Abstract
Coenzyme A (CoA) is an essential cofactor that is emerging as a global regulator of energy metabolism. Tissue CoA levels are tightly regulated and vary in response to different conditions including nutritional state and diabetes. Recent studies reveal the ability of this cofactor to control the output of key metabolic pathways. CoA regulation is important for the maintenance of metabolic flexibility and glucose homeostasis.
Keywords: Coenzyme A, Leptin-deficient mice, fuel oxidation, hyperglycemia
Introduction
CoA is an essential and universally distributed cofactor that plays a central role in energy metabolism. This molecule contains an active thiol moiety that forms a thioester bond with cellular organic acids and acts as the major acyl group carrier. CoA participates in both the synthesis and degradation of all major fuels in the body including lipids and carbohydrates [1]. However, control of intermediary metabolism by this cofactor goes beyond its ability to activate and deliver substrates for hundreds of reactions. Several acyl-CoAs produced as metabolic intermediates allosterically modulate the activity of key enzymes and transcription factors to control flux through specific pathways [2,3]. For example, the ability of malonyl-CoA to potently inhibit carnitine palmitoyltransferase 1 (Cpt1) and fatty acid β-oxidation during lipogenesis, avoids a futile and wasteful cycle of fatty acid synthesis and degradation [3]. Acetyl-CoA occupies a strategic position at the intersection of both anabolic and catabolic pathways within the tricarboxylic acid cycle where it gauges the energy state of the cell and directs the carbon flow [4]. At the same time, acetyl-CoA is the source of the acetyl groups that covalently modify thousands of enzymes, transcription factors and chromatin to modulate their function. Thus, regulation by acetyl-CoA-dependent acetylation coordinates these different activities with cell metabolism [5-7]. CoA levels are themselves regulated and yet flexible to enable adaptation to the metabolic state in response to environmental changes or stimuli. CoA levels are maintained between upper and lower threshold values and vary between these thresholds in response to hormones, diet, drugs, the nutritional state and diabetes [1]. The best characterized mechanism of modulating CoA is through regulation of the biosynthetic pathway at the pantotohenate kinase step. Pantothenate kinase (PanK) catalyzes the phosphorylation of pantothenic acid to phosphopantothenic acid, and feed-back inhibition of this reaction by free CoA and CoA thioesters controls the gross output of the entire pathway [8,9].
Metabolic flexibility is defined as the ability to produce energy and sustain cellular function by adapting the utilization of nutrients to their availability. This term was originally coined by Kelley and Mandarino [10] to describe the ability of skeletal muscle in lean healthy subjects to switch from the preferential uptake, storage and degradation of carbohydrates in the fed state to high rates of fatty acid uptake and oxidation in the fasted state, otherwise known as "fuel switching". During fasting, blood glucose levels fall, while free fatty acids are released from the adipose tissue stores and become a more available energy source for metabolically flexible organs and tissues. The preferential oxidation of fatty acids, instead of glucose, spares glucose for use by the brain. The liver plays an essential role in maintaining whole-body glucose homeostasis during a fast [11]. Liver releases glucose into the blood stream by hydrolyzing its glycogen stores during fasting, and also by synthesizing glucose de novo via gluconeogenesis. In this review we focus on the evidence showing that the ability to adjust CoA levels in the liver is important to maintain the metabolic flexibility of this organ and whole-body glucose homeostasis.
