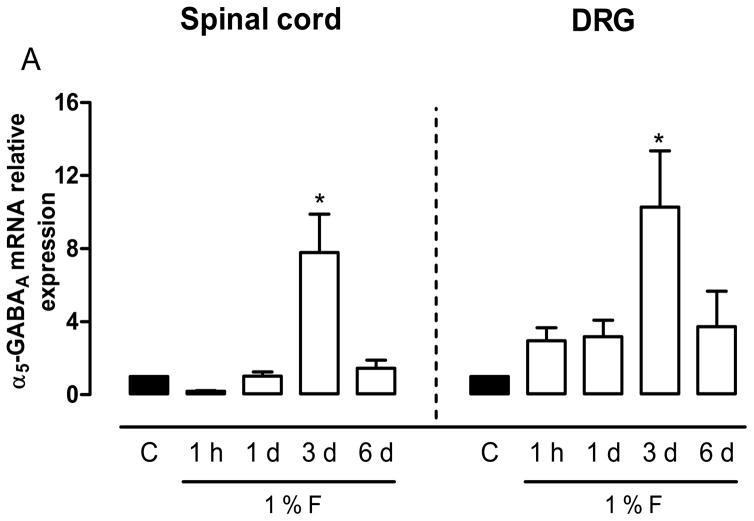
Pharmacological data suggest the presence of α5-GABAA receptors in DRG and spinal cord. Thus, we measured their expression in rats at 1 h and 1, 3 and 6 days after 1% formalin injection by qPCR. α5-GABAA receptors mRNA was detected in the ipsilateral dorsal spinal cord and L4-L6 DRGs (Fig. 6). In addition, formalin injection increased α5-GABAA receptors mRNA levels mainly at 3 days after formalin injection in dorsal spinal cord (F4,15=10.26, P=0.001) and DRG (F4,15=4.07, P=0.02) (Fig. 6).
Figure 6.
Expression of α5-GABAA receptors mRNA is modulated by formalin injection. qPCR data for mRNA relative expression of the α5-GABAA receptors in the dorsal spinal cord and L4-L6 DRGs at 1 h and 1, 3 and 6 days after 1% formalin injection. Data are expressed as mean ± SEM of 3 independent animals. *P<0.05 versus control (C), by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test.
Distribution of α5-GABAA receptors in dorsal horn, DRG and peripheral afferent fibers
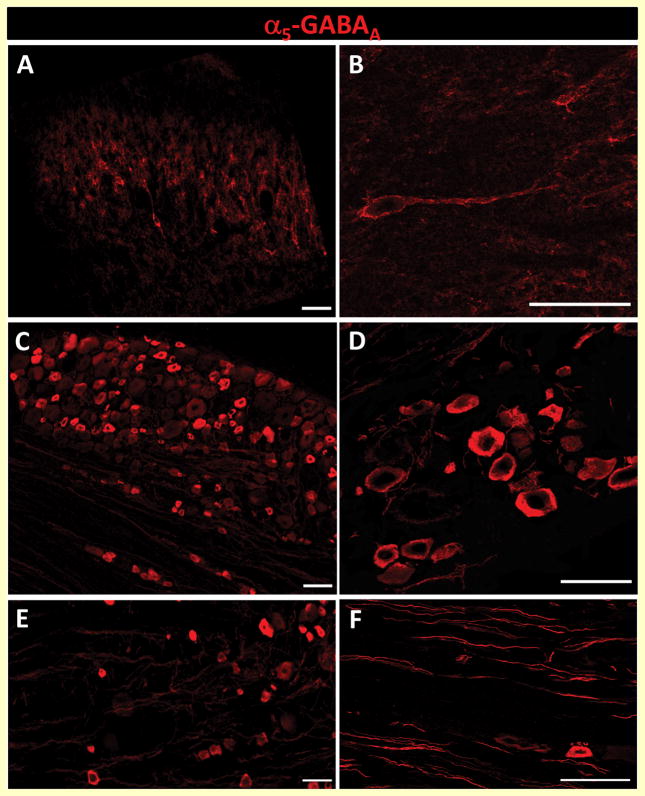
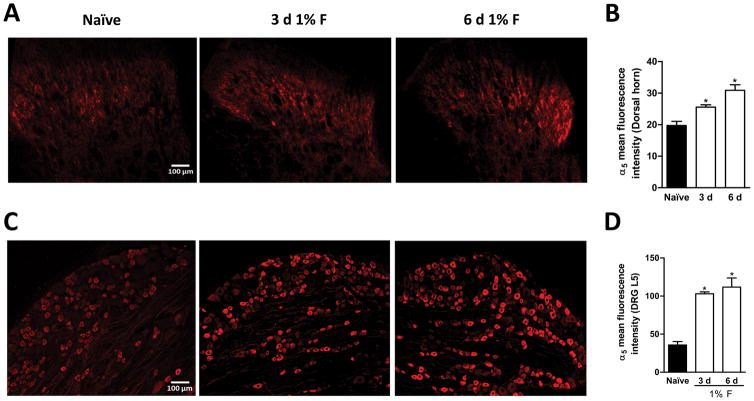
Based on our pharmacological and molecular results, we decided to investigate the distribution of α5-GABAA receptors in dorsal horn spinal cord, DRG and peripheral axons. In agreement with previous reports [9,53], we found α5-GABAA receptors immunoreactivity mainly in lamina II, III, IV of the dorsal horn (Fig. 7A). Furthermore, histological analysis revealed the presence of α5-GABAA receptors in neuronal bodies and neuropil suggesting their presence in specific subsets of neurons and in primary afferent fibers (Figs. 7A/7B). As reported in turtles [41], there was α5-GABAA receptors immunoreactivity in DRG (Figs. 7C/7D) and peripheral afferent fibers of the rat (Figs. 7E/7F). Of note, 1% formalin enhanced α5-GABAA receptors fluorescence intensity in dorsal spinal cord (Figs. 8A–B, F2,6=17.42, P=0.001) and L5 DRG (Figs. 8C–D, F2,12=30.19, P=0.001) at 3 and 6 days after injection.
