Abstract
Circulating microRNAs (miRNAs) have been proposed as promising biomarkers for multiple diseases. miR-126 is reported to be associated with type 2 diabetes mellitus (T2D), diabetic nephropathy (DN) and end stage renal disease. The aim of this study was to investigate the expression of circulating miR-126 and to assess its potential as a blood-based biomarker for DN in T2D patients. In 52 patients with T2D without history of DN (with noromoalbuminuria), 50 patients with T2D and DN (29 with microalbuminuria and 21 with macroalbuminuria), and 50 non-diabetic healthy controls, the expression of circulating miR-126 in peripheral whole blood was evaluated by quantitative polymerase chain reaction. The expression levels of circulating miR-126 were significantly decreased in T2D patients and further decreased in DN patients compared with those in the controls. Multivariate logistic regression analysis confirmed the independent association of lower miR-126 levels with T2D [adjusted odds ratio (OR), 0.797; 95% confidence interval (CI), 0.613–0.960] and DN (adjusted OR, 0.513; 95% CI, 0.371–0.708). miR-126 levels were associated with the degree of albuminuria and showed significantly low expression in DN patients with microalbuminuria (adjusted OR, 0.781; 95% CI; 0.698–0.952) and further lower expression in DN patients with macroalbuminuria (adjusted OR, 0.433; 95% CI, 0.299–0.701), respectively compared with T2D patients with normoalbuminuria. miR-126 levels negatively correlated with albuminuria positively with glomerular filtration rate (P<0.05), and in addition, negatively correlated with fasting glucose, glycated hemoglobin, triglyceride and LDL (P<0.05). Stepwise multiple regression analysis identified albuminuria as a significant predictor of miR-126 (P<0.001). miR-126 in peripheral blood yielded area under the receiver operating characteristic curves of 0.854 (95% CI, 0.779–0.929) and 0.959 (95% CI, 0.916–1.000) in the differentiation of DN patients from T2D patients and DN patients from non-diabetic controls respectively. These data suggest that decreased expression of circulating miR-126 is associated with the development of DN in T2D patients, and may be a promising blood-based biomarker for DN risk estimation.
Keywords: type 2 diabetes mellitus, diabetic nephropathy, circulating miRNAs, miR-126, biomarker
Introduction
Diabetic nephropathy (DN) is a long-term microvascular complication of diabetes mellitus, affecting 30–35% of diabetic patients and is a major cause of end-stage renal disease (ESRD) (1). The incidence of ESRD associated with type 2 diabetes (T2D) has markedly increased because the prevalence of T2D has increased greatly, and accounts for >90% of diabetic cases (2).
The molecular pathophysiology of DN is complex, involving interactions between hyperglycemia-induced metabolic, hemodynamic and inflammatory factors (3,4). These factors modify the function and morphology of blood vessel walls and interact with neighboring cells leading to renal endothelial dysfunction, which plays a central role in the development of DN (4).
The earliest clinical indication of DN is the appearance of abnormally low levels of albumin in the urine (microalbuminuria) (5). The onset of microalbuminuria leads to macroalbuminuria, and the latter is followed by deterioration of renal function with a progressive decline in the glomerular filtration rate (GFR), which eventually leads to ESRD (6). Although microalbuminuria is considered as the gold standard for the diagnosis of DN, it can reveal renal dysfunction only after a long period of a clinically silent phase of the disease (7), and is also independently associated with increased cardiovascular risk (8). In addition, it has been reported that renal impairment occurs in one-third of patients with DN, even prior to the appearance of microalbuminuria (9). Other markers of DN risk are required for optimal clinical management (9), particularly for patients with T2D who pass through a period of pre-diabetes and may experience renal impairment at the time of diagnosis. In this regard, a number of candidate markers have been proposed for the early identification of renal injury, such as creatinine, kidney enzymes, cystatin C and C-reactive protein (10,11).
Genomic regulatory non-coding RNA molecules, namely microRNAs (miRNAs), have been shown to be involved in the control of key biological processes such as proliferation, differentiation, apoptosis and metabolism (12). miRNAs function through inhibition of translation by binding to the 3′ untranslated region (3′-UTR) of their target messenger RNAs (mRNAs) (13). Alterations in the regulation of these miRNAs can lead to serious physiological abnormalities, including chronic diseases such as diabetes (14,15). Studies using in vivo and in vitro models of diabetic renal disease have also linked the dysregulation of miRNA expression with the pathogenicity and progression of DN (16,17).
miRNAs have been found in tissues and several human body fluids, including blood, in a stable form that is protected from endogenous RNase activity (18,19). The sequences of the majority of miRNAs are conserved among different species and their expression is tissue- or biological stage-specific (20). miRNAs are accessible through non-invasive methods, and can be easily assessed by sensitive and specific methods such as quantitative polymerase chain reaction (qPCR) (19,20). This has led to the proposal that circulating miRNAs may be useful biomarkers for the detection of various diseases, including cancer and diabetes (21–23).
miR-126 is highly enriched in endothelial cells and plays a key role in angiogenesis (24). It is the most extensively studied miRNA in diabetes, and a number of studies have shown decreased circulating miR-126 levels in the blood of patients with T2D (25–27). Moreover, changes in circulating miR-126 levels have been reported to be associated with DN and diabetic microvascular damage (28) and with the development of ESRD (29).
