Abstract
Inherited ataxias are a group of heterogeneous disorders in children or adults but their genetic definition remains still undetermined in almost half of the patients. However, CoQ10 deficiency is a rare cause of cerebellar ataxia and ADCK3 is the most frequent gene associated with this defect. We herein report a 48 year old man, who presented with dysarthria and walking difficulties. Brain magnetic resonance imaging showed a marked cerebellar atrophy. Serum lactate was elevated. Tissues obtained by muscle and skin biopsies were studied for biochemical and genetic characterization. Skeletal muscle biochemistry revealed decreased activities of complexes I+III and II+III and a severe reduction of CoQ10, while skin fibroblasts showed normal CoQ10 levels. A mild loss of maximal respiration capacity was also found by high-resolution respirometry. Molecular studies identified a novel homozygous deletion (c.504del_CT) in ADCK3, causing a premature stop codon. Western blot analysis revealed marked reduction of ADCK3 protein levels. Treatment with CoQ10 was started and, after 1 year follow-up, patient neurological condition slightly improved. This report suggests the importance of investigating mitochondrial function and, in particular, muscle CoQ10 levels, in patients with adult-onset cerebellar ataxia. Moreover, clinical stabilization by CoQ10 supplementation emphasizes the importance of an early diagnosis.
Keywords: ADCK3, cerebellar ataxia, CoQ10, mitochondrial disorders, neurodegenerative diseases
Autosomal recessive cerebellar ataxias (ARCAs) are genetically heterogeneous neurodegenerative disorders characterized by slow progressive gait impairment with an onset usually in childhood or adolescence (1–5). ARCAs are quite heterogeneous for age at onset, severity of disease progression and frequency of extra-cerebellar and systemic signs. Mitochondrial dysfunction is often present in ARCAs, and mutations of proteins related to CoQ10 biosynthesis and regulation (ADCK3, Aprataxin, Anoctamin 10) have often been linked to ARCA (6). CoQ10 biosynthesis is a highly conserved mechanism among the species and requires the synergic work of several enzymes. Deficiency of biosynthetic enzymes usually leads to very severe and multisystemic phenotypes.
The ADCK3 gene encodes the homologue of the yeast protein coq8, a putative protein kinase involved in the regulation of CoQ biosynthesis (7). ADCK3 mutations have been identified in patients with early-onset cerebellar ataxia (ARCA2). The clinical course is variable, ranging from mild forms with slowly progressive pure cerebellar ataxia to severe entities with early-onset ataxia, seizures, and stroke-like episodes (8). In patients with mutations in ADCK3, CoQ10 levels are frequently decreased in skeletal muscle and/or fibroblasts (2); adult-onset is very rare and has been reported only in two patients (3) with normal CoQ10 levels in muscle.
We report herein a patient with late-onset cerebellar ataxia, slow progression, severe CoQ10 deficiency in muscle and a new homozygous mutation in ADCK3.
Patient and methods
Clinical history
A 48 year old man with slurred speech and difficult walking was admitted to our department. He was born from an uncomplicated pregnancy; his parents were healthy and non-consanguineous; he had four brothers and five sisters but only one woman showed similar clinical features and died at 50 years of breast cancer. Since he was 20 years old, he started to complain of subtle gait imbalance; symptoms progressed very slowly, and at 48 years of age, he firstly sought medical assistance, because of frequent falling and dysarthria.
Neurological examination revealed mild bilateral ptosis, head tremor, wide-based gait, and slurred speech; he was not able to walk in tandem. Muscle strength was normal at all four limbs. At finger-to-nose test, he had bilateral dysmetria with intentional tremor and dysdiadochokinesia was also present. Deep tendon reflexes were increased at lower limbs with bilateral Babinski sign.
Routine blood tests, including vitamin E, autoantibodies, alpha-fetoprotein, thyroid function and screening for celiac disease, were normal as well as Creatine Kinase (CK) levels. Serum lactate showed normal elevation at rest but resulted abnormally increased after an aerobic exercise reaching 9.3 mmol/l (normal range: 1.5 ± 0.6 mmol/l).
The patient underwent brain magnetic resonance imaging (MRI) on a system operating at 3.0 T (Achieva, Philips Healthcare, Best, Netherlands) that showed millimetric hyper-intense lesions in temporo-parietal bilateral cortical areas and a cerebellar vermis atrophy (Fig. 1a). Moreover, MRI spectroscopy did not reveal any lactate peak in the ventricles or in the cerebellar lobe. Electromyography and nerve conduction studies were unremarkable. Axial and proximal limb muscles MRI was also performed, and no relevant changes were observed.
Fig. 1.