Figure 7.
α5-GABAA receptors are expressed in the dorsal horn and DRG. Representative images of the transverse section of the spinal dorsal horn (panels A and B), sagittal sections of DRG (panels C and D) and primary afferent fibers (panels E and F) showing the distribution of α5-GABAA receptors immunoreactivity (scale bar: 50 μm).
Figure 8.
Formalin injection modulates α5-GABAA receptors immunoreactivity. Representative staining of α5-GABAA on the dorsal spinal cord (panel A) and L5 DRG (panel C) from naïve (left) and formalin-treated rats at 3 (middle) and 6 days (right) after injection. Quantification of immunofluorescence signals for α5-GABAA receptors in naïve and 3 and 6 days after 1% formalin injection (5 sections from 3 independent animals) in the dorsal spinal cord (panel B) and L5 DRG (panel D). *P<0.05 versus naïve, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test.
Expression pattern of α5-GABAA receptors in dorsal horn
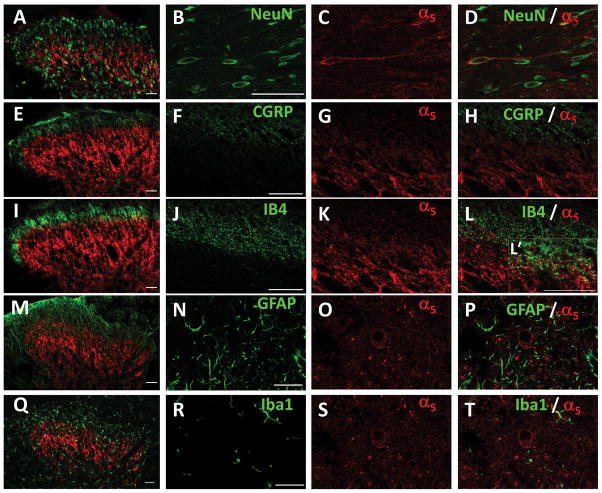
In order to investigate the expression pattern of α5-GABAA receptors, we performed multiple immunolabeling experiments in defined cellular compartments of the dorsal horn. These included neuronal bodies (NeuN), peptidergic (CGRP) and non-peptidergic (IB4) small afferent terminals as well as astrocytes (GFAP) and microglia (Iba1). The specificity of the primary antibodies was confirmed through the immunostaining in the absence of the respective primary antibodies (data not shown). We observed specific neuronal staining for NeuN in the dorsal horn that co-localized with that of α5-GABAA receptors (Figs. 9A–D). We did not observe co-localization of α5-GABAA receptors with CGRP suggesting that these receptors are absent in peptidergic terminals (Figs. 9E–H). In contrast, α5-GABAA receptors co-localized with IB4 suggesting their presence in non-peptidergic afferent terminals (Figs. 9I–L). α5-GABAA receptors did not co-localize with GFAP (Figs. 9M–P) nor Iba1 (Figs. 9Q–T) suggesting their absence in astrocytes and microglia, respectively.
Figure 9.
Distribution of α5-GABAA receptors in dorsal horn spinal cord. Panels A, B, C and D: NeuN (green), α5-GABAA receptors (red) and its co-expression (yellow) in dorsal horn neurons. Of note, α5-GABAA receptors immunoreactivity is present on the membrane and along the axon. Panels H E, F, G and: CGRP (green) and α5-GABAA receptors (red) immunoreactivity is distributed in two different populations of fibers in the dorsal horn. I, J, K, L and L’: IB4 (green), α5-GABAA receptors (red) and their co-expression (yellow) in non-peptidergic fibers. Panels M, N, O and P: GFAP (green) is expressed in astrocytes throughout the spinal cord and α5-GABAA receptors (red) are localized in specific primary afferents fibers and second order neurons. Panels Q, R, S and T: Iba1 (green) is expressed in microglia throughout the spinal cord and α5-GABAA receptors (red) are localized in specific primary afferents fibers and second order neurons. Some α5-GABAA receptors immunoreactive fibers are in close contact with Iba1 microglia (panels Q and T). Scale bar: 50 μm. Confocal images: one optical section 0.2 μM.
