Abstract
Breast cancer is the most common malignant cancer among women. The Hedgehog (Hh) signaling pathway serves a key role in malignant cancer cell growth and migration. However, little is known with regard to the specific function of the Hh signaling pathway in human breast cancer. The current study investigated the specific role of Hh signaling in the human breast cancer cell line MDA-MB-231. Expression of components of Shh-Gli signaling, as well as the Gli-responsive genes B-cell lymphoma 2 (Bcl-2) and cyclin D1, were investigated in MDA-MB-231 cells using western blotting. The effects of Shh-Gli signaling on MDA-MB-231 proliferation were analyzed by MTT assay. The role of E-cadherin in the epithelial-mesenchymal transition process was determined by western blot while matrix metalloproteinase (MMP)-9/MMP-2 secretion was studied by enzyme-linked immunosorbent assay. The results indicated that Shh-Gli signaling was activated in MDA-MB-231 cells, significantly enhancing cell viability. Overexpression of Gli positively regulated the transcription of Bcl-2 and cyclin D1 thereby regulating MDA-MB-231 cell proliferation and survival. Treatment of MDA-MB-231 cells with human sonic hedgehog, n-terminus for 72 h significantly reduced E-cadherin protein levels and enhanced secretion of MMP-9 and MMP-2. These findings suggest that Shh-Gli signaling is significantly activated in human breast cancer cells, and is accompanied by enhanced cell viability, proliferation and migration capacities.
Keywords: hedgehog signaling, breast cancer, MDA-MB-231 cells
Introduction
The Hedgehog (Hh) signaling pathway serves a key role in cell growth and differentiation during embryonic development (1). This signaling is transmitted via binding to the Patched (Ptch) 1 receptor, which is one of two (Ptch1 and 2) that have been identified in vertebrates. When Hh levels are deficient, the binding of Ptch1 and smoothened (Smo) prevents the translocation of Smo into the cell membrane, thereby suppressing the activity of Smo. By comparison, when sufficient levels of Hh are present, Hh phosphorylates the serine/threonine amino acids in the carboxyl terminal of Smo, thereby removing the inhibitory effects of Ptch. The five-zinc finger transcription factor, Gli, is then released from a large protein complex and, following its nuclear translocation, activates numerous downstream target genes (2,3). In the presence of sonic Hh (Shh), full-length Gli3 is released from the large protein complex and transported into the nucleus, thereby activating the downstream genes. As Gli1 is reported to be the target gene of Gli3 (4), Gli1 is a hallmark of the Hh signaling pathway (5).
Recent studies indicate that Hh signaling is closely associated with the progression of human tumors (6). Abnormal activation of the Hh signaling pathway has been identified in several cancer types. In certain brain, skin and muscle tumors, mutations in Ptch1 or Smo apparently lead to ligand-independent activation of the Hh signaling pathway (7–9). Furthermore, in esophageal carcinoma, gastric carcinoma and pancreatic carcinoma, Hh signaling has also been found to be activated in a ligand-dependent manner (10). The widely observed activation of the Hh signaling pathway in a series of solid tumors indicates its importance in tumor initiation and progression.
Cyclopamine is a teratogenic steroidal alkaloid extracted from plants. Research indicates that cyclopamine inhibits the Hh signaling pathway by antagonizing Smo (11). In vitro and in vivo experiments have demonstrated the suppressive effects of cyclopamine on cancer cell growth (12,13).
In mouse mammary gland development, Hh signaling is reportedly constitutively activated (14). Disruption of the Ptch1 or Gli2 gene leads to abnormal ductal morphogenesis, including ductal dysplasias, which are very similar to human breast hyperplasias (15). Meanwhile, in breast tumors, changes in protein levels of Hh signaling components have been identified (16). All these results indicate that the Hh signaling pathway is important in breast cancer development. However, little is known about the specific function of the Hh signaling pathway in human breast cancer. The present study analyzed Hh signaling in the human breast cancer cell line MDA-MB-231 and identified abnormal activation of Hh-related proteins. In addition, inhibition of Hh signaling with cyclopamine confirmed that Hh signaling has a key role in breast cancer cell proliferation and invasion.
