Abstract
Sepsis is characterized by an overwhelming systemic inflammation and multiple organ injury. Toll-like receptors (TLRs) 2 and 4 mediate these inflammatory responses. Sparstolonin B (SsnB), isolated from Chinese herb Scirpus yagara, is a new selective TLR2/4 antagonist. Herein, we report that SsnB inhibited the expression of various inflammatory mediators such as tumor necrosis factor (TNF-α), interleukin (IL)-1β, IL-6, and chemokine (C-C motif) ligand 2 (CCL-2) in lipopolysaccharide (LPS)- or Pam3csk4-stimulated macrophages. Moreover, in LPS-stimulated macrophages, the downregulation of peroxisome proliferator-activated receptor γ (PPAR-γ) was reversed by SsnB dose-dependently; and SsnB had synergistic inhibitory effects with rosiglitazone, a PPAR-γ agonist, on TNF-α and IL-6 expression in LPS-stimulated macrophages. The effects of SsnB were further evaluated in a mouse endotoxin shock model. When intraperitoneal injected in mice 2 days before or 1–2 h after LPS challenge, SsnB attenuated the body temperature reduction and decreased the mortality. SsnB pre-treatment significantly suppressed LPS-induced increase of TNF-α and IL-6 in serum, lungs and livers, and substantially attenuated lung dysfunction in mice. In vivo toxicity test showed that at doses as high as 500 mg/kg, SsnB did not cause death of mice. These results suggest that SsnB protects mice against endotoxin shock by inhibiting production of multiple cytokines and lung dysfunction. In conclusion, our findings indicate that SsnB may be used in the prevention and treatment of endotoxin shock.
Keywords: Sparstolonin B, Inflammation, Toll-like receptor, Sepsis, Cytokine
1. Introduction
Sepsis is a complex systemic inflammatory response to bacterial infection [1]. Both gram negative and gram positive bacterial infections can cause sepsis [5]. Bacterial components such as lipopolysaccharide (LPS), lipoteichoic acid, and peptidoglycan, prompt host immune cells to produce pro-inflammatory cytokines and reactive oxygen species [2,3]. Toll-like receptors (TLRs), which recognize various pathogen-associated molecular patterns derived from bacteria, play a central role in the early immune response during sepsis [4]. Among the TLRs, TLR4 recognizes gram-negative bacteria component LPS; TLR2 dimerizes with TLR1 or TLR6 to detect peptidoglycan and lipopeptides of gram-positive bacteria [6]. Once TLRs were activated, production of cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β, and chemokines such as chemokine C-C motif ligand 2 (CCL2), and other inflammatory mediators such as nitric oxide (NO), were induced [7]. Overwhelming systemic inflammation leads to severe tissue damage, multiple organ failure and death [8]. Sepsis is the most common cause of death among hospitalized patients in intensive care units [9]. Antibiotics alone cannot control the overwhelming systemic inflammation in sepsis [10], thus it is urgent to develop new therapies.
Studies have shown that TLR2 and TLR4 play important roles in the pathogenesis of sepsis, as they are the major signal sensors to recognize the microbial products [11,12]. Sparstolonin B (SsnB) is a newly identified polyphenol with structural features of xanthone and isocoumarin; it is isolated from Chinese herb Scirpus yagara [13,14]. Our previous study showed that SsnB is a selective TLR2 and TLR4 antagonist, and it blocks the early intracellular events in the TLR2 and TLR4 signaling [13].
SsnB has been shown to inhibit TLR2 and TLR4 ligands-induced inflammatory responses in primary mouse macrophages in vitro [13], and attenuate LPS-induce inflammatory responses in endothelial cells [14]. In this current study, we further tested the effects of SsnB on cytokine and chemokine production in two macrophages cell lines in response to LPS (a TLR4 ligand) and Pam3csk4 (a TLR2 ligand). Moreover, we evaluated the therapeutic effects of SsnB on endotoxin shock using a mouse model.
2. Materials and methods
2.1. Animals
Female BALB/c mice (6 weeks old, 18–22 g) were obtained from Nanjing Medical University (SCXK2008-000X, Nanjing, China), and given free access to water and standard rodent chow and were housed in pathogen-free cages. The animals were acclimated for a week before use. ICR mice (6 weeks old, 18–22 g) were obtained from Nanjing Medical University (SCXK2012-0004, Nanjing, China). The mice were given free access to water and standard rodent chow and were housed in pathogen-free cages, and acclimated for 3 days before use. The mouse experiment was under the guidelines of the Committee for Animal Care and Use of Laboratory Animals, College of Pharmacy, Nanjing University of Chinese Medicine.
2.2. Reagents
For in vitro studies, SsnB was dissolved in dimethyl sulfoxide (DMSO) as stock solution. For in vivo studies, SsnB was dissolved in soybean oil to make the stock solution. DMSO was not used as a solvent in the in vivo experiments because of its potential toxicity when it is used at large amounts, which would be necessary to deliver high doses of SsnB to mice. Soybean oil has been widely used as a solvent for water insoluble drugs in in vivo studies [15,16]. LPS (Escherichia coli 055: B5), rosiglitazone (a peroxisome Proliferator-activated receptor γ (PPAR-γ) agonist), Pam3csk4 was purchased from Invitrogen (Grand Island, NY).