Expression pattern of α5-GABAA receptors in DRG and axons
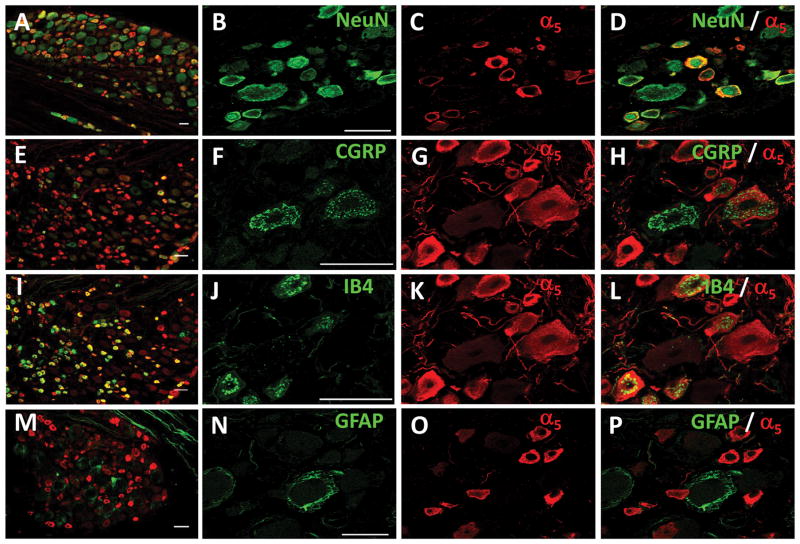
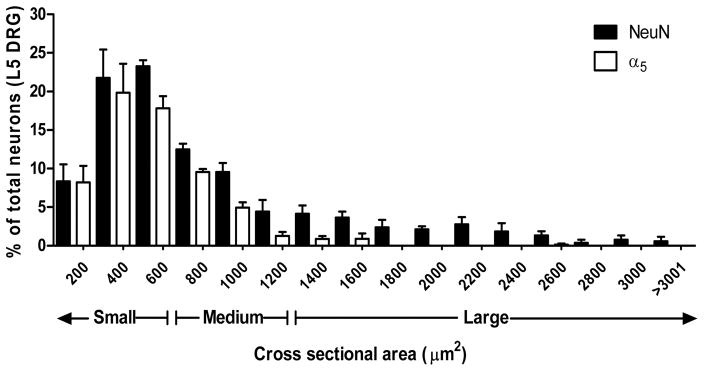
The same staining combinations were performed in DRG. Consistently with the dorsal horn co-labeling, α5-GABAA receptors were observed in small- and medium-sized body neurons (Figs. 10A–D), peptidergic (Figs. 10E–H, 54.7% ± 4.6) and non-peptidergic (Figs. 10I–L, 77.3% ± 4.6) neurons. α5-GABAA receptors did not co-localize with GFAP suggesting that these receptors are absent in satellite cells (Figs. 10M–P). α5-GABAA receptors immunoreactivity was mainly found in small- and medium-size DRG neurons (Fig. 11).
Figure 10.
Distribution of α5-GABAA receptors in DRG. Panels A, B, C and D: NeuN (green), α5-GABAA receptors (red) and their co-localization (yellow) in small-to medium size neurons. Panels E, F, G and H: CGRP (green) and α5-GABAA receptors (red) immunoreactivity are distributed in small to medium size neurons, some neurons are immuoreactive for both. Panels I, J, K and L: IB4 (green), α5-GABAA receptors (red) and their co-expression (yellow) in non-peptidergic small to medium size neurons. Panels M, N, O and P: GFAP (green) is expressed in satellite glial cells and α5-GABAA receptors (red) are localized in specific non-peptidergic small to medium size neurons. Scale bar: 50 μm. Confocal images: one optical section 0.2 μM.
Figure 11.
Size distribution data of α5-GABAA receptors + and NeuN + neurons in L5 DRG. Data were obtain from 5 sections of 3 naïve animas using imageJ software to manually measure cross-sectional area of neurons in 12 μm-thick sections. Data are plotted as % of total number of neurons.
Formalin injection produces GABAergic disinhibition
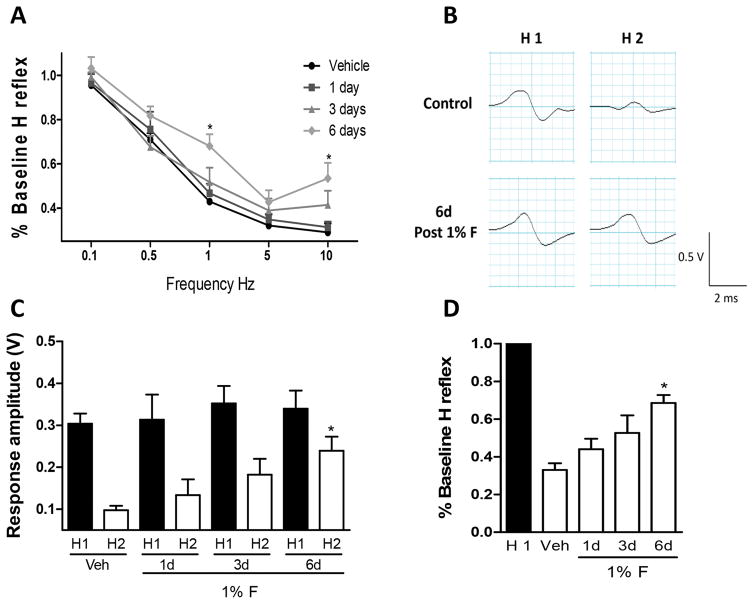
It has been proposed that RDD, mediated by GABAA receptors in normal rats, may be used as a maneuver to investigate alterations in pre- and post-synaptic GABAergic inhibition produced during neuropathic pain [34,35,39]. We, therefore, decided to determine whether RDD of the Hoffmann reflex is modified by formalin injection and the involvement of α5-GABAA receptors in this action. RDD of the Hoffmann reflex was determined at 1, 3 and 6 days after formalin injection. In order to avoid the effect of repetitive electrode insertions, 5 independent groups were used. M-waves were unchanged by stimulation in both vehicle- and formalin-treated rats (data not shown). In contrast, formalin injection impaired the reduction of the RDD of the Hoffmann reflex 6 days after injection, compared to the control group (Figs. 12A–B, F3,130=10.41, P=0.0001). As reported earlier [2], long-lasting secondary allodynia and hyperalgesia was well established at this time after formalin injection. Amplitude of the Hoffmann reflex (H1) was not significantly modified by the formalin injection at any day, suggesting that formalin did not alter the intrinsic excitability of motor neurons (Fig 12C). In contrast, formalin clearly induced a time-dependent impairment of RDD of the Hoffmann reflex (Fig. 12D, F4,40=21.6, P=0.001).