CoA, metabolic flexibility and glucose homeostasis: the link between fatty acid oxidation and glucose production
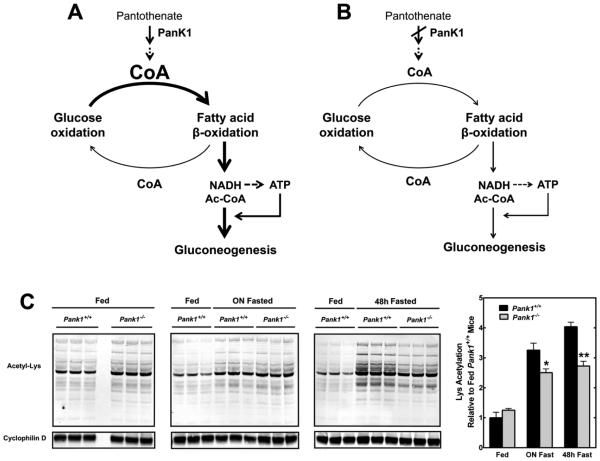
Mammals possess four PanK isoforms and two of them, PanK1α and 1β, are encoded by the Pank1 gene. Pank1 transcripts are highly abundant in the liver, and the hepatic CoA content increases in the fasted state [12,13]. Mice lacking Pank1 exhibit a 40% reduction in hepatic CoA which does not respond to fasting with the characteristic increase observed in wild-type mice [14]. This impairment, in turn, reduces the capacity for β-oxidation which heavily relies on the availability of unacylated CoA (CoASH) to degrade fatty acids. Thus, PanK1 is important to support the metabolic flexibility of the liver and its adaptation to the fasted state. Reduced hepatic CoA and fatty acid oxidation in the Pank1−/− livers are additionally associated with fasting hypoglycemia. This phenotype is milder than the dramatic hypoglycemia observed in mice treated with an inhibitor of all the PanK isoforms [15], and adds to the evidence that supports the tight connection between hepatic CoA and glucose homeostasis. Indeed, further analysis revealed that decreased de novo glucose production by the Pank1−/− livers is the cause for the fasting hypoglycemia of the animals, and led to the identification of CoA as a cofactor that links fatty acid degradation to gluconeogenesis (Fig. 1A and 1B). β-oxidation produces acetyl-CoA and NADH which, in turn, provides reducing equivalents for the synthesis of ATP. Both NADH and ATP are directly required for gluconeogenesis, which is additionally stimulated by acetyl-CoA through the allosteric activation of pyruvate carboxylase, a mitochondrial enzyme that catalyzes the first reaction in the pathway. Although acetyl-CoA was not directly measured in our study, the significant reduction in global mitochondrial protein acetylation observed in fasted Pank1−/− livers suggests that the acetyl-CoA concentration in this subcellular compartment is lower, thus preventing full activation of pyruvate carboxylase and of the gluconeogenic pathway (Fig. 1C). Finally, it is important to mention that no differences in the activation of the transcriptional program that accompanies the transition from the fed to the fasted state are observed in the Pank1−/− livers, indicating that decreased levels of a key small molecule like CoA is sufficient to modify the output of metabolic pathways.
Figure 1.
CoA links between fatty acid β-oxidation to gluconeogenesis. (A) In wild-type livers, the transition from the fed to the fasted state is characterized by a significant increase in CoA levels. This increase is required to support the switch from glucose to fatty acid oxidation that occurs during a fast. Fatty acid oxidation, in turn, feeds the gluconeogenic pathway by supplying NADH, ATP and acetyl-CoA (Ac-CoA), the allosteric regulator of pyruvate carboxylase. (B) Pank1 deficiency prevents the increase in hepatic CoA that occurs in the fasted state, thus decreasing the fatty acid β-oxidation capacity of the liver and the glucose output. (C) Global protein acetylation in liver mitochondria from fed and fasted Pank1+/+ and Pank1−/− mice was measured by western blotting and fluorescent detection using an anti-acetyllysine antibody and cyclophilin D as a loading control. Global protein acetylation increases with fasting in Pank1+/+ but not in Pank1−/− mice, consistent with the decrease in fatty acid β-oxidation and acetyl-CoA production caused by Pank1 deficiency. The immunoblot fluorescence signal was quantified using a Typhoon imaging system, and normalized to the loading control. Data are reported relative to the mean normalized signal of fed Pank1+/+ mice. * p<0.05, ** p<0.01