Materials and methods
Human samples and cell lines
The normal human breast cell line Hs 578Bst and the human breast cancer cell line MDA-MB-231 were purchased from the American Type Tissue Culture Collection (Manassas, VA, USA) and cultured in Hyclone™ Dulbecco's modified Eagle's medium (DMEM)/F12 (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone™; GE Healthcare Life Sciences), 100 U/ml penicillin and streptomycin, in 25 cm2 culture flasks at 37°C in a humidified atmosphere with 5% CO2. Following serum starvation, cells were preincubated with either 100 mM Shh/cyclopamine (Sigma-Aldrich China, Inc., Shanghai, China) or vehicle control [0.1% dimethyl sulfoxide (DMSO)] for 48 h.
Cell viability assay
Cell viability was determined by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO, USA). In order to determine the impacts of Shh signaling on the human breast cancer cell line, MDA-MB-231, cells were cultured to ~70% confluence then starved in serum-free DMEM (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) overnight. The cells were then preincubated with Shh/cyclopamine for 48 h, cultured in fresh medium including 0.5 mg/ml MTT for 4 h, then DMSO was added to the wells to dissolve the blue formazan products and the density was determined spectrophotometrically at a wavelength of 550 nm using a microplate reader. Densitometric analysis was performed in which the level of in Shh-treated cells was normalized against the level in the DMSO group, which was arbitrarily set at 1. Each experiment was independently performed at least three times.
Flow cytometry
Cells were treated with either Shh/Cyclopamine or DMSO as described above. Cells were stained with 25 mg/l ethidium bromide (Sangon Biotech Co., Ltd., Shanghai, China) and the apoptotic status of samples was analyzed by a flow cytometer at an excitation wavelength of 488 nm.
RNA isolation and reverse transcription (RT)-quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from cultured cells with RNAiso Plus (Takara Bio, Inc., Otsu, Japan) following the manufacturer's instructions. Routine DNase (Applied Biosystems; Thermo Fisher Scientific, Inc.) treatment (1 unit DNase I/mg total RNA) was performed prior to RT. A 10 ng aliquot of total RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) with specific primers for SHH, PTCH, SMO, GLI1, snail family zinc finger 2 (SLUG), B-cell lymphoma 2 (BCL-2), cyclin D1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer (Sangon Biotech Co., Ltd.) sequences were as follows: Forward, 5′-TCCTCGCTGCTGGTATG-3′ and reverse, 5′-AAGCGTTCAACTTGTCCTTA-3′ for SHH; forward, 5′-AAATTCAAACCCTCCCTCTG- 3′ and reverse, 5′-AAGAGTCTCTGAAACTTCGC-3′ for PTCH; forward 5′-CTACAACGTGTGCCTGG-3′ and reverse, 5′-GGTCATTCTCACACTTGGG-3′ for SMO; forward, 5′-CAGCGCCCAGACAGA-3′ and reverse, 5′-CGGACATGAGGTTAGCTTG-3′ for GLI1; forward, 5′-ACACATTAGAACTCACACGG-3′ and reverse, 5′-AAGCACTATGTCACAACTTCA-3′ for SLUG; BCL2, forward, 5′-GTGGGAGCTTGCATCAC-3′ and reverse, 5′-ATTTCTACTGCTTTAGTGAACCT-3′ for BCL-2; forward, 5′-TCTATAAATTGAGCCCGCAG-3′ and reverse, 5′-TACCAGAGTTAAAAGCAGCC-3′ for GAPDH. All primers were used at a concentration of 10 µM. qPCR amplifications were performed in reaction volumes of 20 µl containing 10 µl TaqMan 2X Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl 20X TaqMan RNA Assay mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and 1.33 µl template cDNA. The thermal cycling conditions were a hot-start step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. PCR reactions were conducted on a Bio-Rad iQ5 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Relative mRNA expression was normalized against the endogenous control, GAPDH, using the comparative quantification cycle (Cq) method (2−ΔΔCq) (17). Reactions were repeated in triplicate and Bio-Rad CFX Manager Software 1.6 (Bio-Rad Laboratories) was used for quantitative analysis of mRNA expression.
Western blot analysis
For total protein extraction, the cells were treated with radioimmunoprecipitation assay buffer [Beijing Solarbio Science & Technology Co., Ltd., Beijing, China; 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% (v/v) NP-40, 0.1% (w/v) sodium dodecyl sulfate (SDS)] containing 1% (v/v) phenylmethylsulfonyl fluoride (Beijing Solarbio Science & Technology Co., Ltd.), 0.3% (v/v) protease inhibitor (Sigma-Aldrich) and 0.1% (v/v) phosphorylated proteinase inhibitor (Sigma-Aldrich). The lysates were centrifuged at 12,000 rpm (13,400 × g) at 4°C for 15 min, and the relative concentration of total proteins in the supernatant was quantified using a Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc.). Aliquots containing equal amounts of protein (15 µg) were separated by SDS-polyacrylamide gel electrophoresis [10% (v/v)] and transferred onto a polyvinylidene difluoride (PVDF) membrane at 300 mA for 2 h. To block nonspecific binding, the PVDF membrane was incubated in 8% (w/v) milk in Tris-buffered saline with Tween-20 for 2 h at room temperature. The membranes were then incubated overnight at 4°C with the following primary antibodies: Polyclonal rabbit anti-GAPDH (dilution, 1:2,000; #sc-25778), polyclonal rabbit anti-Shh (dilution,1:1,000; #sc-9024) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), polyclonal rabbit anti-Ptch (dilution, 1:1,000; #A01; Abnova Corporation, Taiwan, China), polyclonal rabbit anti-Smo (dilution, 1:1,500; #sc-13943), polyclonal rabbit anti-Gli1 (dilution, 1:1,000; #sc-20687), polyclonal rabbit anti-SLUG (dilution, 1:1,000; #sc-20687), polyclonal rabbit anti-Bcl-2 (dilution, 1:2,000; #sc-492) (Santa Cruz Biotechnology, Inc.), and monoclonal rabbit anti-Cyclin D1 (dilution, 1:1,000; #2978; Cell Signaling Technology, Danvers, MA, USA). The following day, the membranes were washed four times (5 min each) with phosphate-buffered saline with Tween-20, then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Abmart, Arlington, MA, USA; dilution, 1:5,000) for 2 h at room temperature. Following incubation, the membranes were washed four times (5 min each) and the relative protein level was determined using enhanced chemiluminescence (EMD Millipore, Bedford, MA, USA) with the assistance of the ChemiDoc™ Touch Imaging system (Bio-Rad Laboratories, Inc.); each target protein was normalized against GAPDH.
Scratch assay
Cells were grown as a confluent monolayer in 6-well plates. To initiate migration, the cell layer was scratched using a pipette tip. Time-lapse images of cell morphology were captured at 24 and 48 h using a fluorescence stereomicroscope (#MZ16FA; Leica Microsystems, Beijing, China). The migration abilities were quantified by measuring the area of the scratched regions using Image-Pro Plus version 4.5 software (Media Cybernetics, Inc., Rockville, MD, USA). The experiment was performed three times.
Enzyme-linked immunosorbent assay (ELISA)
Following pretreatment with Shh/cyclopamine for 48 h, the culture medium was aspirated, centrifuged at 3,000 rpm (1,006.2 × g) for 20 min, and the levels of matrix metalloproteinase (MMP)-2 and −9 in the supernatant were assayed using human MMP-2 and −9 ELISA kits (Beijing Rui'er Xinde Technology, Beijing, China) according to the manufacturer's instructions.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. The number of independent experiments is represented by ‘n.’ Multiple comparisons were performed using one-way analysis of variance followed by Tukey's multiple-comparisons test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of Shh-Gli signaling molecules in breast cancer cells
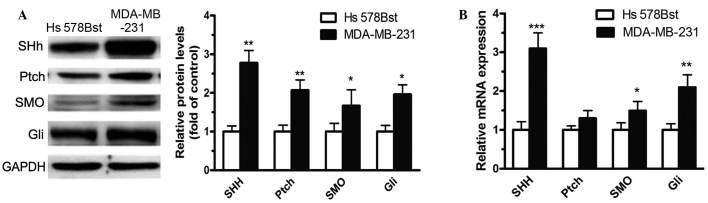
The expression levels of Shh-Gli signaling components were determined in the human breast cancer cell line MDA-MB-231 using western blotting. The Shh-Gli signaling receptors Ptch and Smo, and the target transcription factors Gli1 and Gli2, were found to be highly expressed at the protein level in MDA-MB-231 cells compared with the normal human breast cell line Hs 578Bst (Fig. 1A). Meanwhile, the relative mRNA levels of PTCH, SMO and GLI were also markedly higher in MDA-MB-231 cells relative to Hs 578Bst cells, as determined by RT-qPCR (P<0.01; Fig. 1B). Together, these results indicate that components of Shh-Gli signaling are overexpressed in the human breast cancer cell line MDA-MB-231, as are the target transcripts of this signaling (PTCH, GLI1, and GLI2). This suggests that Shh-Gli signaling is activated in MDA-MB-231 cells.
Figure 1.
Expression of Shh-Gli signaling components in the human breast cancer cell line MDA-MB-231 and the normal human breast cell line Hs 578Bst (control). (A) Protein levels of Shh-Gli signaling molecules in MDA-MB-231 were determined using western blotting. (B) The mRNA levels of Shh-Gli signaling pathway components were quantified using reverse transcription-quantitative polymerase chain reaction. GAPDH expression was used for normalization. Quantitative data are expressed as mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001 compared with control. Shh, sonic hedgehog; Ptch, patched; SMO, smoothened; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effects of Shh-Gli signaling on MDA-MB-231 cell proliferation
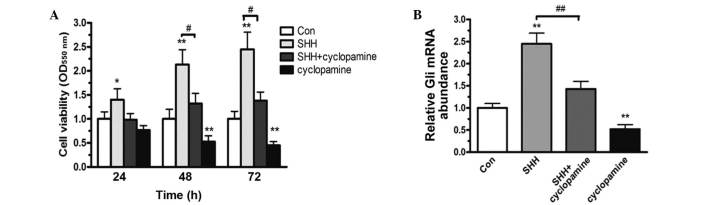
In various types of cancers, Shh-Gli signaling has been reported to be widely activated (18). Exogenous activation of Shh signaling also significantly stimulates cell proliferation (11). Cyclopamine, a specific inhibitor of the Shh-Gli signaling pathway, decreases Shh-induced malignant cancer cell proliferation (19,20). To determine the effects of Shh-Gli signaling on cell proliferation, MDA-MB-231 cells were treated with exogenous Shh, cyclopamine or exogenous Shh plus cyclopamine for 24, 48, or 72 h, and cell viability was determined using an MTT assay. In the presence of exogenous Shh, MDA-MB-231 cell viability was significantly enhanced, while suppression of Shh-Gli signaling with cyclopamine reduced cell viability in a time-dependent manner (P<0.01; Fig. 2A). This result indicates that Shh-Gli signaling regulates human breast cancer cell proliferation. Furthermore, Gli is considered as a hallmark of the Shh-Gli signaling pathway (21). Thus, the transcript level of Gli was determined using RT-qPCR. Compared with the control group, treatment with exogenous Shh significantly increased Gli mRNA levels, while cyclopamine markedly reduced Gli transcription (Fig. 2B). Together, these results indicate that Shh-Gli signaling is important in breast cancer cell proliferation.
Figure 2.
The effects of Shh-Gli signaling on MDA-MB-231 cell proliferation. (A) The influence of exogenous Shh, with or without cyclopamine, was determined in MDA-MB-231 cells at 24, 48, and 72 h using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (B) The effects were also determined using reverse transcription-quantitative polymerase chain reaction. Quantitative data are expressed as mean ± standard error of the mean. *P<0.05 and **P<0.01 compared with control; #P<0.05; ##P<0.01. n=5 independent experiments. Shh, sonic hedgehog; Con, control; OD, optical density.
Effect of Shh-Gli signaling on protein levels of Bcl-2 and cyclin D1
To further explore the effects of Gli on cell proliferation, the protein levels of Bcl-2 and cyclin D1 were analyzed using western blotting. In the presence of Shh, Bcl-2 and cyclin D1 were significantly enhanced (Fig. 3A). By contrast, when Gli expression was inhibited with cyclopamine, Bcl-2 and cyclin D1 were remarkably reduced. Thus Gli may regulate the protein levels of Bcl-2 and cyclin D1 in MDA-MB-231 cells. Meanwhile, cyclopamine treatment significantly reduced Gli, Bcl-2 and cyclin D1 protein levels induced by Shh. Furthermore, cyclopamine significantly enhanced cell apoptosis, while Shh treatment reversed this increase in apoptotic rate (Fig. 3B). These data suggested that overexpression of Gli positively regulates transcription of Bcl-2 and cyclin D1, thereby regulating MDA-MB-231 cell proliferation and survival.
Figure 3.
Effects of Shh and/or cyclopamine on Gli target expression and apoptosis in MDA-MB-231 cells. (A) Expression of Gli, Bcl-2 and cyclin D1 proteins analyzed using western blotting. (B) Effects of Shh and cyclopamine on apoptosis. Quantitative data are expressed as mean ± standard error of the mean. *P<0.05 and **P<0.01 compared with control; #P<0.05. n=5 independent experiments. Shh, sonic hedgehog; Bcl-2, B-cell lymphoma 2; Con, control; NC, negative control; Cyc, cyclopamine.
Shh signaling pathway induces MDA-MB-231 migration through the epithelial-mesenchymal transition (EMT) and MMP-9/MMP-2 secretion
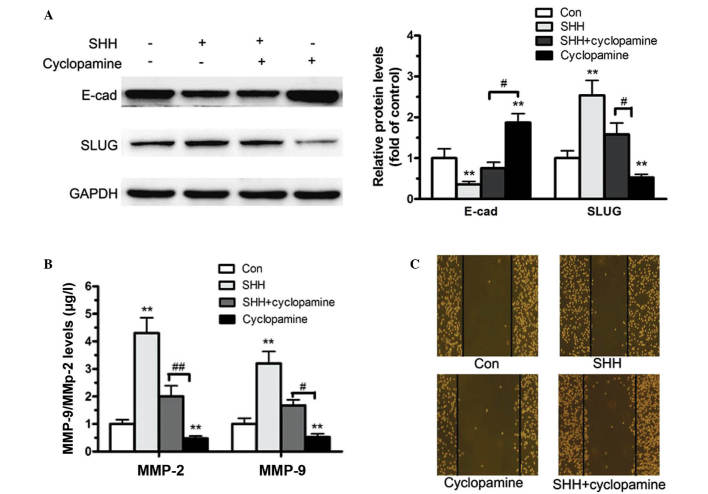
In the tumor progression process, EMT is important in tumor motility and invasion (22). E-cadherin is a hallmark molecule of the EMT process, and its downregulation indicates enhanced cell motility (23). SLUG is a downstream target of Shh-Gli signaling, and is suggested to serve key roles in the EMT process (13). In the current study, western blotting demonstrated that 72 h treatment of MDA-MB-231 cells with human sonic hedgehog, n-terminus (N-Shh) significantly reduces E-cadherin protein levels (Fig. 4A). By contrast, cells treated for 72 h with cyclopamine exhibited enhanced E-cadherin levels, whereas the expression of SLUG was significantly suppressed. These changes were completely restored by pretreatment with cyclopamine prior to N-Shh stimulation (Fig. 4A).
Figure 4.