Figure 12.
Formalin-induced impairment of the rate dependent depression (RDD) of the Hofmann reflex. Panel A: % Baseline of the Hofmann reflex showing the presence (control rats) and loss (1, 3 and 6 days after 1% F) of RDD induced by the increment of stimulation frequency (0.1–10 Hz). Note the significant impairment of RDD at 1 Hz measured 6 days after formalin treatment, as compared to control rats. Panel B: Representative recording in one control rat (top, control) and 6 days post-formalin injection (bottom, 6 days, post 1%F) comparing the first stimulus (H1) versus the second stimulus (H2) at a 1 Hz. Note that the amplitude of H2 was not different from that of H1 in the formalin-treated rats. Panel C: Response amplitude comparing (H1) versus (H2) in rats treated with vehicle or 1, 3, 6 days after formalin injection (1%F). Note that H1 does not change with time or formalin treatment while H2 is restored in a time-dependent manner. Panel D: Time-dependent impairment of the RDD of the Hofmann reflex (H reflex) in H2 compared to % Baseline H reflex normalized to H1 in rats treated with vehicle or 1, 3, 6 days after formalin injection. Data are expressed as the mean (n= 7 rats) ± SEM. *P<0.05 versus vehicle (Veh), by one-way ANOVA followed by the Student Newman Keuls post-hoc test.
L-655,708 restores RDD of the Hoffmann reflex in formalin-treated rats
Formalin-treated rats showed long-lasting secondary allodynia/hyperalgesia and impairment of the RDD of the Hoffmann reflex 6 days post-injection. This phenotype is similar to that observed after spinal cord injury or neuropathic pain in rats [39,58]. Since the selective α5-GABAA receptors antagonist L-655,708 prevented and reversed long-lasting formalin-induced secondary allodynia and hyperalgesia, we investigated its effect on RDD of the Hoffmann reflex in formalin-treated rats (Fig. 13). Both intrathecal pre-treatment (F3,14=5.97, P=0.008, Fig. 13B) and post-treatment (F3,17=10.05, P=0.001, Fig. 13C) with L-655,708 prevented and reversed, respectively, formalin-induced alteration of RDD of the Hoffmann reflex.
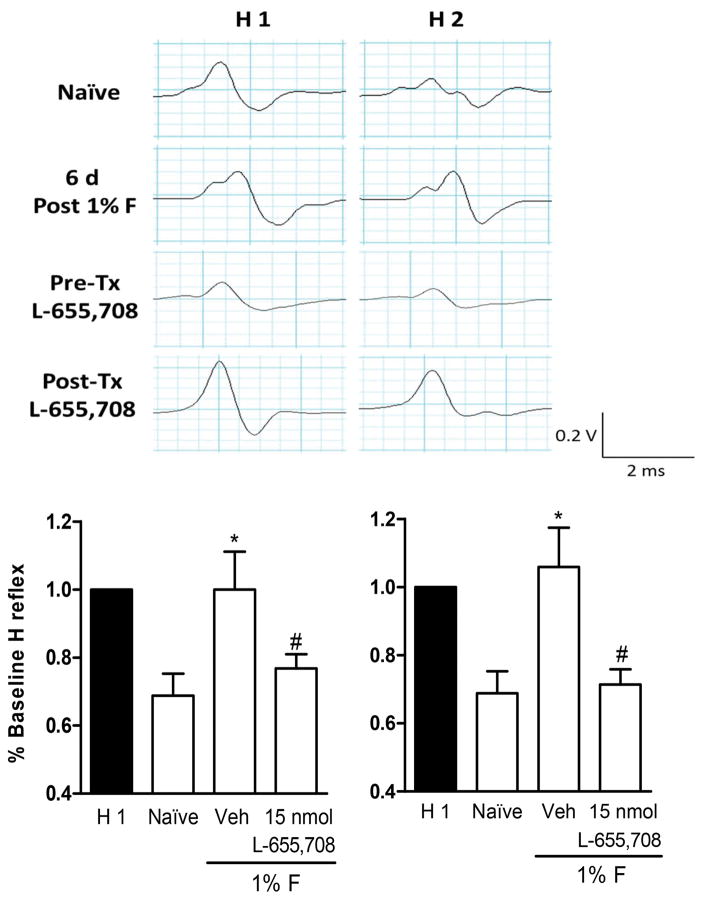
Figure 13.
L-655,708 restores RDD of the Hofmann reflex in formalin-treated rats. Panel A: Representative recordings of RDD of the Hofmann reflex in naïve (first row), 6 days post-formalin injection (second row), pre-treated with L-655,708 + 1% formalin (third row) and post-treated with L-655,708 + 1% formalin (last row) rats. Panel B: Intrathecal L-655,708, delivered as a pre-treatment (10 min), significantly prevented the loss of RDD of the Hofmann reflex 6 days after 1% formalin administration. Panel C: Intrathecal L-655708, delivered as a post-treatment, reversed the loss of RDD of the Hofmann reflex 6 days after 1% formalin administration. Data are expressed as the rate dependent depression of the Hofmann reflex (% baseline H reflex) comparing the H1 versus H2 at 1 Hz for 5 animals per group *P<0.05 versus naïve, #P<0.05 versuss Vehicle (Veh), by one-way ANOVA followed by the Student Newman Keuls post-hoc test.