The aim of the present study was to investigate the expression of circulating miR-126 in the peripheral whole blood of patients with T2D without history of DN (with normoalbuminuria), DN patients (with microalbuminuria/macroalbuminuria) and non-diabetic healthy control individuals, and to evaluate the potential of miR-126 as a blood-based biomarker for DN in patients with T2D.
Materials and methods
Participants and clinical data
The study included 152 individuals: 52 patients with T2D without history of DN (with normoalbuminuria), 50 DN patients who had a history of albuminuria (microalbuminuria/macroalbuminuria) and 50 non-diabetic healthy controls. All subjects were recruited from King Abdullah University Medical Centre (Arabian Gulf University, Kingdom of Bahrain). The study was approved by the Research and Ethics Committee in the College of Medicine and Medical Sciences, Arabian Gulf University. The participants provided written consent for research use of their blood samples.
Age, gender, body mass index (BMI), blood pressure and other clinical parameters were collected by reviewing the medical records of the participants.
The diagnosis of T2D was in accordance with the World Health Organization (WHO) criteria (30) using combinations of the following parameters: Fasting glucose (FG) levels ≥7.0 mmol/l (126 mg/dl) or 2-h glucose levels ≥11.1 mmol/l (200 mg/dl) in an oral glucose tolerance test, and glycated hemoglobin (HbA1c) levels >6.5%. Normoalbuminuria was defined as an albumin/creatinine ratio (ACR) <2.5 mg/mmol for men and ACR <3.5 mg/mmol for women, with an albumin excretion rate (AER) of <25 mg/day. Microalbuminuria was defined as an ACR of 2.5–25 mg/mmol for men and 2.8–28 mg/mmol for women, with an AER of 30–300 mg/day. Macroalbuminuria was defined as an ACR of >25 mg/mmol for men and >28 mg/mmol for women, with an AER of >300 mg/day. GFR was assessed using the Modification of Diet in Renal Disease (MDRD) formula (31). Hypertension was defined as a systolic pressure of ≥140 mmHg, and/or a diastolic pressure of ≥90 mmHg. T2D patients without nephropathy had ≥10 years of diabetes duration, were without history of albuminuria, and had normal renal function and normal blood pressure (≤130/80 mmHg). Healthy control individuals had a FG level of 4.8–5.2 mmol/l (<110 mg/dl), were without history of albuminuria, and had normal renal function and normal blood pressure. General exclusion criteria were a known history of vascular events (stroke, unstable angina, acute myocardial infarction or coronary artery disease), or evidence of hepatic dysfunction and neoplastic diseases.
Extraction of miRNA
Whole blood samples were collected in tubes containing ethylenediamine tetraacetic acid, and total RNA including miRNA was extracted using a Norgen Blood RNA kit (Norgen Biotek Corporation, Thorold, ON, Canada) following the manufacturers' protocol. Samples were stored at −80°C until RNA extraction. RNA concentration and quality were assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Reverse transcription (RT)-qPCR
RT-qPCR analysis was performed using the TaqMan® MicroRNA Reverse Transcription kit and Applied Biosystems Real-Time PCR detection system (Thermo Fisher Scientific, Inc.) as previously described (22). The small nuclear RNU6B was used for normalization (22). The PCR primer sequences were as follows: Mature hsa-miR-126 (target), UCGUACCGUGAGUAAUAAUGC and RNU6B (reference), CGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTTT (Thermo Fisher Scientific, Inc.). qPCR was performed using a 7900HT Fast Real Time PCR system (Thermo Fisher Scientific, Inc.) with the following cycling conditions: 95°C for 10 min, followed by 95°C for 15 sec and 60°C for 60 sec for a total of 40 cycles. All reactions were performed in duplicate under identical experimental conditions. Results were analyzed using Sequence Detection Software, version 1.7 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The expression levels of miR-126 were normalized to those of RNU6B, and relative expression values were calculated using the 2−ΔΔCt method as previously described (22). The Ct values from qPCR assays with >35 cycles were treated as not expressed.
Data analysis
Data were analyzed using SPSS version 19 (IBM, Armonk, NY, USA). Chi-square and Student's t-tests were used to compare the expression levels of miR-126, and the differences in clinical parameters between T2D patients or DN patients and non-diabetic healthy controls. One-way analysis of variance was used to compare differences in clinical parameters among the subject groups. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to assess the association of miR-126 with T2D and DN, with multivariate logistic regression models after adjusting for multiple factors. Pearson's correlation coefficient was used to test the correlation between miR-126 and albuminuria, estimated GFR (eGFR), and other clinical variables. A stepwise multiple regression analysis was performed to identify the predictors of miR-126 (dependent variable). Receiver operating characteristic (ROC) analysis was used to assess the biomarker potential of miR-126 for DN, and areas under the curves (AUCs) were reported. All statistical analyses were two-sided, and a P-value <0.05 was considered significant.