Pank1−/− mice contain a global deletion of the gene, and two additional features related to fuel utilization in these animals are noteworthy. First, circulating fasting ketone bodies in the Pank1−/− mice are similar to those measured in wild-type controls, and actually tend to be higher, in spite of the reduction in hepatic fatty acid oxidation [14]. Ketone bodies are synthesized from acetyl-CoA in the liver, and their blood level is commonly considered an indirect measurement of hepatic acetyl-CoA and fatty acid β-oxidation during a prolonged fast. The steady state concentration of ketone bodies in the blood depends on the rates of synthesis in the liver and degradation in extrahepatic tissues. The lack of Pank1 in tissues other than the liver could be responsible for the decreased CoASH-dependent utilization of ketones. This is more clearly observed in double knockout mice lacking both Pank1 and Pank2 that exhibit a substantial accumulation of serum ketone bodies and a more severe hypoglycemic phenotype compared to the Pank1−/− mice [16]. Second, Pank1−/− mice exhibit a significant reduction in exercise capacity compared to controls when tested for their ability to run on a treadmill until exhaustion (R. Leonardi and S. Jackowski, unpublished data). Skeletal muscle adjusts fuel selection between carbohydrates and lipids according to the intensity and duration of the exercise it is required to perform. The muscle from Pank1−/− mice may be unable to switch fuels efficiently because of lower CoASH and impaired fatty acid β-oxidation capacity during exercise. More studies will be required to fully characterize this additional aspect of the Pank1−/− metabolic phenotype.
PANK1, CoA and insulin
An association between insulin levels and single nucleotide polymorphisms (SNPs) on chromosome 10 in an intronic region of the human PANK1 gene was recently identified in a genome-wide association study (GWAS) on specific metabolic traits in the Northern Finland Birth Cohort 1966 [17]. The enrolled participants were born in 1966 in a genetically isolated region to eliminate differences in age and provide a relatively homogeneous genetic background. This study analyzed the influence of environmental exposures over several decades and genetic variation on nine metabolic traits that represent heritable risk factors for cardiovascular disease and type 2 diabetes. The parameters were serum triglycerides, high-density lipoprotein, low-density lipoprotein, glucose, insulin, C-reactive protein, body mass index (BMI) and systolic and diastolic blood pressure. Analysis of about 4200 people revealed that fasting insulin levels were exclusively associated, after correction for the BMI, with two SNPs in PANK1, rs11185790 and rs1075374. All other traits were associated with SNPs in 3-7 genes. Both SNPs are located in an intronic region of PANK1 and the effects of these mutations on PANK1 expression are currently not known. However, this GWAS suggested that insulin levels would be altered in Pank1−/− mice and, indeed, Pank1 deficiency causes a 50% reduction in fasting blood insulin. The Pank1−/− mice become hypoglycemic during fasting and the reduction in circulating insulin is likely an adaptive response of the pancreatic β-cells to prevent a more severe drop in fasting blood glucose. The combination of hypoglycemia and hypoinsulinemia is often associated with increased insulin sensitivity and improved responses to a bolus of glucose (glucose tolerance test, GTT) or insulin (insulin tolerance test, ITT) [18-20]. Glucose levels during GTT and ITT fall faster in Pank1−/− mice compared to control mice, indicating improved insulin sensitivity. These data extend the association between Pank1 expression, and glucose metabolism to include insulin homeostasis.