Discussion
Role of α5-GABAA receptors in formalin-induced secondary allodynia and hyperalgesia
Here we show that α5-GABAA receptors, located at peripheral and spinal sites, are involved in formalin-induced long-lasting secondary allodynia and hyperalgesia. This conclusion is based on the following observations: i) local peripheral injection of L-838,417 (α2-, α3- and α5-GABAA receptor partial agonist) increased formalin-induced long-lasting secondary hyperalgesia; ii) local peripheral L-655,708 (selective α5-GABAA receptor antagonist) diminished the pronociceptive effect of L-838,417; iii) local peripheral or intrathecal injection of L-655,708 prevented and reversed long-lasting formalin-induced hypersensitivity; and iv) local peripheral or intrathecal injection of L-655,708 was effective at 3 and 6 days after formalin injection. To our knowledge, this is the first report showing that peripheral and spinal α5-GABAA receptors play a role in the development and maintenance of the long-lasting secondary hypersensitivity. Our data extend previous observations showing that local peripheral GABAA receptors agonists increase formalin-induced acute nociception and those effects are prevented by the peripheral injection of bicuculline or L-655,708 [11]. In contrast, it has been reported that L-838,417 reduces incision-induced mechanical and thermal hyperalgesia [59]. Though the reason for this discrepancy is unknown, the antinociceptive effect induced by L-838,417 could be mediated via the α2 and/or α3 subunits [23,36,37,52,59], whereas the pronociceptive effect may be mediated via the α5 subunit (this study). In support of our data, intrathecal administration of L-655,708 reversed CFA- or nerve injury-induced tactile allodynia at 3, 7 and 14 days. These data suggest that α5-GABAA receptors are also involved in the development and maintenance of other chronic inflammatory and neuropathic pain models.
The role of GABAA receptors in chronic pain is a matter of debate. In naïve animals, GABAA receptors agonists and antagonists produce antinociception and pronociception, respectively [5,24,65]. However, tissue or nerve injury appears to switch GABAA receptors function [18,19,55]. Our results indicate that the α5-GABAA receptors antagonist L-655,708 produces antinociception implying that GABAergic inhibition might be switched to excitation and/or loss of inhibition [16,55,74,75]. It is worth noting that extrasynaptic α5-GABAA receptors are present in DRG and axons [20,22,48]. In those sites, these receptors could be tonically activated by low extracellular GABA concentrations [25] leading to a tonic state of excitability and lowering the threshold for activation of the dorsal root reflex [41]. In such conditions, the α5-GABAA receptors antagonist L-655,708 could diminish dorsal root reflexes [41] or restore GABAergic inhibition. The net effect could be a reduced excitability of the primary afferents. In support of this, there is evidence that dorsal root reflexes that follow GABAA receptors activation in the spinal cord also occur in the periphery [13].
Expression of α5-GABAA receptors
Besides the pharmacological evidence, our study demonstrates that α5-GABAA receptors mRNA is expressed in the dorsal spinal cord and DRG of naïve rats (Fig. 6). This result agrees with previous observations showing expression of α5-GABAA receptors in those sites [11,54,41,45]. Further, formalin injection increased α5-GABAA receptors mRNA expression in the dorsal spinal cord and DRG of rats mainly at 3 days post-injury. Other studies have reported that peripheral axotomy also enhances α5-GABAA receptors expression but at longer times (14 and 28 days) [71,73]. Differences in the time to increase α5-GABAA receptors mRNA expression could be due to the model (formalin test versus nerve axotomy). Our results suggest that the transient modulation of α5-GABAA receptors could be required for the initiation but also for maintenance of the hypersensitivity. In support of this, behavioral data showed that α5-GABAA receptors play a role in development and maintenance of chronic nociception.
Immunofluorescence studies reveal that α5-GABAA receptors are localized in laminae II–IV, DRG and primary afferent fibers. Their presence in DRG has been reported previously [6,29,41,53,54,64]. Interestingly, formalin increased fluorescence intensity in dorsal spinal cord and DRG at 3 and 6 days after injection. These data agree with the enhancement of α5-GABAA receptors mRNA expression and suggest that these receptors are important in the development and maintenance or formalin-induced hypersensitivity.
We observed immunoreactivity in multipolar neurons that were NeuN-positive and neuropil within non-peptidergic fibers in the dorsal horn. A similar pattern was obtained in DRGs confirming that α5-GABAA receptors are present in small- to medium-size non-peptidergic neurons. We also observed co-localization of α5-GABAA receptors with CGRP, suggesting that these receptors are present in some peptidergic neurons. To our knowledge, this is the first report describing the presence of extrasynaptic α5-GABAA receptors in non-peptidergic and, at a lesser extent, peptidergic neurons in dorsal spinal cord and DRG.