Results
Baseline characteristics of subjects
The baseline characteristics of the subjects in this study are shown in Table I. There was a significant difference in age between T2D patients, DN patients and non-diabetic healthy controls (P<0.05), but no statistically significant difference was found for gender (P>0.05). FG, HbA1c and total cholesterol levels differed significantly in T2D patients and DN patients compared with controls (P<0.05). Patients with DN had significantly higher blood pressure and triglyceride levels than patients with T2D and controls (P<0.05). There was no significant difference between the T2D patient and DN patient groups in terms of FG, HbA1c, diabetes duration, LDL and HDL (P>0.05). Patients with DN exhibited increased albuminuria, ACR and serum creatinine and decreased eGFR compared with T2D patients (P<0.05).
Table I.
Baseline characteristics of subjects.
| Characteristic | T2D (normoal-buminuria) | All DN microalbuminuria/macroalbuminuria | DN (microal-buminuria) | DN (macro-albuminuria) | Controls |
|---|---|---|---|---|---|
| Number of subjects | 52 | 50 | 29 | 21 | 50 |
| Age (years) | 62.0±10.5a | 64.6±6.3a | 62.5±6.5 | 67.0±5.4 | 56±5.2 |
| Gender (male/female) | 27/25 | 24/26 | 16/13 | 8/13 | 22/28 |
| BMI (kg/m2) | 25.2±4.5 | 26.3±5.2a | 26.7±5.1 | 25.8±4.7 | 24.2±4.1 |
| Mean blood pressure (mmHg) | 86.2±11.3 | 96.2±12.6a,c | 92.8±10.5 | 99.5±13.4 | 83.9±2.5 |
| Diabetes duration (years) | 15.2±4.4 | 17.5±4.6 | 16.4±4.4 | 19.0±4.1 | – |
| FG (mmol/l) | 8.8±2.2b | 8.7±1.2b | 8.6±1.4 | 8.8 ±1.1 | 4.3±0.6 |
| HbA1c (%) | 8.9±2.7b | 9.3±1.8b | 8.1±1.1 | 10.6±1.4 | 4.9±0.7 |
| Total cholesterol (mmol/l) | 4.4±1.2a | 4.6±1.1b | 4.3±1.2 | 4.9±0.9 | 4.02±0.8 |
| LDL (mmol/l) | 2.8±1.4 | 4.4±1.5a | 3.7±1.2 | 4.3±1.0 | 2.14±0.8 |
| HDL (mmol/l) | 1.3±0.3 | 1.3±0.3 | 1.4±0.2 | 1.2±0.3 | 1.3±0.2 |
| Triglyceride (mmol/l) | 1.7±0.6 | 2.5±1.3b,c | 1.8±1.3 | 3.2±1.1 | 1.6±0.6 |
| Albuminuria (mg/day) | 6.2±3.3 | 223.3±117.9b,c | 113.4±52.2 | 332.8±28.7 | 5.4±1.7 |
| ACR (mg/mmol) | 1.0±0.7 | 28.3±12.0b,c | 22.2±15.8 | 34.3±7.7 | 0.9±0.4 |
| Serum creatinine (mm/l) | 66.3±15.3a | 119.2±45.8b,c | 121.2±41.1 | 176.8±41.2 | 54.7±11.7 |
| eGFR (ml/min/1.73 m2) | 95.0±9.4 | 66.0±14.8b,c | 78.2±15.1 | 53.7±7.7 | 104.3±13.2 |
Data are presented as numbers for categorical data, or mean ± standard deviation for parametrically distributed data. T2D, type 2 diabetes mellitus; DN, diabetic nephropathy; BMI, body mass index; FG, fasting glucose; HbA1c, glycated hemoglobin; LDL, low density lipoprotein; HDL, high density lipoprotein; ACR, albumin/creatinine ratio; eGFR, estimated glomerular filtration rate.
P<0.05
P<0.001 compared with controls
P<0.05 compared with T2D.
Expression of circulating miR-126 in T2D patients, DN patients and healthy controls
The expression levels of circulating miR-126 in peripheral whole blood were quantified by qPCR in T2D patients, DN patients and non-diabetic healthy individuals; and were expressed relative to the endogenous control RNU6B as mean ± standard deviation (SD).
In comparison with those in non-diabetic healthy control individuals, the expression levels of circulating miR-126 were significantly decreased in T2D patients and further decreased in DN patients (P<0.05; Fig. 1). miR-126 levels were 2.5-fold lower in T2D patients and 5-fold lower in DN patients compared with controls. Notably, DN patients had significantly lower miR-126 levels (2-fold lower) compared with those in T2D patients (P<0.05). The means (± SD) of relative quantification for miR-126 were 10.5±4.3 for T2D patients, 5.3±7.6 for DN patients and 26.4±10.0 for controls.
Figure 1.