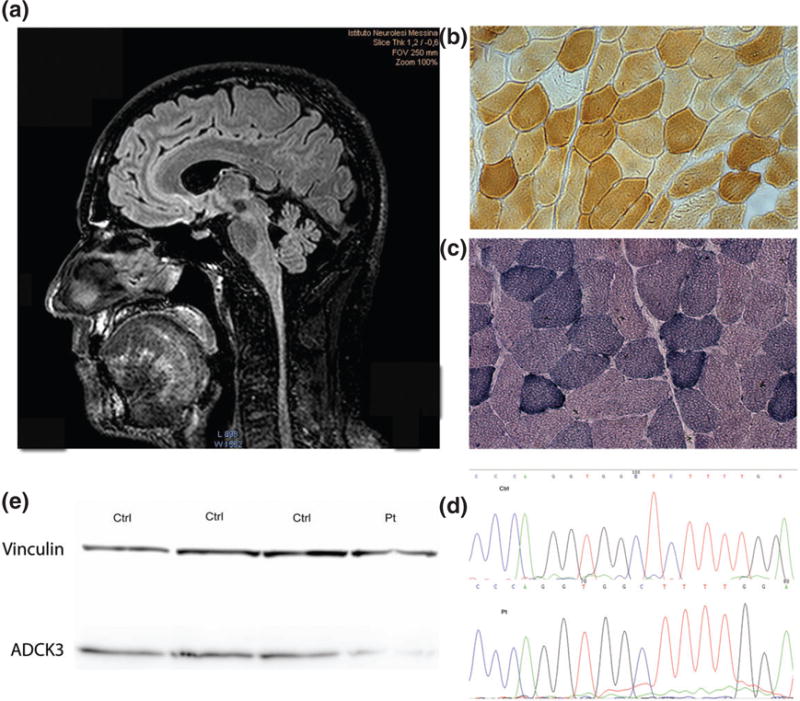
(a) Brain magnetic resonance imaging (MRI) showing cerebellar atrophy. (b, c) Muscle biopsy with rare COX-negative fibers (b) and few hyper-reactive SDH fibers (c). (d) Electropherogram of one control (Ctrl) and the patient (Pt). (e) Western blot of ADCK3 protein from patients’ fibroblasts and three controls.
At neuropsychological assessment, the patient showed a mild impairment in working memory and executive functions. Genetic tests for Friedreich’s ataxia, spinocerebellar ataxias (SCA 1, 2, 3, 6, 17), and apraxia with oculomotor ataxia types 1 and 2 were negative.
Muscle biopsy
After informed consent, an open biopsy of vastus lateralis was performed. Frozen samples were processed for histological (H&E and Gomori trichrome) and histochemical for cytochome oxidase (COX) and succinate dehydrogenase (SDH) studies, as well as for biochemical analysis according to standard techniques (9).
Cell culture
Primary fibroblasts were obtained from skin biopsy. Fibroblasts from our patient and from five age- and passage-matched controls were cultured in Dulbecco’s modified Eagle medium, supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin and maintained at 37°C humidified and 5% CO2.
Biochemical studies
To measure mitochondrial respiratory chain enzymes activities in skeletal muscle, 40 mg of tissue was homogenized in 9 volumes of 0.15M KCl, 50mM Tris-HCl, pH 7.4 (CPT medium), and centrifuged at 2500 g for 20 min at 4°C. The supernatant was used also for protein determination. About 1.5 × 106 cells were harvested and suspended in phosphate buffer saline pH 7, sonicated in ice for 10 s and protein was determined by Bradford assay. Activities of mitochondrial respiratory chain complexes were assessed spectrophotometrically (9).
CoQ10 assessment in fibroblasts and skeletal muscle was performed as previously described (9). Oxygen consumption rate (OCR) in fibroblasts was measured using a XF24 Seahorse® instrument as previously described (10)
Molecular genetic studies
ADCK3 gene amplification and Sanger sequencing were performed following standard protocols (6). To measure ADCK3 mRNA expression levels, total RNA was extracted from patient’s skin fibroblasts, and quantitative Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using TaqMan assays for ADCK3 and GAPDH (Applied Biosystems Foster City, CA).
To assess ADCK3 protein level, 30 μg of proteins extracted from the patient’s skin fibroblasts were loaded in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and immunoblotted with anti ADCK3 antibody (CABC1 monoclonal antibody M04A, clone 7G1) (1:200) and with control anti-vinculin antibody (ab73412).