Effects of Shh signaling on the epithelial-mesenchymal transition, MMP-9/MMP-2 activity, and cell invasion. (A) MDA-MB-231 cells were treated with N-Shh (0.5 mg/ml) alone or together with cyclopamine (10 mmol/l) for 72 h. The protein levels of E-cad and SLUG were analyzed using western blotting. (B) Cells treated with N-Shh (0.5 mg/ml) alone or in combination with cyclopamine (10 mmol/l) for 24 h were collected and the levels of MMP-9/MMP-2 were analyzed by enzyme-linked immunosorbent assay. (C) Cell invasion capacity was analyzed using the scratch assay following treatment with N-Shh (0.5 mg/ml) alone or in combination with cyclopamine (10 mmol/l). Quantitative data are expressed as mean ± standard error of the mean. **P<0.01 compared with control; #P<0.05; ##P<0.01. n=5 independent experiments. Shh, sonic hedgehog; N-Shh, human sonic hedgehog, n-terminus; MMP, matrix metalloproteinase; E-cad, E-cadherin; SLUG, snail family zinc finger 2; Con, control.
Subsequently, Shh-mediated cell migration and invasion were evaluated to determine whether these processes involved elevated levels of MMPs, which are associated with tumor invasiveness (24). ELISA indicated that MDA-MB-231 cells treated with Shh had significantly enhanced MMP-9 and MMP-2 levels. By contrast, inhibition of the Shh-Gli signaling pathway with cyclopamine led to significantly reduced MMP-9 and MMP-2 levels (Fig. 4B). Cell invasion capacity was determined using a scratch assay, which revealed that pretreatment with Shh enhanced, while cyclopamine treatment reduced, MDA-MB-231 cell invasion (Fig. 4C). However pretreatment with cyclopamine prior to Shh stimulation significantly reduced Shh-induced cell invasion (Fig. 4C). These findings indicate that Shh-Gli activation enhances the EMT process and increases MMP-9/MMP-2 secretion, thereby promoting cell migration and invasion in breast cancer cells.
Discussion
Breast cancer is the most common malignant cancer among women (25). Although a number of effective therapies have been reported, the majority of breast cancers are difficult to cure, resulting in a high mortality rate (26). The Hh signaling pathway serves a key role in cell proliferation, survival and differentiation. In cells in the normal state, Hh signaling is active and effectively regulates micro-environmental homeostasis (27). In a subset of malignant tumors, including those of the skin, brain, lung and pancreas, Hh signaling has been identified to be aberrantly activated (28,29). In the mammary gland, abnormal Hh signaling activation leads to enhanced tumor formation and progression (30). However, little is known with regard to the specific role of Hh signaling in breast cancer. In the present study, the specific mechanisms of Hh signaling in the development of breast cancer were explored.
In vertebrates, three Hh homologs have been identified: Shh, Desert hedgehog and Indian hedgehog (1). Ptch1 is a 12-pass transmembrane protein that inhibits the 7-pass transmembrane protein, Smo. Through binding with Hh, Smo is released from the inhibitory action of Ptch1, thereby activating the downstream Hh signaling pathway (31). Subsequently, the zinc finger transcription factors Gli1, Gli2 and Gli3 are activated, among which Gli1 and Gli2 serve as the primary targets (32). The present study examined the protein levels of members of the Shh-Gli signaling pathway and identified that Shh, Ptch, Smo and Gli1 were all significantly enhanced in the human breast cancer cell line MDA-MB-231 compared with the normal human breast cell line Hs 578Bst. In addition, the role of Shh signaling on cell viability was investigated. As shown in Fig. 2, when Gli was stimulated with exogenous Shh, breast cancer cell viability was significantly enhanced. By contrast, when Gli expression was suppressed with the specific inhibitor, cyclopamine, cell viability was significantly decreased. These data indicate that Shh signaling serves key functions in human breast cancer.