Contribution of α5-GABAA receptors to formalin-induced loss of the RDD of the Hoffmann reflex
In order to address how GABAergic signaling was participating in the development and maintenance of long-lasting secondary allodynia and hyperalgesia, we used the RDD of the Hoffmann reflex as a paradigm. Interestingly, formalin injection impaired, in a time-dependent fashion, the RDD of the Hoffmann reflex. A similar effect has been reported in spinal cord injured and diabetic rats [34,58] and it has been attributed to the inversion of the GABAA receptors function [35,39]. It has been reported that extrasynaptic GABAA receptors mediate tonic inhibition in substantia gelatinosa neurons [67,68] and in primary afferents terminals [41]. The most likely subunit composition of GABAA receptors in substantia gelatinosa neurons mediating tonic inhibition may be α5βxγ2 [4,67]. Furthermore, the charge transfer by the GABAergic tonic current in substantia gelatinosa neurons has been estimated to be 6 times of that carried by phasic inhibition [4], which is similar to that reported in hippocampus and cerebral neurons [25] and suggests a role for tonic inhibition in modulation of neuron excitability. The switch from tonic inhibition to excitation and/or loss of function could disrupt neuronal network activity involved in the control of nociceptive information. Our finding that blockade of α5-GABAA receptors restores the RDD of the Hoffmann reflex suggest that these receptors contribute to GABAA receptors-mediated tonic inhibition switching to excitation and/or loss of function in the long-lasting nociception induced by formalin and, likely, CFA or nerve injury.
At the pre-synaptic level, extrasynaptic α5-GABAA receptors may contribute to these behaviors by tonically depolarizing the axonal membrane, without altering the primary afferent depolarization [41] and facilitating the dorsal root reflex activity which might be reinforced with an over-expression of NKCC1 [31,47]. At the post-synaptic level, extrasynaptic α5-GABAA receptors would tonically depolarize second order neurons contributing to long-lasting nociception. An alternative hypothesis would be that α5-GABAA receptors, on primary afferents, drive increased spinal input and brain derived neurotrophic factor (BDNF) release. Elevated synaptic BDNF drives depression of post-synaptic KCC2 expression and thus the switch to GABA-driven excitation [34]. In support of this, our immunofluorescence studies showed a broad presence of this receptor in dorsal horn neurons and DRG while formalin injection increased α5-GABAA receptors fluorescence intensity.
In conclusion, our study shows that peripheral and spinal extrasynaptic α5-GABAA receptors play an important role in formalin-induced GABAergic disinhibition and long-lasting secondary hypersensitivity. Spinal extrasynaptic α5-GABAA receptors also participate in the maintenance of chronic nociception induced by CFA or spinal nerve ligation.
Supplementary Material
Peripheral and spinal α5-GABAA receptors are involved in the development of formalin-induced long-lasting secondary allodynia and hyperalgesia at 3 days. Effect of peripheral (panel A) and intrathecal (panel B) pre-treatment (10 min) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Rats were evaluated 3 days after formalin injection. Data are expressed as the mean (n=6) ± SEM of paw withdrawal response to the applications of von Frey filaments (10 and 250 mN) on the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
Peripheral and spinal α5-GABAA receptors are involved in the maintenance of formalin-induced long-lasting secondary allodynia and hyperalgesia. Effect of peripheral (panel A) and intrathecal (panel B) post-treatment (3 days after 1% formalin injection) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the applications of von Frey filaments (10 and 250 mN) on the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
Effect of intrathecal pre-treatment (10 min) (Panel A) or post-treatment (6 days after formalin injection) (Panel B) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on naïve rats. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the application of von Frey filaments (10 and 250 mN) to the plantar surface of rat paws before (control, C) and after saline plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
Acknowledgments
This work is part of the Ph.D. dissertation of Mariana Bravo-Hernández. Authors are grateful with Dr. Jean-Marc Fritschy (University of Zurich) for the generous gift of the α5 subunit-containing GABAA receptor antibody. We greatly appreciate the technical assistance of Michael R. Navarro, Guadalupe C. Vidal-Cantú and Mercedes C. Urbán-Nuñez. Mariana Bravo-Hernández, Jorge B. Pineda-Farias and Paulino Barragán-Iglesias are Conacyt fellows. Ricardo González-Ramírez was supported by a post-doctoral fellowship from Conacyt (CB-2012/179294). This work was partially supported by Conacyt (CB-2012/179294 to VG-S, RF and RD-L) and NIH (DK057629 to NAC) grants.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Ambriz-Tututi M, Cruz SL, Urquiza-Marín H, Granados-Soto V. Formalin-induced long-term secondary allodynia and hyperalgesia are maintained by descending facilitation. Pharmacol Biochem Behav. 2011;98:417–24. doi: 10.1016/j.pbb.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Ambriz-Tututi M, Rocha-González HI, Castañeda-Corral G, Araiza-Saldaña CI, Caram-Salas NL, Cruz SL, Granados-Soto V. Role of opioid receptors in the reduction of formalin-induced secondary allodynia and hyperalgesia in rats. Eur J Pharmacol. 2009;619:25–32. doi: 10.1016/j.ejphar.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABAA receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–38. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol Pain. 2006;2:36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–30. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 6.Barolet AW, Kish SJ, Morris ME. Identification of extrasynaptic binding sites for [3H]-GABA in peripheral nerve. Brain Res. 1985;358:104–9. doi: 10.1016/0006-8993(85)90953-9. [DOI] [PubMed] [Google Scholar]