Results
Muscle biopsy showed increased fiber size variation, some SDH hyperactive fibers and few COX-negative fibers (Fig. 1b,c). Muscle mitochondrial respiratory chain activities evidenced reduction of complex I + III (34.9; normal range 50 ± 6 nmol/min/mg prot) and II + III activity (3.5; normal range: 17.5 ± 4.02 nmol/min/mg prot), whereas SDH and COX activities were normal (Table 1). Muscle CoQ10 was severely decreased (1.14 μg/g; normal range 32 ± 6 μg/g). Activities of complex I + III and II + III as well as CoQ10 concentration resulted normal in fibroblasts (Table 1). However, OCR measured by XF24 Seahorse® in the patient’s fibroblasts, showed maximal respiration capacity in the lower range of controls.
Table 1.
Biochemical feature of fibroblasts and muscle
| Fibroblasts
|
Muscle
|
|||
|---|---|---|---|---|
| Pt | Ctr | Pt | Ctr | |
| CoQ10 | 45.2 ± 1.3 | 47.4 ± 2.4 (μg/mg Prot) | 1.14* | 32 ± 6 (μg/mg tissue) |
| I + III | 0.08 ± 0.02 | 0.18 ± 0.08 (nmol/min/mg Prot) | 34.9* | 50 ± 6 (nmol/min/mg Prot) |
| II + III | 0.01 ± 0.001 | 0.01 ± 0.003 (nmol/min/mg Prot) | 3.5* | 17.5 ± 4 (nmol/min/mg Prot) |
| COX | 0.044 ± 0.004 | 0.048 ± 0.002 (nmol/min/mg Prot) | 55.8 | 48 ± 10 (nmol/min/mg Prot) |
| SDH | 0.064 ± 0.003 | 0.059 ± 0.004 (nmol/min/mg Prot) | 11.3 | 11 ± 3 (nmol/min/mg Prot) |
| CS | 0.089 ± 0.017 | 0.091 ± 0.001 (nmol/min/mg Prot) | 98 | 130 ± 45 (nmol/min/mg Prot) |
Ctrl, control; Pt, patient.
abnormal values
Molecular studies revealed a homozygous novel deletion (c1511_1512delCT) in ADCK3, which causes a premature stop codon in the kinase domain of the protein (p.Ala504fs) (Fig. 1d). The mutation was heterozygous in two asymptomatic sisters of the proband and absent in 104 controls. Analysis of cDNA did not show reduction of ADCK3 expression in cultured skin fibroblasts from the patient, thus excluding mRNA decay. However, Western blot analysis showed about 60% reduction of ADCK3 protein levels when normalized over beta-actin (Fig. 1e).
The patient started supplementation with 400 mg/day of CoQ10 and, at 1 year follow-up, he reported clinical improvement as showed by Scale for the Assessment and Rating of Ataxia (SARA) score, more evident at speech and gait items (total SARA score 13 at baseline and 10 after 1 year).
Discussion
ARCAs are a heterogeneous group of diseases, and genetic diagnosis is a real challenge for neurologists. ADCK3 biochemical function has been only partially characterized; phylogenic analysis showed that ADCK3 is an atypical kinase orthologous to yeast protein Coq8p (2). Coq8p is known to stabilize the complex of CoQ biosynthetic proteins in yeast, but the underlying mechanism is still undefined.
Mutations in ADCK3, even if rare, represent the most common cause of primary CoQ10 deficiency (8). So far, only 33 patients with ADCK3 mutation have been reported (2, 3, 11, 12). In these patients, usually central nervous system involvement is the predominant feature, gait ataxia being the most frequent sign at disease onset. In some patients, hand clumsiness, myoclonus and choreic movements have been also observed. Individuals rarely present seizures, stroke-like episodes, and developmental delay. In almost half of the patients, involvement of the corticospinal tract is present, but spasticity seldom reaches clinical significance.
We herein report a new mutation in ADCK3 in a patient with late-onset cerebellar ataxia; such late-onset is quite uncommon, considering that the majority of ADCK3 patients develop symptoms in childhood or adolescence. In 2012, Horvath et al. described two patients with an adult-onset form, who were still ambulating in their late forties, thus resembling our patient from the clinical point of view. However, biochemical analysis in the reported case disclosed normal CoQ10 levels in muscle, whereas in our patient CoQ10 level was severely reduced.
We observed slow progression of the disease in accordance with previous observations (11) and a moderate response to CoQ10 supplementation. The absence of any stroke-like lesions at MRI confirms the heterogeneity of this disorder and the lack of correlation between CoQ10 residual levels and disease severity. Our patient had a mild alteration in the executive functions, suggesting that, in ARCA2, cognitive alteration might not be related to the stroke-like episodes.
In ARCA2, exercise intolerance and muscle weakness have been mentioned (3) but our patient, although presenting with a severe CoQ10 deficiency in muscle, did not exhibit any clinical sign of myopathy. This surprising finding supports the hypothesis that tissue damage in CoQ10 deficiency is a complex phenomenon that depends upon the delicate balance between electron transport respiratory chain defects and excessive reactive oxygen species (ROS), buffering activity of CoQ10. In particular, it has been shown that very low levels of CoQ (<30% of normal) lead to a dramatic reduction of mitochondrial bioenergetics but no increased ROS levels, while intermediate values of CoQ10 (40–50% residual), cause increased ROS production, mitochondrial hyperpolarization and more prominent cell death (13).
By direct sequencing, we identified a homozygous two base pair deletion in ADCK3: mutation is localized in the protein kinase-like fold residue of the protein that recently has been documented to be responsible for the kinase activity (14). Moreover, the deletion causes a shift of the reading frame and leads to a shorter protein, lacking 130 amino acids at the C-terminal domain with loss of the F-helix region. Surprisingly, for the first time we observed, in human tissues, that, although mRNA was normal, protein levels were severely reduced. These results suggest that the mutation impairs protein stability, causing a posttranslational degradation. The wild-type size band observed in the western blot analysis may represent a protein generated by stop codon read through, or a posttranslational modified mutant protein.
Diagnosis of ataxias because of mitochondrial impairment is quite difficult, and these cases are easily underestimated because of the lack of reliable biomarkers (15). In our patient, although skin fibroblasts showed only mild decreased bioenergetic capacity by high-resolution respirometry analyses, CoQ10 was severely decreased in muscle, raising a strong suspicion of mitochondrial disease (16). These features also support the usefulness of a muscle biopsy in such cases.
Treatment with CoQ10 caused a mild improvement of symptoms in our patient. Up to day, cerebellar ataxia due to CoQ10 deficiency includes a group of disorders where an etiological approach is available (8), for which a prompt identification and characterization of patients is required.
Acknowledgments
The authors acknowledge the Ministry of Health GR2009-1606283.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366:636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 2.Lagier-Tourenne C, Tazir M, Lopez LC, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath R, Czermin B, Gulati S, et al. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J Neurol Neurosurg Psychiatry. 2012;83:174–178. doi: 10.1136/jnnp-2011-301258. [DOI] [PubMed] [Google Scholar]
- 4.Mollet J, Delahodde A, Serre V, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degardin A, Dobbelaere D, Vuillaume I, et al. Spinocerebellar ataxia: a rational approach to aetiological diagnosis. Cerebellum. 2012;11:289–299. doi: 10.1007/s12311-011-0310-1. [DOI] [PubMed] [Google Scholar]
- 6.Quinzii CM, Emmanuele V, Hirano M. Clinical presentations of coenzyme q10 deficiency syndrome. Mol Syndromol. 2014;5:141–146. doi: 10.1159/000360490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He CH, Xie LX, Allan CM, Tran UC, Clarke CF. Coenzyme Q supplementation or over-expression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants. Biochim Biophys Acta. 2014;1841:630–644. doi: 10.1016/j.bbalip.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmanuele V, Lopez LC, Berardo A, et al. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol. 2012;69:978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musumeci O, Naini A, Slonim AE, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- 10.Invernizzi F, D’Amato I, Jensen PB, Ravaglia S, Zeviani M, Tiranti V. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 2012;12:328–335. doi: 10.1016/j.mito.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mignot C, Apartis E, Durr A, et al. Phenotypic variability in ARCA2 and identification of a core ataxic phenotype with slow progression. Orphanet J Rare Dis. 2013;8:173. doi: 10.1186/1750-1172-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YT, Hersheson J, Plagnol V, et al. Autosomal-recessive cerebellar ataxia caused by a novel ADCK3 mutation that elongates the protein: clinical, genetic and biochemical characterisation. J Neurol Neurosurg Psychiatry. 2014;85:493–498. doi: 10.1136/jnnp-2013-306483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinzii CM, Tadesse S, Naini A, Hirano M. Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts. PLoS One. 2012;7:e30606. doi: 10.1371/journal.pone.0030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefely JA, Reidenbach AG, Ulbrich A, et al. Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme Q biosynthesis. Mol Cell. 2015;57:83–94. doi: 10.1016/j.molcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMauro S, Schon EA, Carelli V, Hirano M. The clinical maze of mitochondrial neurology. Nat Rev Neurol. 2013;9:429–444. doi: 10.1038/nrneurol.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terracciano A, Renaldo F, Zanni G, et al. The use of muscle biopsy in the diagnosis of undefined ataxia with cerebellar atrophy in children. Eur J Paediatr Neurol. 2012;16:248–256. doi: 10.1016/j.ejpn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