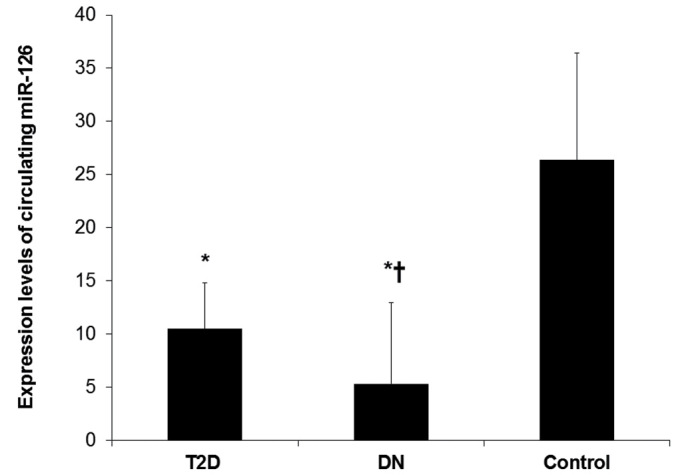
Expression of circulating miR-126 in T2D patients, DN patients and healthy controls. Expression levels of circulating miR-126 were quantified by quantitative polymerase chain reaction in the peripheral whole blood of T2D patients with normoalbuminuria (n=52), DN patients with microabuminuria or macroalbuminuria (n=50) and non-diabetic healthy control individuals (n=50). Relative expression levels of miR-126 normalized to those of RNU6B are expressed as the mean ± standard deviation (SD). *P<0.05 vs. the control group, †P<0.05 vs. the T2D group.
On the application of further analysis using multivariate logistic regression with adjustment for age, gender, BMI and blood pressure, additionally for FG and HbA1c, and further for triglyceride and LDL (Table II), low miR-126 levels were independently associated with T2D (OR, 0.797; 95% CI, 0.613–0.960; P=0.003) and with DN (OR, 0.513; 95% CI, 0.371–0.708; P=0.002).
Table II.
Association of circulating miR-126 expression levels with T2D and DN.
| T2D | DN | |||
|---|---|---|---|---|
| Circulating miR-126 | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Crude | 0.778 (0.708–0.853) | <0.001 | 0.557 (0.467–0.664) | <0.001 |
| Adjusted, model 1a | 0.869 (0.761–0.901) | 0.002 | 0.601 (0.496–0.728) | 0.030 |
| Adjusted, model 2b | 0.817 (0.669–0.923) | 0.046 | 0.540 (0.415–0.702) | 0.005 |
| Adjusted, model 3c | 0.797 (0.613–0.960) | 0.003 | 0.513 (0.371–0.708) | 0.002 |
Model 1: Adjusted for age, gender, BMI and blood pressure.
Model 2: Adjusted for model 1, FG and HbA1c.
Model 3: Adjusted for model 2, triglyceride and LDL. OR, odds ratio; CI, confidence interval; T2D, type 2 diabetes mellitus; DN, diabetic nephropathy; BMI, body mass index; FG, fasting glucose; HbA1c, glycated hemoglobin; LDL, low density lipoprotein.
Association of circulating miR-126 expression with the degree of albuminuria
To evaluate the association between circulating miR-126 and albuminuria, a sub-analysis was performed in T2D patients with normoalbuminuria (n=52), and DN patients with microalbuminuria (n=29) or macroalbuminuria (n=21) to determine the association between circulating miR-126 expression levels and the development of DN.
As shown in Fig. 2, miR-126 levels were significantly lower in DN patients with microalbuminuria, and were further decreased in DN patients with macroalbuminuria than in T2D patients with normoalbuminuria (P<0.05). The means (± SD) of relative quantification for miR-126 were 10.5±4.3 for normoalbuminuria, 6.9±3.2 for microalbuminuria and 3.4±2.2 for macroalbuminuria.
Figure 2.
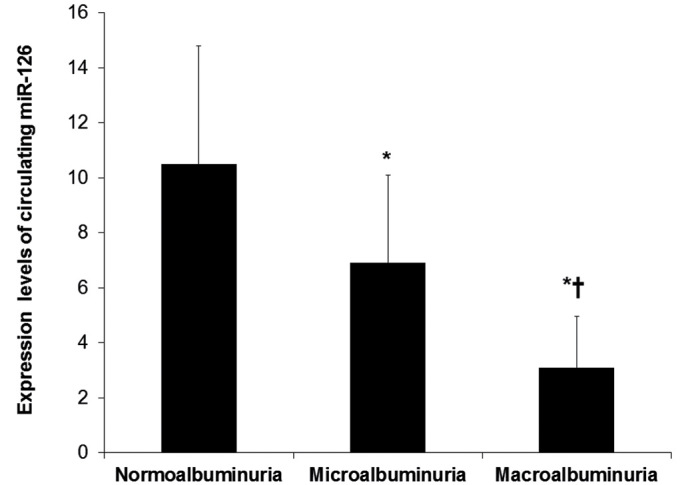
Association of circulating miR-126 levels with the degree of albuminuria. The association of circulating miR-126 expression levels with the degree of albuminuria was determined in a sub-analysis in T2D patients with normoalbuminuria (n=52), DN patients with microalbuminuria (n=29) and DN patients with macroalbuminuria (n=21) to determine the association between circulating miR-126 expression levels and the development of DN. *P<0.05 vs. the normoalbuminuria group, †P<0.05 vs. the microalbuminuria group.
The association of circulating miR-126 with the degree of albuminuria was also confirmed in multivariate logistic regression analysis after adjustment for age, gender, BMI and blood pressure, additionally for FG and HbA1c, and further for triglyceride and LDL. The adjusted OR was found to be 0.781 (95% CI, 0.698–0.952; P=0.04) for microalbuminuria and the adjusted OR was 0.433 (95% CI, 0.299–0.701; P=0.03) for macroalbuminuria (Table III).
Table III.
Association of circulating miR-126 expression levels with the degree of albuminuria.
| Microalbuminuria | Macroalbuminuria | |||
|---|---|---|---|---|
| Circulating miR-126 | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Crude | 0.758 (0.643–0.894) | 0.013 | 0.394 (0.275–0.564) | <0.001 |
| Adjusted, model 1a | 0.756 (0.641–0.892) | 0.046 | 0.347 (0.226–0.532) | <0.001 |
| Adjusted, model 2b | 0.769 (0.647–0.931) | 0.05 | 0.426 (0.291–0.624) | 0.03 |
| Adjusted, model 3c | 0.781 (0.698–0.952) | 0.04 | 0.433 (0.299–0.701) | 0.03 |
Model 1: Adjusted for age, gender, BMI and blood pressure.
Model 2: Adjusted for model 1, FG and HbA1c.
Model 3: Adjusted for model 2, triglyceride and LDL. OR, odds ratio; CI, confidence interval; T2D, type 2 diabetes mellitus; DN, diabetic nephropathy; BMI, body mass index; FG, fasting glucose; HbA1c, glycated hemoglobin; LDL, low density lipoprotein.
Correlation and multivariate analysis
Pearson's correlation coefficient was calculated in patients with DN to determine the correlation between circulating miR-126 and renal function parameters, including albuminuria and eGFR, as well as other clinical variables including FG, HbA1c, triglyceride and LDL.
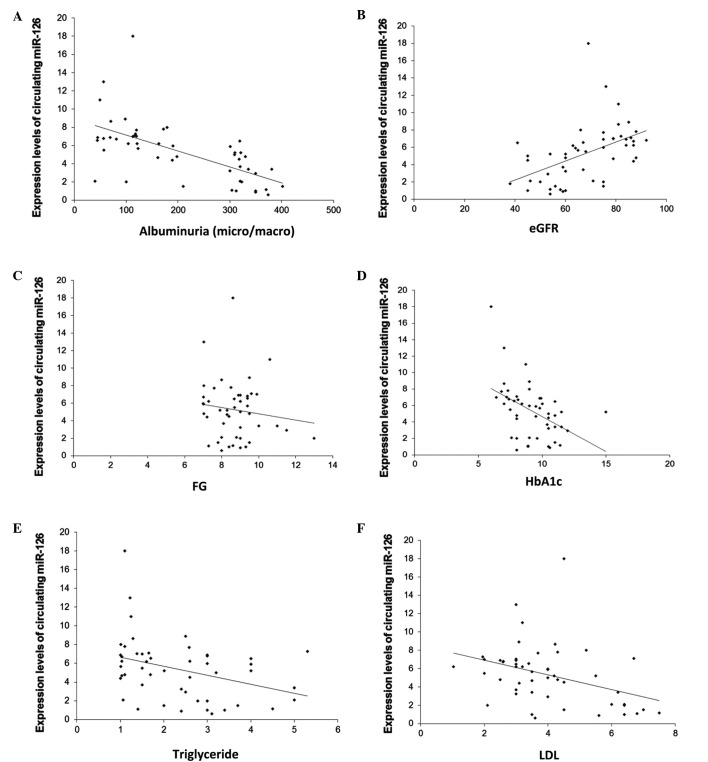
The results showed that the expression levels of circulating miR-126 were significantly and negatively correlated with albuminuria (r=−0.63; P<0.001; Fig. 3A), and were significantly and positively correlated with eGFR (r=0.48; P<0.001; Fig. 3B). Moreover, miR-126 levels were negatively correlated with FG (r=−0.63; P<0.001; Fig. 3C), HbA1c (r=−0.63; P<0.001; Fig. 3D), triglyceride (r=−0.63; P<0.001; Fig. 3E) and LDL (r=−0.63; P=0.01; Fig. 3F).
Figure 3.
Correlation between circulating miR-126 and renal function parameters and other clinical variables. Pearson's coefficient correlation analysis was undertaken in patients with DN to determine the correlation between circulating miR-126 levels and albuminuria, eGFR, FG, HbA1c, triglyceride and LDL. (A) Circulating miR-126 negatively correlated with albuminuria. (B) Circulating miR-126 positively correlated with eGFR. (C) Circulating miR-126 negatively correlated with FG. (D) Circulating miR-126 negatively correlated with HbA1c. (E) Circulating miR-126 negatively correlated with triglyceride. (F) Circulating miR-126 negatively correlated with LDL.
In addition, stepwise multiple regression analyses applied in these subjects, which included albuminuria, eGFR as well as age, gender, BMI, blood pressure, FG, HbA1c, total cholesterol, triglyceride and LDL, identified albuminuria as a significant predictor of circulating miR-126 (P<0.001).
ROC analysis
ROC analysis was performed to evaluate the usefulness of circulating miR-126 as a potential blood-based biomarker for DN in T2D patients.
First, the expression levels of circulating miR-126 were compared between DN patients and T2D patients. The ROC analysis of miR-126 yielded an AUC of 0.854 (95% CI, 0.779–0.929; P<0.001) in the differentiation of DN patients from T2D patients (Fig. 4A).
Figure 4.
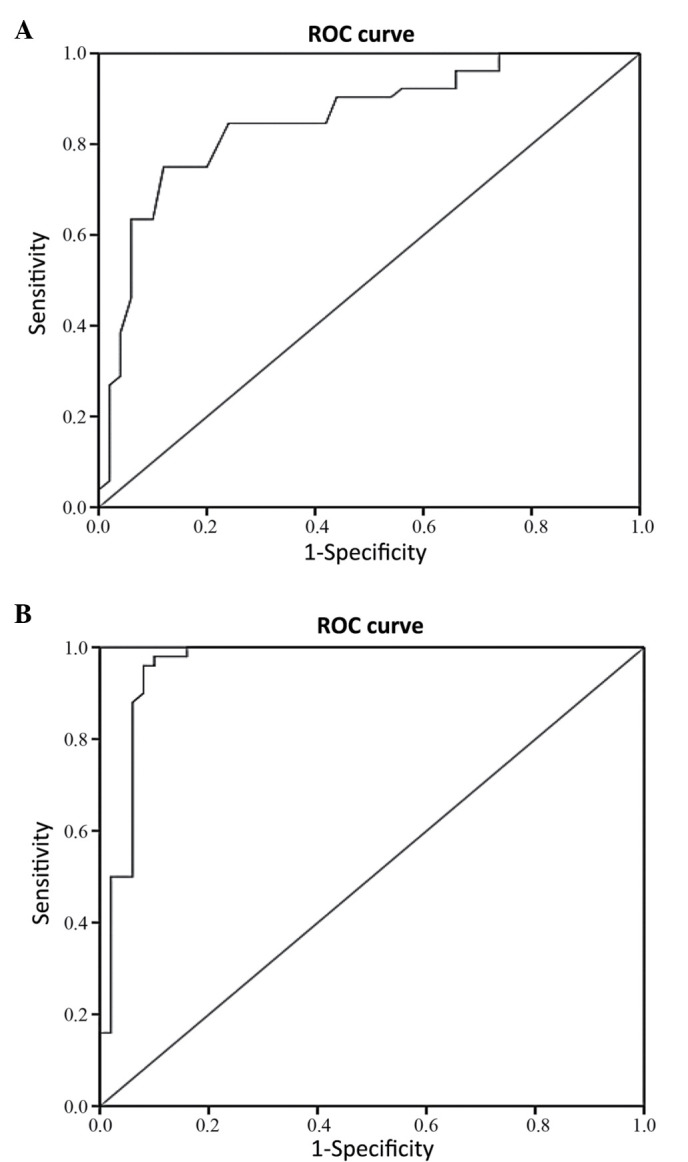
Receiver operating characteristic (ROC) curves of circulating miR-126. The receiver operating characteristic (ROC) analysis was used to assess the biomarker potential of miR-126, and the area under the curve (AUC) was reported. (A) MiR-126 differentiated between DN patients and T2D patients (AUC 0.854; 95% CI 0.779–0.929). (B) MiR-126 differentiated between DN patients and healthy controls (AUC 0.959; 95% CI 0.916–1.000).
Next, the expression levels of circulating miR-126 were compared between DN patients and non-diabetic healthy control individuals. The AUC of the ROC curve was found to be 0.959 (95% CI, 0.916–1.000; P<0.001) in the differentiation of DN patients from healthy controls (Fig. 4B).
Discussion
MicroRNAs (miRNAs) have emerged as key players in the modulation of gene expression in several biological processes (12), and have been implicated in the manifestation of various diseases, including diabetes and kidney diseases (14–17). miRNAs can be encapsulated in exosomes, microparticles, or apoptotic bodies (32,33), and actively released from cells and enter the circulation. It is believed that circulating miRNAs mediate cell-to-cell communication (33,34), and correlate well with diseases or injurious conditions. Changes in the levels of circulating miRNAs have been observed in several diseases, suggesting that miRNAs could serve as a new class of biomarkers (21–23).
In the present study, the expression of circulating miR-126 was investigated in the peripheral whole blood of T2D patients without history of DN (with normoalbuminuria), T2D patients with DN (with microalbuminuria/macroalbuminuria), and non-diabetic healthy control individuals; and the possibility of using circulating miR-126 as a blood-based biomarker for DN was evaluated.
The results showed significantly reduced expression levels of circulating miR-126 in T2D patients, with further reduction in DN patients as compared with non-diabetic healthy controls. The results also showed a significant association of lower circulating miR-126 levels with T2D and DN after controlling for possible confounders.
Previous studies on diabetes have shown decreased circulating miR-126 levels in the blood of patients with T2D (25–27), and the results of the present study are in agreement with these reports. However, in contrast to previous clinical data for DN which showed increased miR-126 levels in blood samples of DN patients with type 1 diabetes (T1D) (28) and in urine samples of DN patients of T2D (35) compared with healthy individuals, the results of the present study revealed decreased expression levels of circulating miR-126 in the peripheral blood of T2D patients with DN compared with the levels in non-diabetic healthy controls. Osipova et al (36) reported lower miR-126 levels in the urine of T1D pediatric patients compared with corresponding controls, but they found no differences in the levels of miR-126 in plasma from the two groups, whereas Wang et al (29) observed decreased circulating miR-126 levels in the plasma of patients with chronic kidney disease, which was associated with the development of ESRD.
miR-126, a highly enriched miRNA in endothelial cells and apoptotic bodies (24), promotes pro-angiogenic actions by repressing two negative regulators of the vascular endothelial growth factor (VEGF) pathway, namely Sprouty-related protein (SPRED1) and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2/p85-β), and enhances blood vessel formation (24,37). It has been also shown that apoptotic bodies containing miR-126, derived from endothelial cells, are taken up by neighboring vascular cells and induce vascular protection (38). Moreover, miR-126 has been shown to be expressed in glomerular and peritubular endothelial cells and targets negative repressors (such as PIK3R2 and SPRED1) of the VEGF pathway (39). In human umbilical vein endothelial cells, loss of miR-126 was observed when cultured with a high glucose concentration (25), and downregulation of endothelial miR-126 was reported to impair the functional properties of endothelial progenitor cells from diabetic patients via VEGF signaling (40).
Besides its role in endothelial homeostasis and angiogenesis, miR-126 additionally controls vascular inflammation through targeting and suppressing vascular cell adhesion molecule 1 (VCAM-1), thereby limiting leukocyte adherence to endothelial cells (41). miR-126 also protects against atherosclerosis by inhibiting VCAM-1 during inflammation (42,43) and contributes to renal microvascular heterogeneity of VCAM-1 protein expression in acute inflammation (44). Diabetes-induced endothelial dysfunction has been proposed as a major mechanism implicated in the pathogenesis and development of DN, occurring through activation of signal transducers of metabolic, hemodynamic and inflammatory factors (3,4), which modifies the function and morphology of neighboring cells, triggering a cascade of inflammatory, proliferative and profibrotic responses in progressive DN (4).
The results of the current study, indicating that T2D patients and DN patients exhibit significantly low expression levels of circulating miR-126 are consistent with the suggestion that decreased miR-126 levels are associated with a reduced response to VEGF and endothelial dysfunction (24,37). Furthermore, as miR-126 is an important regulator of vascular homeostasis and vascular inflammatory pathways (24,37,41), the results of the preset study suggest that decreased circulating miR-126 levels in patients with DN may be the consequence of diabetes-associated renal endothelial damage.
The progression of DN is commonly defined by an increase in albuminuria from normoalbuminuria to microalbuminuria and from microalbuminuria to macroalbuminuria. On average, 20–40% of patients with diabetes develop renal dysfunction (45), but type 2 diabetics with ESRD are rapidly increasing because of the continuing increase in the prevalence of T2D (2). Indeed, T2D patients represent the large majority of macroalbuminuric patients at risk of ESRD (2).
In the present study, a significant association of miR-126 expression with the degree of albuminuria was observed, as miR-126 levels were significantly low in DN patients with microalbuminuria and were even lower in DN patients with macralbuminuria than in patients with T2D and macralbuminuria. These results suggest a possible link between miR-126 and the development of DN.
In the DN patients, it was found that circulating miR-126 levels were correlated negatively with albuminuria, and positively with eGFR, suggesting that decreased miR-126 levels may be associated with the development of renal impairment. Notably, a previous study conducted in patients with ESRD has also shown a positive correlation between blood miR-126 levels and eGFR (29).
Additionally, the observation in the present study that circulating miR-126 levels negatively correlated with FG and HbA1c in DN patients, further supports the involvement of miR-126 in long-term hyperglycemia-induced diabetic renal damage. Furthermore, the negative correlation between circulating miR-126 and either triglyceride or LDL, may indicate a possible involvement of miR-126 in lipid metabolism in DN. In the same subject groups, the present study found that albuminuria is a significant predictor for circulating miR-126, by conducting a stepwise multiple regression analysis with different variables including albuminuria, eGFR as well as age, gender, BMI, blood pressure, FG, HbA1c, total cholesterol, triglyceride and LDL. The present study indicates that a significant reduction in miR-126 may be used as a biomarker for DN, as shown in the ROC curve analysis of its ability to discriminate DN patients from T2D patients, in addition to its ability to differentiate between DN patients and non-diabetic healthy control individuals.
Although the circulating level of miR-126 appears to have potential as a biomarker for DN, there are certain limitations to the current study. The sample size was relatively small and larger samples are required for the further validation of miR-126 as a biomarker for DN. Moreover, additional investigations and validation studies are required for the prognostic evaluation of circulating miR-126 levels in a clinical setting.
In conclusion, our results suggest that decreased expression of circulating miR-126 is implicated in the development of DN and may serve as a potential blood-based biomarker for the identification of T2D patients at risk of developing DN.
Acknowledgements
The authors would like to thank the staff of the Clinical Laboratory of King Abdullah University Medical Centre in the Kingdom of Bahrain. This study was supported by a research grant from the College of Medicine and Medical Sciences, Arabian Gulf University, Kingdom of Bahrain (grant no. 81).