Bcl-2 and cyclin D1 are considered important target genes of the Shh signaling pathway, directly affecting cell proliferation and survival (33). Research indicates that high levels of cyclin D1 are associated with activation of the transcription factor Gli (34). Furthermore, the pro-survival protein Bcl-2 is upregulated in various tumors, including human breast cancers (35). Thus the Hh pathway has been suggested to promote cancer cell proliferation, possibly by the upregulation of Bcl-2 and cyclin D1 proteins. Consequently, the present study explored the protein levels of Bcl-2 and cyclin D1 under the influence of exogenous Shh, alone or in combination with cyclopamine treatment. Shh significantly stimulated the enhanced expression of Bcl-2 and cyclin D1, while suppression of Shh signaling by cyclopamine inhibited Bcl-2 and cyclin D1 expression. These data indicate that the Shh signaling pathway promotes cell survival by increasing protein levels of Bcl-2 and cyclin D1.
SLUG is a downstream target of Shh signaling and is closely associated with the EMT (36). The Shh signaling pathway has also been established to be a key contributor to the process of EMT (37). EMT is characterized by changes in cell morphology and physiology, accompanied by reduced epithelial-cell specific gene expression and enhanced mesenchymal markers (38). E-cadherin is an important cell-cell adhesion molecule that is significantly reduced during the EMT process, enabling cells to break the structural constraints between cells (39). In malignant tumors, the EMT process converts normal cells into malignant ones. In the present study, the impact of Shh signaling on the EMT process was investigated. The results indicated that the addition of exogenous Shh significantly reduced the level of E-cadherin, while pretreatment with cyclopamine reversed such effects. These data indicate that Shh signaling promotes the EMT, thereby enhancing malignant cell migration. Enhanced secretion of MMP-2 and MMP-9 was also identified when MDA-MB-123 cells were pretreated with exogenous Shh, further enhancing cell migration capacity. To confirm this, cell migration capacity was assessed using the scratch test. The results indicated that exogenous Shh significantly enhances MDA-MB-123 cell migration ability.
In conclusion, the current results indicate that Shh-Gli signaling is significantly activated in MDA-MB-231 human breast cancer cells. Activation of the Shh-Gli signaling pathway enhances MDA-MB-123 cell viability in a time-dependent manner. Thus increased Bcl-2 and cyclin D1 expression significantly enhances MDA-MB-231 cell proliferation capacity. Furthermore, activation of Shh-Gli signaling enhances cell migration capacity by reducing E-cadherin expression and increasing MMP-9/MMP-2 secretion. These results indicate that Shh-Gli signaling may be a potential therapeutic target in clinical practice for the treatment of breast cancer.
Acknowledgements
This work was supported by grants from the Social Development Project, Science and Technology Department of Shaanxi Province, Xi'an, China (2010k01-140).
References
- 1.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):S19–S24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 3.Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, Thérond PP. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol. 2003;5:907–913. doi: 10.1038/ncb1052. [DOI] [PubMed] [Google Scholar]
- 4.Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, Sasaki H, Schier AF. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130:1549–1564. doi: 10.1242/dev.00364. [DOI] [PubMed] [Google Scholar]
- 5.Dunaeva M, Michelson P, Kogerman P, Toftgard R. Characterization of the physical interaction of Gli proteins with SUFU proteins. J Biol Chem. 2003;278:5116–5122. doi: 10.1074/jbc.M209492200. [DOI] [PubMed] [Google Scholar]
- 6.Abidi A. Hedgehog signaling pathway: A novel target for cancer therapy: Vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3–12. doi: 10.4103/0253-7613.124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott MP. Cancer: A twist in a hedgehog's tale. Nature. 2003;425:780–782. doi: 10.1038/425780a. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 9.Cowan R, Hoban P, Kelsey A, Birch JM, Gattamaneni R, Evans DG. The gene for the naevoid basal cell carcinoma syndrome acts as a tumour-suppressor gene in medulloblastoma. Br J Cancer. 1997;76:141–145. doi: 10.1038/bjc.1997.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 11.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 12.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 13.Che J, Zhang FZ, Zhao CQ, Hu XD, Fan SJ. Cyclopamine is a novel Hedgehog signaling inhibitor with significant anti-proliferative, anti-invasive and anti-estrogenic potency in human breast cancer cells. Oncol Lett. 2013;5:1417–1421. doi: 10.3892/ol.2013.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Hui C, Daniel CW. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- 15.Lewis MT. Hedgehog signaling in mouse mammary gland development and neoplasia. J Mammary Gland Biol Neoplasia. 2001;6:53–66. doi: 10.1023/A:1009516515338. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Johnson RL, Zhang X, Bare JW, Waldman FM, Cogen PH, Menon AG, Warren RS, Chen LC, Scott MP, Epstein EH., Jr Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–2372. [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Kayed H, Kleeff J, Osman T, Keleg S, Büchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–129. doi: 10.1097/01.mpa.0000202937.55460.0c. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Yu Y, Deng C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fluorosis. Toxicol Lett. 2014;225:318–324. doi: 10.1016/j.toxlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: Fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/S0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury AK, Ghosh S, Rudin CM, Mukherjee A, Basu A, Dhara S. Hedgehog signaling pathway is active in GBM with GLI1 mRNA expression showing a single continuous distribution rather than discrete high/low clusters. PLoS One. 2015;10:e0116390. doi: 10.1371/journal.pone.0116390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ailles L, Siu LL. Targeting the Hedgehog pathway in cancer: Can the spines be smoothened? Clin Cancer Res. 2011;17:2071–2073. doi: 10.1158/1078-0432.CCR-11-0211. [DOI] [PubMed] [Google Scholar]
- 23.Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, Wouters BG, Lammering G, Vooijs M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392–397. doi: 10.1016/j.radonc.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;44–46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwabo Kamdje AH, Seke Etet PF, Vecchio L, Muller JM, Krampera M, Lukong KE. Signaling pathways in breast cancer: therapeutic targeting of the microenvironment. Cell Signal. 2014;26:2843–2856. doi: 10.1016/j.cellsig.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Qiao A, Gu F, Guo X, Zhang X, Fu L. Breast cancer-associated fibroblasts: their roles in tumor initiation, progression and clinical applications. Front Med. 2016;10:33–40. doi: 10.1007/s11684-016-0431-5. [DOI] [PubMed] [Google Scholar]
- 27.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 28.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Maitah MY, Ahmad A, Kong D, Bao B, Sarkar FH. Targeting the Hedgehog signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:49–66. doi: 10.1517/14728222.2011.617367. [DOI] [PubMed] [Google Scholar]
- 30.Di Magno L, Coni S, Di Marcotullio L, Canettieri G. Digging a hole under Hedgehog: Downstream inhibition as an emerging anticancer strategy. Biochim Biophys Acta. 2015;1856:62–72. doi: 10.1016/j.bbcan.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui M, Cazet A, Nair R, Watkins DN, O'Toole SA, Swarbrick A. The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013;15:203. doi: 10.1186/bcr3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: Mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Shahi MH, Afzal M, Sinha S, Eberhart CG, Rey JA, Fan X, Castresana JS. Regulation of sonic hedgehog-GLI1 downstream target genes PTCH1, Cyclin D2, Plakoglobin, PAX6 and NKX2.2 and their epigenetic status in medulloblastoma and astrocytoma. BMC Cancer. 2010;10:614. doi: 10.1186/1471-2407-10-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F. Activation of the Bcl2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 36.Joannes A, Grelet S, Duca L, Gilles C, Kileztky C, Dalstein V, Birembaut P, Polette M, Nawrocki-Raby B. Fhit regulates EMT targets through an EGFR/Src/ERK/Slug Signaling axis in human bronchial cells. Mol Cancer Res. 2014;12:775–783. doi: 10.1158/1541-7786.MCR-13-0386-T. [DOI] [PubMed] [Google Scholar]
- 37.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi HW, Xin LY, Xu X, Ji XX, Fan LH. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J Transl Med. 2014;12:22. doi: 10.1186/1479-5876-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]