Deletion of Pank1 in diabetic mice and paradigms challenged
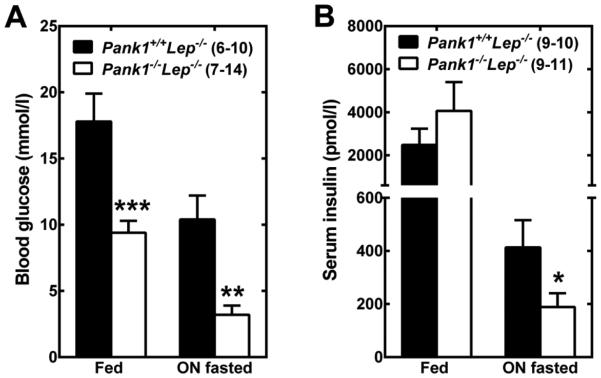
Type 2 diabetes is a complex, chronic, multiorgan disease characterized by abnormally high blood glucose levels. The etiology of type 2 diabetes is multifactorial with strong genetic and lifestyle components and, although the pathogenesis of type 2 diabetes is not fully understood, three key defects are responsible for the appearance of the hyperglycemia: impaired insulin action, hyperinsulinemia followed by reduced insulin secretion, and increased hepatic glucose production [21,22]. Lep−/− (ob/ob) mice are deficient in the hormone leptin and are an established model for human type 2 diabetes. These animals are hyperphagic, obese and exhibit hyperglycemia, hyperinsulinemia, insulin resistance and increased gluconeogenesis [23]. Liver and skeletal muscle from Lep−/− animals in the fed state contain almost twice the amount of CoA found in nonobese (Lep+/+ or Lep+/−) littermate controls [24]. The abnormally high liver CoA content results from higher PanK activity and reduced CoA degradation through Nudt7, a nudix hydrolase that specifically degrades CoA [25]. Nudt7 expression is significantly reduced in fed Lep−/− livers. While fasting of the non-obese controls is characterized by a rise in both hepatic and muscle CoA, this flexibility is lost in the Lep−/− mice and in the closely related db/db mice [26]. The observation of higher hepatic CoA and deregulated gluconeogenesis in the Lep−/− mice led us to test the hypothesis that deleting Pank1 in the leptin-deficient background might improve the diabetic phenotype. Pank1−/− Lep−/− (dKO) mice were derived by mating Pank1−/− and Lep+/− mice [24]. CoA levels, mitochondrial β-oxidation and gluconeogenesis are significantly decreased in the livers of the dKO mice compared to the Pank1+/+ Lep−/− controls, leading to a dramatic reduction in blood glucose both in the fed and in the fasted state (Fig. 2A). Additionally, correction of the fasting hyperglycemia reduced the insulin levels (Fig. 2B) and altered fuel utilization at the whole-body level. Serum acylcarnitine profiling and indirect calorimetry show that the dKO mice have reduced metabolism of fatty acid and amino acids, while relying more heavily on carbohydrates for energy production. This is likely a metabolic adaptation to the significant reduction in blood glucose and insulin since glycogen stores are significantly depleted in skeletal muscle from fasted dKO mice. The dramatic improvement in hyperglycemia and hyperinsulinemia resulting from Pank1 deletion occurs in spite of persistent obesity, fatty liver and insulin resistance in both liver and muscle.
Figure 2.
Deletion of Pank1 in the Lep−/− mice dramatically decreases hyperglycemia and hyperinsulinemia. Blood from 10-16 week-old male mice in the fed state or after an overnight (ON) fast was analyzed for glucose (A) and insulin (B) levels. The number of mice used for the measurement is indicated in brackets. * p<0.05, ** p<0.01, *** p<0.001. Animal handling procedures were approved under protocol 323 by the St. Jude Institutional Animal Care and Use Committee.
Obesity has long been associated with insulin resistance, as accumulation of toxic lipids in insulin-sensitive organs like skeletal muscle and liver has been shown to interfere with glucose uptake and insulin signaling [27,28]. In the liver, insulin resistance is manifested as a defect in insulin-stimulated glycogen synthesis and an abnormally high rate of gluconeogenesis which, in turn, is a major contributor to the elevated fasting blood glucose observed in diabetic patients. Although it remains unclear whether hepatic steatosis preceeds insulin resistance or whether fatty liver invariably leads to impaired insulin action [29,30], strategies that reduce hepatic lipogenesis effectively correct steatosis and insulin resistance [28]. Deletion of Pank1 in the leptin-deficient background has no effect on obesity, hepatic steatosis or insulin resistance, supporting the conclusion that reduced hepatic CoA is sufficient to restrict de novo glucose production independent of insulin signaling. Thus, Pank1 deletion effectively uncouples obesity and/or fatty liver-associated insulin resistance from hyperglycemia. The contribution of fatty acid β-oxidation to hepatic insulin resistance, if any, is still unclear. