Abstract
Pluripotent embryonic stem cells (ESCs) can be derived from blastocyst-stage mouse embryos. However, the exact in vivo counterpart of ESCs has remained elusive. A combination of expression profiling and stem cell derivation identifies epiblast cells from late-stage blastocysts as the source, and functional equivalent, of ESCs.
In 1981, the laboratories of Martin Evans and Gail Martin reported the first successful derivation of pluripotent embryonic stem cells from pre-implantation blastocyst-stage mouse embryos1,2. When maintained under defined culture conditions, ESCs possess the unique ability to self-renew indefinitely, as well as a propensity for generating germline chimaeras when introduced into host pre-implantation-stage embryos. These features established ESCs as the de facto tool for engineering changes in the mouse genome. Germline transmission of genetically modified ESCs produces animals that can be used in the investigation of gene function and disease modelling. Harnessing their developmental potential, protocols have been developed for the directed differentiation of ESCs to generate therapeutically relevant cell types for potential future use in tissue replacement therapies in regenerative medicine. Recently, ESCs have also been adopted as an in vitro model for early mammalian development, but precisely how well they mimic in vivo lineage progression remains an open question.
Following on from the pioneering work in the mouse, ESC-like lines were derived from several mammalian species, including human3. However, it soon became apparent that the developmental potential of mouse ESCs was more robust than that of ESC-like lines from other mammals. Indeed, only mouse and rat ESCs possess the ability to generate germline-transmitting chimaeras when introduced into embryonic day 2.5 (E2.5) morulae or E3.5 blastocysts. The recent derivation of pluripotent cell lines from isolated epiblast tissue of post-implantation mouse embryos (referred to as epiblast stem cells, EpiSCs) using human ESC derivation and culture conditions4,5 has led to the concept of two distinct pluripotent states: naive and primed6. Mouse ESCs represent the naive state, which is associated with the pre-implantation epiblast and is characterized by the ability to give rise to all somatic cell types and germline competency. In contrast, mouse EpiSCs (and human ESCs) represent a primed state, which in mice resembles the early post-implantation (E5.5) epiblast, and are unable to contribute to chimaeras when introduced into pre-implantation host embryos. Thus mouse EpiSCs and human ESCs are functionally distinct from naive-state ESCs, and represent a different embryonic stage. However, this distinction does not clarify why mouse ESCs differ in their developmental potential and culture requirements from ESCs of other mammalian species, as all are, in principle, derived from the blastocyst stage. Nor does it provide evidence to support the notion that mouse ESCs (or EpiSCs) are the in vitro counterpart of a cell type resident in vivo.
The true identity of ESCs has long been debated. On the one hand, it has been suggested that ESCs might represent an artefactual state: an in vitro culture adaptation with no true in vivo counterpart. A counterpoint argument draws comparison between ESCs and the cells of the inner cell mass (ICM) in the pre-implantation blastocyst-stage embryo. In this issue, Nichols and colleagues7 address this question and demonstrate that self-renewing embryonic stem cell potential arises following epiblast specification.
They begin by determining the gene expression profiles of cells present across a range of pre-implantation and early post-implantation stages of mouse embryo development (Fig. 1), and compared these to the expression profiles of mouse ESCs. As baseline conditions for ESC culture, the authors used two culture conditions that promote naive-state pluripotency within ESC populations and facilitate the derivation of ESCs from permissive and non-permissive strains of mice8. These culture conditions are based on the dual inhibition of the extracellular signal related kinase (ERK) and glycogen synthase kinase-3 (GSK3) so as to suppress cellular differentiation while maintaining self-renewal — termed the 2i condition. The authors also used 2i medium supplemented with the cytokine LIF (2i-LIF). Marker profiling revealed that ESCs most closely resembled pre-implantation E4.5 epiblast cells.
Figure 1.