- 7.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–97. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Medicine. 2010;16:302–7. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 10.Bravo-Hernández M, Cervantes-Durán C, Pineda-Farías JB, Barragán-Iglesias P, López-Sánchez P, Granados-Soto V. Role of peripheral and spinal 5-HT3 receptors in development and maintenance of formalin-induced long-term secondary allodynia and hyperalgesia. Pharmacol Biochem Behav. 2012;101:246–57. doi: 10.1016/j.pbb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Bravo-Hernández M, Feria-Morales LA, Torres-López JE, Cervantes-Durán C, Delgado-Lezama R, Granados-Soto V, Rocha-González HI. Evidence for the participation of peripheral α5 subunit-containing GABAA receptors in GABAA agonists-induced nociception in rats. Eur J Pharmacol. 2014;734:91–7. doi: 10.1016/j.ejphar.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Caram-Salas NL, Reyes-García G, Bartoszyk GD, Araiza-Saldaña CI, Ambriz-Tututi M, Rocha-González HI, Arreola-Espino R, Cruz SL, Granados-Soto V. Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur J Pharmacol. 2007;573:75–83. doi: 10.1016/j.ejphar.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Carlton SM, Zhou S, Coggeshall RE. Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–22. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 14.Castro A, Aguilar J, González-Ramírez R, Loeza-Alcocer E, Canto-Bustos M, Felix R, Delgado-Lezama R. Tonic inhibition in spinal ventral horn interneurons mediated by α5 subunit-containing GABAA receptors. Biochem Biophys Res Commun. 2011;412:26–31. doi: 10.1016/j.bbrc.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes-Durán C, Pineda-Farías JB, Bravo-Hernández M, Quiñonez-Bastidas GN, Vidal-Cantú GC, Barragán-Iglesias P, Granados-Soto V. Evidence for the participation of peripheral 5-HT2A, 5-HT2B, and 5-HT2C receptors in formalin-induced secondary mechanical allodynia and hyperalgesia. Neuroscience. 2013;232:169–81. doi: 10.1016/j.neuroscience.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Cervero F, Laird J, García-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–51. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 18.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 19.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 20.De Groat WC, Lalley PM, Saum WR. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 1972;44:273–7. doi: 10.1016/0006-8993(72)90383-6. [DOI] [PubMed] [Google Scholar]
- 21.Delgado-Lezama R, Loeza-Alcocer E, Andrés C, Aguilar J, Guertin PA, Felix R. Extrasynaptic GABAA receptors in the brainstem and spinal cord: structure and function. Curr Pharm Des. 2013;19:4485–97. doi: 10.2174/1381612811319240013. [DOI] [PubMed] [Google Scholar]
- 22.Deschenes M, Feltz P, Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976;118:486–93. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- 23.Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–32. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirig DM, Yaksh TL. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J Pharmacol Exp Ther. 1995;275:219–27. [PubMed] [Google Scholar]
- 25.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 26.Fritschy JM, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–94. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 27.Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–35. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 28.Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- 29.Furuyama T, Sato M, Sato K, Araki T, Inagaki S, Takagi H, Tohyama M. Co-expression of glycine receptor beta subunit and GABAA receptor gamma subunit mRNA in the rat dorsal root ganglion cells. Brain Res Mol Brain Res. 1992;12:335–8. doi: 10.1016/0169-328x(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 30.Godínez-Chaparro B, Barragán-Iglesias P, Castañeda-Corral P, Rocha-González HI, Granados-Soto V. Role of peripheral 5-HT4, 5-HT6, and 5-HT7 receptors in development and maintenance of secondary mechanical allodynia and hyperalgesia. Pain. 2011;152:687–97. doi: 10.1016/j.pain.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann N Y Acad Sci. 2010;1198:168–72. doi: 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- 32.Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–8. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang YL, He XF, Shen YF, Yin XH, DUJY, Liang YI, Fang JQ. Analgesic roles of peripheral intrinsic met-enkephalin and dynorphin A in long-lasting inflammatory pain induced by complete Freund’s adjuvant in rats. Exp Ther Med. 2015;9:2344–48. doi: 10.3892/etm.2015.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jolivalt C, Lee C, Ramos M, Calcutt N. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakinohana O, Hefferan MP, Nakamura S, Kakinohana M, Galik J, Tomori Z, Marsala J, Yaksh TL, Marsala M. Development of GABA-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: a qualitative and quantitative electrophysiological and histopathological study. Neuroscience. 2006;141:1569–83. doi: 10.1016/j.neuroscience.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 36.Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–4. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 37.Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–8. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee-Kubli C, Calcutt N. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain. 2014;155:250–60. doi: 10.1016/j.pain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leem JW, Willis WD, Weller SC, Chung JM. Differential activation and classification of cutaneous afferents in the rat. J Neurophysiol. 1993;70:2411–24. doi: 10.1152/jn.1993.70.6.2411. [DOI] [PubMed] [Google Scholar]
- 41.Loeza-Alcozer E, Canto-Bustos M, Aguilar J, González-Ramírez R, Felix R, Delgado-Lezama R. α5 GABAA receptors mediate primary afferent fiber tonic excitability in the turtle spinal cord. J Neurophysiol. 2013;110:2175–84. doi: 10.1152/jn.00330.2013. [DOI] [PubMed] [Google Scholar]
- 42.LoPachin RM, Rudy TA, Yaksh TL. An improved method for chronic catheterization of the rat spinal subarachnoid space. Physiol Behav. 1981;27:559–61. doi: 10.1016/0031-9384(81)90350-4. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva Al, De Koninck Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABAA receptors. J Neurosci. 2014;34:8300–17. doi: 10.1523/JNEUROSCI.0159-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machelska H, Schopohl JK, Mousa SA, Labuz D, Schäfer M, Stein C. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J Neuroimmunol. 2003;141:30–9. doi: 10.1016/s0165-5728(03)00213-3. [DOI] [PubMed] [Google Scholar]
- 45.Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Develop. 2004;149:143–51. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 46.McCabe C, Shaw D, Atack JR, Street JL, Wafford KA, Dawson GR, Reynolds DS, Leslie JC. Subtype-selective GABAergic drugs facilitate extinction of mouse operant behavior. Neuropharmacology. 2004;46:171–8. doi: 10.1016/j.neuropharm.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Morales-Aza BM, Chillingworth NL, Payne John A, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis. 2004;17:62–9. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Morris ME, Di Costanzo GA, Fox S, Werman R. Depolarizing action of GABA (γ-aminobutyric acid) on myelinated fibers of peripheral nerves. Brain Res. 1983;278:117–26. doi: 10.1016/0006-8993(83)90230-5. [DOI] [PubMed] [Google Scholar]