2.3. Cells culture
The mouse macrophage cell line RAW264.7 was purchased from ATCC (Manassas, VA). Cells were cultured in DMEM medium (Gibco, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum (Gibco, USA), penicillin (100 U/ml), and streptomycin (100 µg/ml) at 37 °C under a humidified atmosphere with 5% CO2. RAW264.7 cells (density 1 × 105 cells/well) were cultured in serum-free DMEM for 16 h before treatment was started. The cells were then stimulated with LPS (1 µg/ml) or Pam3csk4 (500 ng/ml) in the presence or absence of SsnB for additional 16 h in serum-free DMEM.
2.4. RNA isolation and quantitative real-time PCR (qPCR)
Total RNA was isolated and purified with Trizol reagent (Invitrogen) and RNeasy™ Mini kit (Qiagen) according to the manufacturers’ instructions.
Total RNA was reverse transcribed into cDNA using a First-strand cDNA Synthesis System (Marligen Bioscience, MD). qPCR analysis was performed using the Fast Start Universal SYBR Green Master (Rox) (Roche Applied Science) on an Eppendorf Realplex2 Mastercycler (Eppendorf, Hamburg, Germany). The primers used in qPCR were: mouse 18 s RNA (internal control) 5′-CGCGGTTCT ATTTTGTTGGT-3′ (forward) and 5′-AGTCGGCATCGTTTATGGTC-3′ (reverse); mouse TNF-α, 5′-CGTCAGCCGATTTGCTATCT-3′ (forward) and 5′-CGGATCCGC-AAAGTCTAAG-3′ (reverse); mouse IL-6, 5′-AGTTGCCTTCTTGGGACTGA-3′ (forward) and 5′-TCCACGATTT CCCAGAGAAC-3′ (reverse); mouse CCL-2, 5′-GGCTCAGCCAGATG CAGT-TAA-3′ (forward) and 5′-CCAGCCTACTCATTGGGATCA-3′ (reverse); mouse PPAR-γ, 5′–GCAGTGGGGATGTCTCATAATGC-3′ (forward) and 5′–CAGGGGGGTGATGTGTTTGA-AC-3′ (reverse). Equal amounts of cDNA were taken from each reverse transcription reaction mixture for real-time PCR amplification using gene specific primers for TNF-α, IL-6, CCL-2 and PPAR-γ. Samples were amplified using the following program: 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s, 60 °C for 15 s, and 68 °C for 20 s, then a melting curve analysis from 60 °C to 95°C every 0.2 °C was obtained. The abundance of each gene product was calculated by relative quantification, with values for the target genes normalized with 18 s RNA.
2.5. Enzyme-linked immunosorbent assay (ELISA)
The concentrations of cytokines in the cell culture medium, mouse serum and lung and liver tissue homogenates were determined by specific enzyme-linked immunosorbent assay (ELISA) for mouse IL-6, TNF-α, and IL-1β (eBioscience, San Diego, CA) following the manufacturer’s instructions. The 96-well microplates were read using a PowerWave X340 microplate reader (Bio-TEK).
2.6. Animals treatment
LPS was dissolved in saline at a concentration of 1.5 mg/ml. For the lethal endotoxin shock model, mice were intraperitoneally injected with LPS at a dose of 15 mg/kg. In pre-treatment experiment, mice received an intraperitoneal injection of 3 or 9 mg/kg of SsnB (LPS + SsnB groups) or soybean oil alone (LPS group) once a day for 2 days before administration of LPS. In post-treatment experiment, mice received 3 or 9 mg/kg of SsnB 1 h or 2 h after LPS administration. The vehicle group animals received an intraperitoneal injection of soybean oil alone. For non-survival experiments, mice were sacrificed at 2 or 6 h after LPS administration; blood, lungs and liver were collected for analysis. Mice in the vehicle group received intraperitoneal injections of soybean oil alone. For analysis of cytokines, mouse blood was allowed to clot at room temperature for 1 h. Then blood was centrifuged, serum was separated and stored at −80 °C until analysis.
2.7. Histological analysis
The liver and lungs were harvested at sacrifice; half of the lung tissue and parts of liver tissues were homogenized and centrifuged, and the supernatant was stored at −80 °C for cytokines measurement; the other half of lung was fixed in 10% neutral buffered formalin for 24 h, subsequently routinely processed, and embedded in paraffin. Sections of 5 µm in thickness were cut and stained with hematoxylin and eosin (H&E).
2.8. Body temperature measurement
Body temperature was measured rectally at appropriate times using a digital temperature probe (MC-342FL).
2.9. Acute toxicity evaluation
The mice were randomly divided into four groups (4 mice per group) and fasted for 12 h before experiments. Within the following 24 h, all mice received one or more intraperitoneal injections of SsnB of varied concentrations in 0.4 ml volume. Mice in control group were injected with soybean oil once every 3 h for six times. For the 50 mg/kg SsnB group, each mouse received a single injection of 2.5 mg/ml SsnB. For the 150 mg/kg SsnB group, each mouse was injected with 2.5 mg/ml SsnB once every 3 h for 3 times. For the 500 mg/kg SsnB group, each mouse was administrated 4.2 mg/ml SsnB once 3 h for 6 times. Every SsnB group after injection was continuously observed for 72 h, and then the injection of the next higher dose group was initiated. Mice were monitored for 6 days.
2.10. Statistical analysis
GraphPad Prism 5 software was used to carry out all statistical analysis. One-way analysis of variance was used for multiple group comparison; when only two groups were compared, Student’s t test was performed. The mice survival rate was analyzed using Kaplan–Meier. P values of <0.05 were considered statistically significant.
3. Results
3.1. Effects of SsnB on inflammatory cytokine expression in LPS-treated RAW264.7 cells
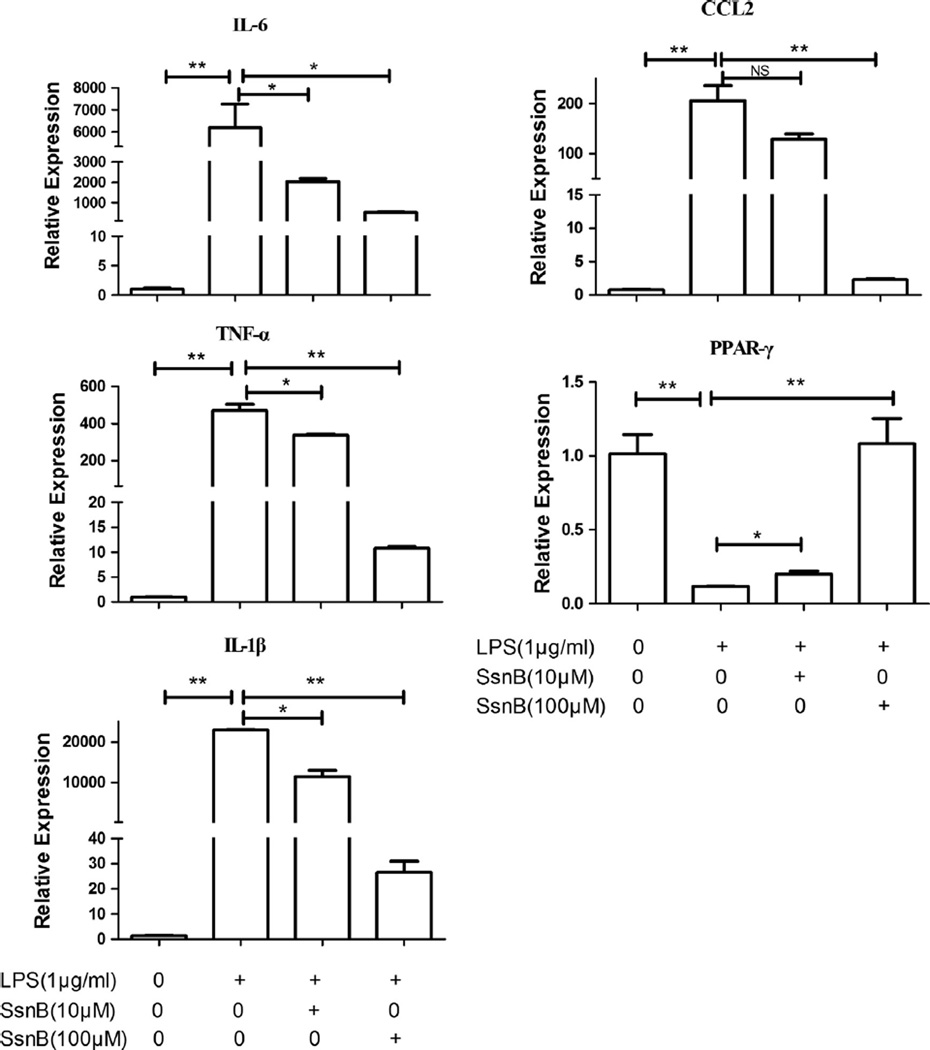
To investigate if SsnB has any effects on LPS-induced expression of inflammatory molecules in macrophages, qPCR was performed to measure mRNA levels of cytokines IL-6, IL-1β and TNF-α, and chemokine CCL-2 in LPS-treated RAW264.7 cells. As shown in Fig. 1, LPS (1 µg/ml) treatment for 6 h significantly induced mRNA levels of IL-6, IL-1β, TNF-α and CCL-2. However, co-treatment with 10 µM or 100 µM SsnB reduced the IL-6 mRNA levels by 3.0 or 12.0-fold, IL-1β mRNA levels by 2.0 or 865.0-fold, TNF-α mRNA levels by 1.4 or 43.5-fold, and CCL-2 mRNA levels by 1.6 or 90.2-fold, respectively.
Fig. 1.
Effects of SsnB on the expression of inflammatory mediator and PPAR-γ in LPS-treated RAW264.7 cells. RAW264.7 macrophages were treated with SsnB and LPS either alone or in combination as indicated for 6 h, the mRNA expression of indicated inflammatory mediator and transcription factor PPAR-γ was measured by quantitative real-time PCR. Bars represent the mean ± SEM. For each treatment, n = 3. *p < 0.05, **p < 0.01, NS: not significant.
We also examined the effects of SsnB on the expression of PPAR-γ in RAW264.7 cells. We found that, LPS (1 µg/ml) markedly reduced PPAR-γ mRNA level by 8.6-fold compared with the control, but co-treatment with 10 or 100 µM SsnB significantly increased the expression of PPAR-γ by 1.7 and 9.2-fold, respectively (versus LPS group, p < 0.05) (Fig. 1).