References
- 1.Declèves AE, Sharma K. New pharmacological treatments for improving renal outcomes in diabetes. Nat Rev Nephrol. 2010;6:371–380. doi: 10.1038/nrneph.2010.57. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Remuzzi G. Nephropathy of type 1 and type 2 diabetes: Diverse pathophysiology, same treatment? Nephrol Dial Transplant. 2000;15:1900–1902. doi: 10.1093/ndt/15.12.1900. [DOI] [PubMed] [Google Scholar]
- 3.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets. 2014;14:22–33. doi: 10.2174/1871529X14666140401110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 6.Rossing K, Christensen PK, Hovind P, Tarnowl L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–1605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J Diabetes. 2013;4:245–255. doi: 10.4239/wjd.v4.i6.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillege HL, Janseen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE. Prevend Study Group: Microalbuminuria is common, also in a nondiabetic, no hypertensive population and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 9.Tabaei BP, Al-Kassab AS, Ilag LL, Zawacki CM, Herman WH. Does microalbuminuria predict diabetic nephropathy? Diabetes Care. 2001;24:1560–1566. doi: 10.2337/diacare.24.9.1560. [DOI] [PubMed] [Google Scholar]
- 10.Jeon YK, Kim MR, Huh JE, Mok JY, Song SH, Kim SS, Kim BH, Lee SH, Kim YK, Kim IJ. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci. 2011;26:258–263. doi: 10.3346/jkms.2011.26.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oddoze C, Morange S, Portugal H, Berland Y, Dussol B. Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis. 2001;38:310–316. doi: 10.1053/ajkd.2001.26096. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Ardekani AM, Naeini M. The role of microRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey AK, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: Tiny players in big disease. Cell Physiol Biochem. 2009;23:221–232. doi: 10.1159/000218169. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt K, Mi QS, Dong Z. MicroRNAs in kidneys: Biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300:F602–F610. doi: 10.1152/ajprenal.00727.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–1266. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: A new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo L, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 22.Al-Kafaji G, Al-Mahroos G, Alsayed NA, Hasan ZA, Nawaz S, Bakhiet M. Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Mol Med Rep. 2015;12:7485–7490. doi: 10.3892/mmr.2015.4416. [DOI] [PubMed] [Google Scholar]
- 23.Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hameddani F, Kayvanpour E, Just S, Borries A, Rudloff J, Leidinger P, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 26.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, Xifra G, Martínez C, Ricart W, Rieusset J, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37:1375–1383. doi: 10.2337/dc13-1847. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X. The role of circulating microRNA-126 (miR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci. 2014;15:10567–10577. doi: 10.3390/ijms150610567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijkerk R, Duijs JM, Khairoun M, Ter Horst CJ, van der Pol P, Mallat MJ, Rotmans JI, de Vries AP, de Koning EJ, de Fijter JW, et al. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am J Transplant. 2015;15:1081–1090. doi: 10.1111/ajt.13072. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Peng W, Shen X, Huang Y, Ouyang X, Dai Y. Circulating levels of inflammation-associated miR-155 and endothelial-enriched miR-126 in patients with end-stage renal disease. Braz J Med Biol Res. 2012;45:1308–1314. doi: 10.1590/S0100-879X2012007500165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Stoves J, Lindley EJ, Barnfield MC, Burniston MT, Newstead CG. MDRD equation estimates of glomerular filtration rate in potential living kidney donors and renal transplant recipients with impaired graft function. Nephrol Dial Transplant. 2002;17:2036–2037. doi: 10.1093/ndt/17.11.2036. [DOI] [PubMed] [Google Scholar]
- 32.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 33.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X. Stability of miR-126 in urine and its potential as a biomarker for renal endothelial injury with diabetic nephropathy. Int J Endocrinol. 2014;2014:393109. doi: 10.1155/2014/393109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osipova J, Fischer DC, Dangwal S, Volkmann I, Widera C, Schwarz K, Lorenzen JM, Schreiver C, Jacoby U, Heimhalt M, et al. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: A cross-sectional cohort study. J Clin Endocrinol Metab. 2014;99:E1661–E1665. doi: 10.1210/jc.2013-3868. [DOI] [PubMed] [Google Scholar]
- 37.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 39.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfer BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Wang HY, Li Y, Guo SH, Zhang L, Cai JH. Peripheral blood miRNAs as a biomarker for chronic cardiovascular diseases. Sci Rep. 2014;4:5026. doi: 10.1038/srep05026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asgeirsdóttir SA, van Solingen C, Kurniati NF, Zwiers PJ, Heeringa P, van Meurs M, Satchell SC, Saleem MA, Mathieson PW, Banas B, et al. MicroRNA-126 contributes to renal microvascular heterogeneity of VCAM-1 protein expression in acute inflammation. Am J Physiol Renal Physiol. 2012;302:F1630–F1639. doi: 10.1152/ajprenal.00400.2011. [DOI] [PubMed] [Google Scholar]
- 45.Hostetter TH. Prevention of the development and progression of renal disease. J Am Soc Nephrol. 2003;14(7 Suppl 2):S144–S147. doi: 10.1097/01.ASN.0000070150.60928.06. [DOI] [PubMed] [Google Scholar]