- 49.Notter T, Panzanelli P, Pfister S, Mircsof D, Fritschy J. A protocol for concurrent high-quality immunohystochemical and biochemical analyses in adult mouse central nervous system. Eur J Neurosci. 2014;39:165–75. doi: 10.1111/ejn.12447. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien DE, Alter BJ, Satomoto M, Morgan CD, Davidson S, Vogt SK, Norman ME, Gereau GB, Demaro JA, 3rd, Landreth GE, Golden JP, Gereau RW., 4th ERK2 Alone Drives Inflammatory Pain But Cooperates with ERK1 in Sensory Neuron Survival. J Neurosci. 2015;35:9491–507. doi: 10.1523/JNEUROSCI.4404-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen RW, Sieghart W International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid (A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update Pharmacol Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul J, Yévenes GE, Benke D, Di Lio A, Ralvenius WT, Witschi R, Scheurer L, Cook JM, Rudolph U, Fritschy JM, Zeilhofer HU. Antihyperalgesia by α2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacol. 2014;39:477–87. doi: 10.1038/npp.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABAA receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol. 2012;520:3895–911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- 54.Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- 55.Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain. Mol Pain. 2006;13:32. doi: 10.1186/1744-8069-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price T, Cervero F, Gold M, Hammond D, Prescott S. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–70. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708 a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–5. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 58.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- 59.Reichl S, Augustin M, Zahn PK, Pogatzki-Zahn EM. Peripheral and spinal GABAergic regulation of incisional pain in rats. Pain. 2012;153:129–41. doi: 10.1016/j.pain.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 60.Rodgers FC, Zarnowska ED, Laha KT, Engin E, Zeller A, Keist R, Rudolph U, Pearce RA. Etomidate impairs long-term potentiation in vitro by targeting α5-subunit containing GABAA receptors on nonpyramidal cells. J Neurosci. 2015;35:9707–16. doi: 10.1523/JNEUROSCI.0315-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- 62.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–90. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 63.Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha 5 subunit in the rat brain. J Comp Neurol. 2006;499:458–70. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer E, Placheta P. Reduction of [3H]-muscimol binding sites in rat dorsal spinal cord after neonatal capsaicin treatment. Brain Res. 1980;202:484–7. doi: 10.1016/0006-8993(80)90160-2. [DOI] [PubMed] [Google Scholar]
- 65.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–79. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 66.Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric acid A receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–33. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi A, Tokunaga A, Yamanaka H, Mashimo T, Noguchi K, Uchida I. Two types of GABAergic miniature inhibitory postsynaptic currents in mouse substantia gelatinosa neurons. Eur J Pharmacol. 2006;553:120–8. doi: 10.1016/j.ejphar.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 68.Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol. 2010;588:2571–87. doi: 10.1113/jphysiol.2010.188292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tokumine J, Kakinohana O, Cizkova D, Smith DW, Marsala M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J Neurosci Res. 2003;74:552–61. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- 70.Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 71.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang H. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. PNAS. 2002;99:8360–5. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto T, Yaksh TL. Effects of intrathecal strychnine and bicuculline on nerve compression-induced thermal hyperalgesia and selective antagonism by MK-801. Pain. 1993;54:79–84. doi: 10.1016/0304-3959(93)90102-U. [DOI] [PubMed] [Google Scholar]
- 73.Yang L, Zhang FX, Huang F, Lu YJ, Li GD, Bao L, Xiao HS, Zhang X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–83. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]
- 74.Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: Pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–33. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y, Dua S, Gold MS. Inflammation-induced shift in spinal GABAA signaling is associated with a tyrosine - kinase dependent increase in GABAA current density in nociceptive afferents. J Neurophysiol. 2012;108:2581–93. doi: 10.1152/jn.00590.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral and spinal α5-GABAA receptors are involved in the development of formalin-induced long-lasting secondary allodynia and hyperalgesia at 3 days. Effect of peripheral (panel A) and intrathecal (panel B) pre-treatment (10 min) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Rats were evaluated 3 days after formalin injection. Data are expressed as the mean (n=6) ± SEM of paw withdrawal response to the applications of von Frey filaments (10 and 250 mN) on the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
Peripheral and spinal α5-GABAA receptors are involved in the maintenance of formalin-induced long-lasting secondary allodynia and hyperalgesia. Effect of peripheral (panel A) and intrathecal (panel B) post-treatment (3 days after 1% formalin injection) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on 1% formalin-induced mechanical secondary allodynia and hyperalgesia. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the applications of von Frey filaments (10 and 250 mN) on the plantar surface of rat paws before (control, C) and after formalin plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.
Effect of intrathecal pre-treatment (10 min) (Panel A) or post-treatment (6 days after formalin injection) (Panel B) with the selective α5-GABAA receptors antagonist L-655,708 (15 nmol/rat) on naïve rats. Data are expressed as the mean (n=6) ± SEM of paw withdrawal ipsilateral response to the application of von Frey filaments (10 and 250 mN) to the plantar surface of rat paws before (control, C) and after saline plus vehicle (Veh) or drug injection. *P<0.05 versus C group, #P<0.05 versus Veh group, by one-way ANOVA followed by the Student–Newman–Keuls post-hoc test. CL: Contralateral.