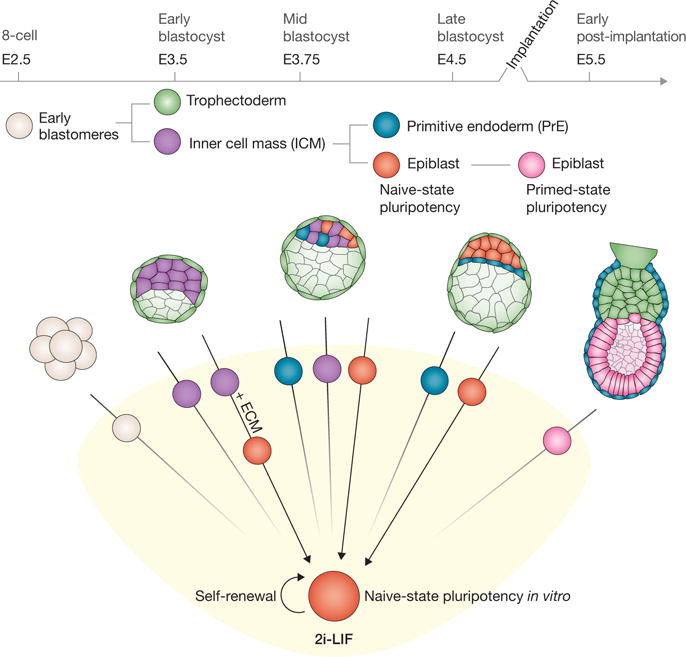
Direct in vitro capture of embryo-derived epiblast cells. Single ICM cells were isolated from pre- and post-implantation embryos, and either pooled and expression profiled (not shown), or plated in medium containing 2i-LIF (see text for details) for the derivation of self-renewing naive-state ESCs. Arrows indicate the capacity of individual embryo-derived cells to give rise to ESCs.
The expression data presented by Nichols and colleagues7 supports previous studies that demonstrated that the blastocyst is not a singular stage, but instead comprises a dynamic heterogeneous population of cells encompassing several sequential steps of embryonic development9,10. In the late blastocyst (E4.0–E4.5), the ICM contains two distinct cell types: the pluripotent epiblast, which includes the precursors of almost all somatic and germ cells, and the primitive endoderm (PrE), a predominantly extra-embryonic lineage (Fig. 1)11. The epiblast exclusively expresses pluripotency-associated transcription factors such as Nanog, Sox2 and Klf2, whereas PrE precursors are characterized by their expression of Gata6, Gata4, Sox17 and Sox7 (ref. 11).
It has long been known that it is ICM cells of the blastocyst that give rise to ESCs, but, surprisingly, the precise cellular origin of ESCs has not been elucidated until now7. The capacity for clonal propagation in 2i-LIF conditions is a distinct feature of naive ESCs, and Nichols and colleagues7 hypothesized that if cells resident in the late blastocyst are functionally equivalent to naive ESCs, it would be possible to derive ESC lines from single cells isolated from E4.5 embryos. Lineage-specific green fluorescent protein (GFP) reporter mouse lines facilitated the identification and isolation of individual epiblast and PrE cells from E4.5 embryos. These single cells were subjected to an ESC derivation protocol, with ESC colonies emerging from epiblast, but not PrE, precursors. Surprisingly, the number of clonal ESC lines obtained per embryo approximated to the number of epiblast cells within the ICM at E4.5. Thus, every epiblast cell present within an E4.5 blastocyst has the potential to produce naive, germline, competent ESCs. By contrast, ICM cells isolated from embryos staged earlier than E3.75, and older (E5.5) post-implantation epiblast, lacked the capacity to generate ESCs. Indeed, based on marker expression, ICM cells from early blastocyst stages (~E3.5) cannot be categorized as epiblast or PrE because of their co-expression of markers for both of these two ICM lineages9,12. Presumably, early ICM cells represent an immature state of multi-lineage potential. Conversely, early post-implantation epiblast cells have developed beyond the stage of naive pluripotency. Interestingly, 2i-LIF could not revert the post-implantation epiblast cells from a primed to a naive state of pluripotency.
These data suggest that a naive pluripotent state gradually develops within the ICM between E3.75 and E4.0. However, it is unlikely that all ICM cells develop at the same rate. Some epiblast cells are likely to acquire the naive state and the ability to self-renew as ESCs ahead of others. This asynchronous progression of the ICM population to a naive state could account for the reduced efficiency of ESC derivation from E3.75 embryos compared with E4.5 embryos. By E4.5, virtually all epiblast cells had acquired a naive pluripotent state making them ‘ESC competent’, and, accordingly, ESC derivation efficiencies were maximal. Collectively, these data suggest that the window of opportunity for when naive mouse ESCs can be captured in vitro from blastocyst-stage embryos is narrow. This may also hold true for other mammalian species.
The detailed transcriptional and functional characterization of the naive state in the mouse should assist in the identification of an equivalent stage in other species, and facilitate the derivation of naive ESCs from non-rodent mammals. Recently, the generation of naive human ESC lines independent of transgenes for stable culture was reported13,14. However, the conditions of stem cell derivation and the culture requirements differed between studies. Moreover, the requirement for fibroblast growth factor (FGF) supplementation in one of the reports begs the question of whether a bona fide naive human ESC state was captured in vitro, as FGF is not required for the culture of naive mouse ESCs (Fig. 1).
Interestingly, early (E3.5) ICM cells failed to progress to a naive state of pluripotency when cultured under 2i conditions. Hypothesizing that interactions between cells and the extracellular matrix (ECM) within the embryo might facilitate progression of ICM cells (which are initially responsive to FGF and ERK (ref. 11)) to a state of ERK independence, Nichols and colleagues demonstrated that culture of early ICM cells in the presence of ECM components did indeed promote their acquisition of naive pluripotency. These data highlight the critical role played by the ECM in the maturation of ICM cells, and support the beneficial influence of ECM components for ESC propagation. If early ICM cells have multi-lineage potential, these observations also raise the question of whether they can all be equally programmed to a naive pluripotent state when exposed to ECM.
Mounting evidence suggests that FGF-producing epiblast precursors are necessary for the correct specification and maturation of extra-embryonic lineages10,15. The data presented in the current study reveal that the proper maturation of the epiblast may depend on the adjacent extra-embryonic lineages. Extra-embryonic PrE cells express genes encoding ECM components. They are therefore responsible for the deposition of ECM proteins within the ICM, and the assembly of a basement membrane at the PrE–epiblast tissue interface by E4.5. Thus, although the pre-implantation period of embryonic development is characterized by a high degree of cell fate plasticity, mutual cooperation between all three emergent lineages of the blastocyst is a requisite for the correct execution of the developmental program. This cooperation leads to the timely specification of the two ICM lineages, and the progression of epiblast progenitors from a naive to a primed state of pluripotency.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Berenika Plusa, Email: Berenika.Plusa@manchester.ac.uk, Faculty of Life Sciences, The University of Manchester, Oxford Road, Manchester M13 9PT, UK.
Anna-Katerina Hadjantonakis, Email: hadj@mskcc.org, Developmental Biology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, New York 10065, USA.
References
- 1.Evans MJ, Kaufman MH. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Odorico JS. Trends Biotech. 2000;18:53–57. doi: 10.1016/s0167-7799(99)01410-9. [DOI] [PubMed] [Google Scholar]
- 4.Tesar PJ, et al. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 5.Brons IG, et al. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 6.Nichols J, Smith A. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Boroviak T, Loos R, Berton P, Smith A, Nichols J. Nat Cell Biol. 2014;16:513–525. doi: 10.1038/ncb2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying QL, et al. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnishi Y, et al. Nat Cell Biol. 2014;16:27–37. doi: 10.1038/ncb2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrode N, et al. Genesis. 2013;51:219–233. doi: 10.1002/dvg.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Gafni O, et al. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 14.Ware CB, et al. Proc Natl Acad Sci USA. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bin GC, et al. Development. 2014;141:1001–1010. doi: 10.1242/dev.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]