3.2. Effects of SsnB on protein production of inflammatory cytokines in TLR ligands-treated macrophages
To examine the effects of SsnB on protein production of cytokines, RAW264.7 cells were incubated with LPS or Pam3csk4 in the presence of different concentrations of SsnB for 16 h, media were collected for ELISA assay for TNF-α and IL-6 protein concentrations. As shown in Fig. 2A, SsnB significantly inhibited the TNF-α and IL-6 productions in LPS-treated cells in a dose-dependent manner. And also, Pam3csk4 treatment markedly increased the pro-inflammatory cytokine levels, whereas co-treatment with SsnB reduced the levels in a dose-dependent manner (Fig. 2B). The effects of SsnB on TNF-α and IL-1β induction by LPS (Fig. 2C) and Pam3csk4 (Fig. 2D) in human THP-1 macrophages were also examined, and the results were similar to those in RAW264.7 macrophages.
Fig. 2.
Effects of SsnB on protein production of inflammatory cytokines in macrophages. RAW264.7 cells were plated at a density of 1 × 105 cells/well in a 96-well culture plates and incubated overnight. The cells were stimulated with LPS (1 µg/ml) (A) or Pam3csk4 (500 ng/ml) (B) in presence or absence of SsnB for 16 h. The concentrations of cytokines in the culture medium were determined by ELISA. Bars represent the mean ± SEM. For each treatment, n = 3. Similarly, the effects of SsnB on cytokines productions induced by LPS (C) or Pam3csk4 (D) in human THP-1 macrophages were examined.
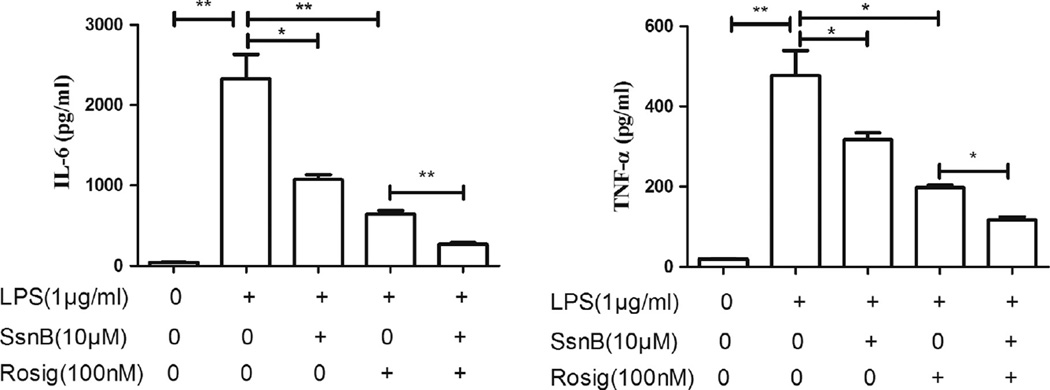
3.3. The synergistic effects of SsnB with rosiglitazone on inflammatory cytokine expression in macrophages
To investigate if SsnB and rosiglitazone, a PPAR-γ agonist, have synergistic inhibitory effects on the production of LPS-induced inflammatory cytokines, SsnB, rosiglitazone alone or in combination were add with LPS to treat RAW264.7 cells for 16 h; ELISA was performed to measure the protein levels in the culture medium. The results showed that LPS (10 ng/ml) significantly increased TNF-α and IL-6 expression. Addition of 10 µM SsnB or 100 nM rosiglitazone reduced IL-6 by 2.2 and 3.6-fold, and reduced TNF-α by 1.5 and 2.4-fold, respectively, compared to LPS alone group. Interestingly, SsnB and rosiglitazone co-treatment reduced IL-6 and TNF-α more than either one of them. The co-treatment decreased IL-6 and TNF-α by 2.4 and 1.7-fold, respectively, compared to rosiglitazone alone group (p < 0.01) (Fig. 3).
Fig. 3.
The synergistic effects of SsnB with rosiglitazone on inflammatory cytokine expression in macrophages. RAW264.7 cells were treated with LPS in the presence of SsnB and Rosiglitazone either alone or in combination as indicated for 16 h. Bars represent the mean ± SEM. For each treatment, n = 3. *p < 0.05, **p < 0.01.
3.4. Effects of SsnB on endotoxin shock in mice
3.4.1. Effects of SsnB pre-treatment on the mortality of LPS-challenged mice
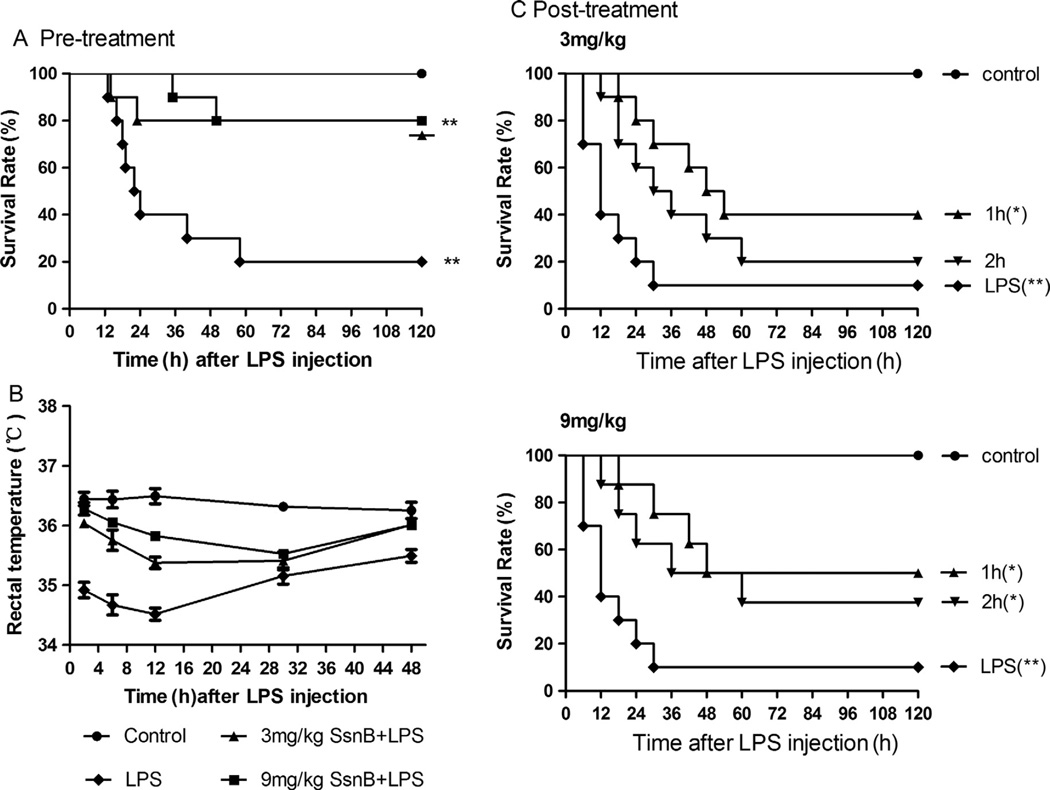
Mice received an intraperitoneal injection of 3 or 9 mg/kg of SsnB (LPS + SsnB groups) or soybean oil alone (LPS group) once a day for 2 days before LPS (15 mg/kg) was administrated. Survival rate of mice was monitored for 7 days. In LPS alone group, within 24 h after LPS administration, 60% of mice died, and on Day 7 post LPS injection, only 20% mice survived. Pre-treatment with 3 mg/kg or 9 mg/kg SsnB increased survival rate to 80% on Day 7 post LPS injection. And pre-treatment with 9 mg/kg SsnB substantially delayed the death of mice compared to the pre-treatment with 3 mg/kg SsnB (Fig. 4A).
Fig. 4.
Effects of SsnB on endotoxin shock in mice. (A) Protective effects of SsnB on LPS-induced lethality in mice. The mice were given intraperitoneal injection of SsnB (3 or 9 mg/kg), once a day for two days, before they were injected LPS (15 mg/kg i.p.). (B) Effects of SsnB pre-treatment on LPS-induced hypothermia. Rectal temperature of mice was measured at indicated times with a digital thermometer probe. (C) Therapeutic effects of SsnB on LPS-induced lethality. SsnB (3 or 9 mg/kg) was intraperitoneally administered 1 h or 2 h after LPS challenge. Data are expressed as means ± SEM. *, #p < 0.05 compared with LPS and control group, respectively. N = 10 in each group.
3.4.2. Effects of SsnB pre-treatment on LPS-induced hypothermia
The rectal temperature of mice decreased significantly after LPS injection. At 24 h after LPS injection, the temperature of mice in LPS alone group was slightly increased, but was still below the normal level. Pre-treatment with SsnB at doses of 3 mg/kg or 9 mg/kg significantly improved this LPS-induced hypothermic response in a dose-dependent manner (Fig. 4B). The body temperature of SsnB-treated mice returned close to normal level within 48 h, while the temperature of LPS alone mice remain substantially below the normal level.
3.4.3. Effects of SsnB post-treatment on the mortality of LPS-challenged mice
Mice were i.p. injected with LPS (15 mg/kg); and 1 or 2 h later, SsnB (3 mg/kg or 9 mg/kg) was injected. As shown in Fig. 4C, SsnB rescued mice when administered after LPS challenge. SsnB treatment at 3 mg/kg 1 h after LPS challenge increased the survival rates of mice from 10% to 40%; and injection of 9 mg/kg SsnB 1 or 2 h after LPS challenge led to 50% or 40% survival (p < 0.05 vs. LPS-treatment alone), respectively.
3.4.4. Effects of SsnB pretreatment on mouse cytokine production
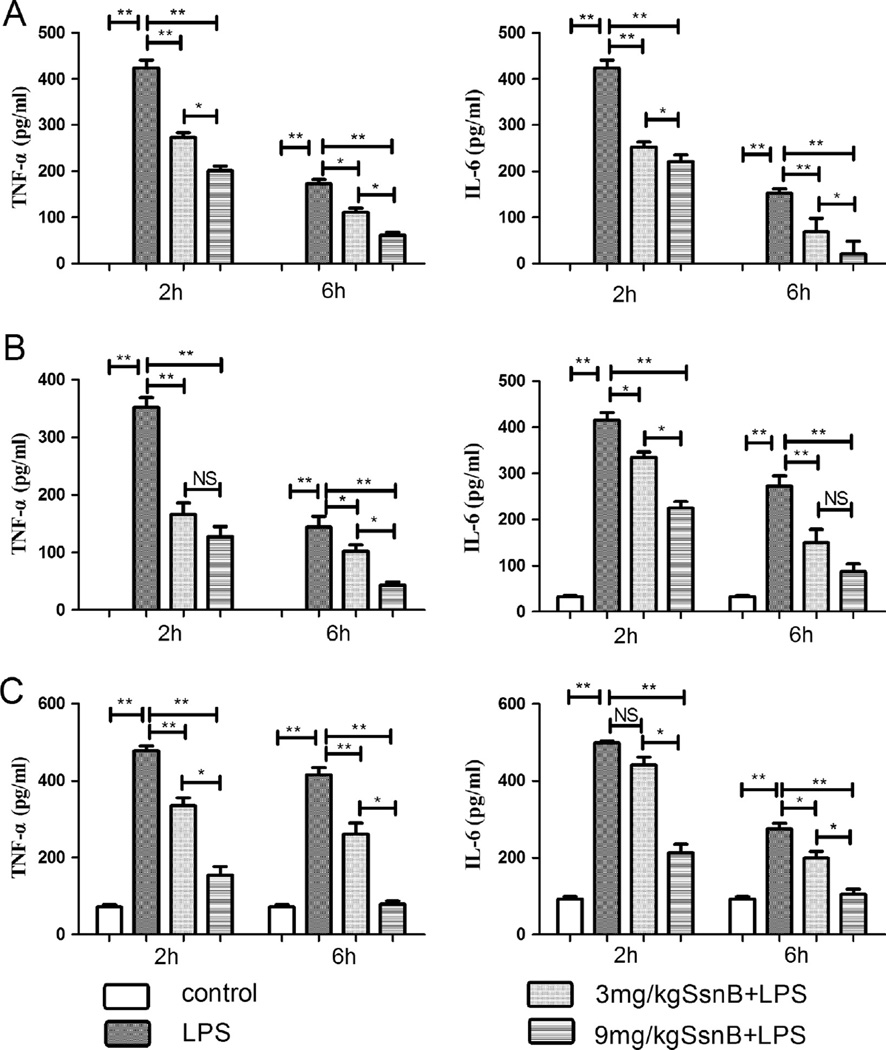
Peritoneal injection of LPS into mice resulted in a significantly increase in serum IL-6 and TNF-α protein levels. SsnB pre-treatment decreased LPS-induced TNF-α levels by 1.70 and 3.45-fold at 3 mg/kg and 9 mg/kg doses, respectively, and reduced LPS-induced IL-6 by 1.67 and 1.92-fold at these two doses, respectively, at 2 h after LPS injection. At 6 h post LPS administration, the 3 mg/kg and 9 mg/kg SsnB treated groups had significantly lower serum TNF-α levels (1.74 and 3.12-fold reduction, respectively), and lower serum IL-6 levels (2.22 and 7.29-fold reduction, respectively), compared with LPS alone group (Fig. 5A).
Fig. 5.
Effects of SsnB pretreatment on pro-inflammatory cytokine levels in LPS-treated mice. Two hours or six hours after LPS challenge, some mice were sacrificed to measure the cytokine concentrations. The concentrations of TNF-α and IL-6 in serum (A), lung homogenate (B), and liver homogenate (C) of the septic mice were measured by ELISA. Data are presented as means ± SEM. For each treatment, n = 10. *p < 0.05, **p < 0.01, NS: not significant.
Tissue homogenate of lungs was also used for pro-inflammatory cytokine measurement. LPS treatment caused a marked increase in TNF-α and IL-6 levels in the lungs compared with the control group. Pre-treatment with SsnB dose-dependently attenuated this increase. Two hours after LPS injection, similar to the trend in serum, pretreatment with 3 mg/kg and 9 mg/kg reduced TNF-α levels in lungs by 2.12 and 2.76-fold, and IL-6 levels by 1.24 and 1.85-fold, respectively, compared to LPS alone group. At the 6 h time point, 3 mg/kg and 9 mg/kg SsnB pretreatment reduced the TNF-α levels by 1.40 and 3.27-fold, and IL-6 levels by 1.82 and 3.14-fold, respectively, compared to LPS alone group (Fig. 5B).
Pro-inflammatory cytokines in the liver were also analyzed. LPS administration induced a significant increase in TNF-α and IL-6 levels in the liver compared with the control group. The increase in TNF-α and IL-6 levels was attenuated by SsnB dose-dependently. Two hours after LPS injection, pretreatment with 3 mg/kg and 9 mg/kg reduced TNF-α levels by 43% and 2.1-fold, and IL-6 levels by 13% and 1.34-fold, respectively, compared to LPS group. At the 6 h time point, 3 mg/kg and 9 mg/kg SsnB treatment reduced the TNF-α levels by 59% and 4.26-fold, and IL-6 levels by 38% and 1.63-fold, respectively, compared to LPS group (Fig. 5C).
3.4.5. Effects of SsnB on LPS-induced lung injury
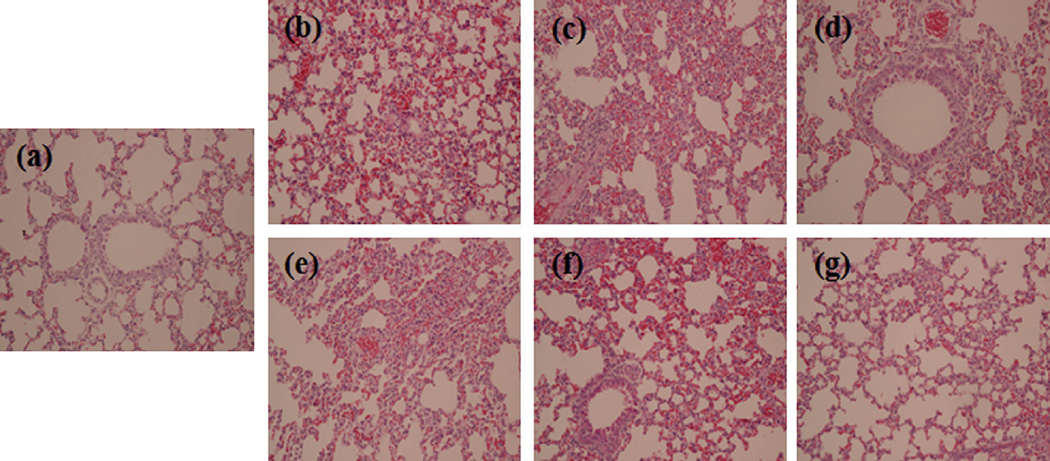
As lung injury is a main cause of mortality in sepsis, we examined the histological alterations of the lungs. Lungs were harvested at 2 h or 6 h after injection of LPS. As shown in Fig. 6, compared with the control group, LPS induced a severe pathological abnormality in lungs, including pulmonary interstitial hyperemia, edema, alveolar collapse, thickening of the alveolar wall, as well as infiltration of inflammatory cells. The lung pathology was more severe in the mice sacrificed at 6 h post LPS injection than in those sacrificed at 2 h time point. Pretreatment with SsnB (3 mg/kg and 9 mg/kg) significantly alleviated the lung pathology in the mice at both time points.
Fig. 6.
Effects of SsnB pre-treatment on lung histopathology in LPS-treated mice. Two hours or six hours after LPS challenge, mice were sacrificed to obtain lung tissue for histological analysis. H&E staining was performed using lung tissue specimens from control (a), 2 h-LPS (b), 2 h-LPS + 3 mg/kg SsnB (c), 2 h-LPS + 9 mg/kg SsnB (d), 6 h-LPS (e), 6 h-LPS + 3 mg/kg SsnB (f), and 6 h-LPS + 9 mg/kg SsnB (g) mice, respectively. Magnification 300×.
3.5. Toxicity of SsnB in mice
To evaluate the in vivo toxicity of SsnB in mice, after intraperitoneal injection of various doses of SsnB, mice were observed for 6 days. None of mice, including those injected with the highest dose of SsnB (500 mg/kg), showed any obvious abnormality or died. And the histological examination did not reveal any obvious alterations in organs and tissues including the lung, liver, spleen, and intestine (Data not shown).
4. Discussion
Sepsis is a syndrome resulted from an excessive host response to bacterial infection. Both Gram-negative and gram-positive bacteria cause septic shock [17]. TLRs signaling triggered by bacteria-derived ligands contributes to the pathogenesis of sepsis [18]. Suppression of TLR2 and TLR4 pathways can reduce pro-inflammatory cytokines and attenuate tissue injury in mouse endotoxin shock models [17,19]. Our previous study demonstrated that SsnB selectively inhibited inflammatory responses to TLR2 and TLR4 ligands [13]. In this current study, we found that SsnB suppressed LPS (a TLR4 ligand)-induced production of multiple inflammatory cytokines, including TNF-α, IL-1β, IL-6 and chemokine CCL-2, in mouse and human macrophages (Figs. 1 and 2). Similar inhibition on cytokines productions was also observed in macrophages stimulated by a synthetic TLR2 ligand Pam3csk4 (Fig. 2). These findings confirmed our previous report and suggested that SsnB is a TLR2/4 antagonist.
PPAR-γ is involved in regulating of inflammatory response in sepsis [20]. In septic mice, treatment of PPAR-γ ligands suppresses the systemic inflammatory response and improves survival [20,21]. Activation of PPAR-γ induces an anti-inflammatory M2 macrophage phenotype [22,23]. We found that in LPS-induced RAW264.7 macrophages, SsnB markedly reversed the LPS-induced decrease in mRNA level of PPAR-γ (Fig. 1). Furthermore, we found that SsnB and PPAR-γ agonist rosiglitazone had synergistic inhibitory effects on LPS-induced inflammatory responses in macrophages. These results suggest that, anti-inflammatory effects of SsnB may be partially through the activation of PPAR-γ. However, SsnB and PPAR-γ agonist rosiglitazone also exert their anti-inflammatory effects through distinct pathways, suggesting combined treatment with SsnB and rosiglitazone may improve the anti-sepsis therapeutic efficacy.
Sepsis is characterized by an overwhelming systemic hyper-inflammatory condition that leads to multiple organ dysfunctions [24]; and the failure of the lungs is the most common cause of mortality in sepsis [25]. In this study, we assessed the suppressive effects of SsnB on LPS-induced systemic inflammation and lung injury. Acute increase in cytokines (TNF-α, IL-6) levels leads to endothelial cell-leukocyte adhesion, induces organ edema, and initiates multiple tissue injury and eventually multiple organ dysfunctions [26]. In mouse endotoxin shock model, we showed that SsnB inhibited LPS-induced hypothermia and protected mice from death (Fig. 4). SsnB also attenuated lung injury and inflammatory cytokines in the lungs and livers in a dose-dependent manner (Fig. 5). On the other hand, SsnB did not show any obvious toxicity in naïve mice even when a dose as high as 500 mg/kg was used.
Since sepsis leads to a complicated inflammatory cascade of cytokines [27], suppression of individual inflammatory mediator has not shown pronounced efficacy in clinical trials [18]. In our mouse model of sepsis, SsnB could significantly reduce the levels of multiple cytokines and chemokines in serum, livers and lungs, suggesting that SsnB may be a better choice of therapy for sepsis than strategies targeting individual inflammatory mediators.
In summary, we evaluated the effects of SsnB on macrophage inflammatory responses in vitro and mouse endotoxin shock in vivo. SsnB significantly suppressed cytokines production induced by LPS or Pam3csk4 in transcriptional and translational levels in macrophages. When co-treated with PPAR-γ agonist, SsnB synergistically decreased LPS-induced TNF-α and IL-6 production. SsnB, without obvious toxicity even at much higher dose than that could achieve significant therapeutic effects, protected mice against LPS-induced death by inhibiting cytokines production and decreasing lung injury. Thus, SsnB may be developed as a new therapy for sepsis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81173515 to QL), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADA), and the National Institute of Health (R21AT006767 and R01HL116626 to DF).
References
- 1.Matot I, Sprung CL. Definitions of sepsis. Intensive Care Med. 2001;27:83–89. doi: 10.1007/pl00003795. [DOI] [PubMed] [Google Scholar]
- 2.Van Aemersfoort ES, Van Berkel TJC, Kuiper J. Receptor, mediators and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–565. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Toll-like receptors: their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:5517–5524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 6.Tomohiro K, Masayuki I, Tomoyuki K, Yuji I, Hiroyuki K. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:140–148. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Silverman N, Fitzgerald K. DUBbing down innate immunity. Nat Immunol. 2004;5:1010–1012. doi: 10.1038/ni1004-1010. [DOI] [PubMed] [Google Scholar]
- 8.Ritcirsch D, Flier MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UIIoa L, Tracey KJ. The cytokine profile: a code for sepsis. Trends Immunol. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Sharma A. Is neuroimmunomodulation a future therapeutic approach for sepsis? Int Immunopharmacol. 2010;10:9–17. doi: 10.1016/j.intimp.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimira A, Kobayashi S, et al. Inhibition of endotoxin response by E5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:31093–31102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of Toll-like receptors in the development of sepsis. Shock. 2008;29:315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 13.Qiaoli L, Qinan W, Jihong J, Jin’ao D, Chao W, Mark DS, et al. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. J Biol Chem. 2011;286:3026470–3026479. doi: 10.1074/jbc.M111.227934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Liang Q, Kong L, Wu Q, Duan J. Study on chemical constituents of Scirpus yagara Ohwi. Chin Pharm J. 2012;47:1987–1989. [Google Scholar]
- 15.Medina J, Salvadó A, del Pozo A. Use of ultrasound to prepare lipid emulsions of lorazepam for intravenous injection. Int J Pharm. 2001;216:1–8. doi: 10.1016/s0378-5173(00)00664-5. [DOI] [PubMed] [Google Scholar]
- 16.Vita E, Schroeder DJ. Intralipid in prophylaxis of amphotericin B nephrotoxicity. Ann Pharmacother. 1994;28:1182–1183. doi: 10.1177/106002809402801010. [DOI] [PubMed] [Google Scholar]
- 17.Bin L, Jun L, Xichun P, Guofu D, Hongwei C, Weiwei J, et al. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-α release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-κB activation. Int Immunopharmacol. 2010;10:3344–3350. doi: 10.1016/j.intimp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:774–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takukyu S, Mie S, Tomoyuki K, Jun S, Masaruki I, Yuji I. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, et al. The anti-inflammatory regulation of curcumin in an experimental model of sepsis is mediated by upregulation of peroxisome proliferator–activated receptor-gamma. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CL, Hui TY, Yu CH, Yen SC, Wan CC. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced live injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. Nutr Biochem. 2014;25:119–125. doi: 10.1016/j.jnutbio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta (12,4)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–6837. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 23.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen I. The immunophathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 25.In Duk J, Min-Goo L, Jeong Hyun C, Jun Sik L, Young-II J, Chang-Min L, et al. Blockade of Indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J Immunol. 2009;182:53146–53154. doi: 10.4049/jimmunol.0803104. [DOI] [PubMed] [Google Scholar]
- 26.Mayer Sagy MD, Yasir Al-Qaqaa MD, Paul Kim MD. Definitions and Pathophysiology of sepsis. Curr Probl Pediator Adolesc Health Care. 2013;43:260–263. doi: 10.1016/j.cppeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trails and lesson learned. Crit Care Med. 2001;29(Suppl.):s121–s125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]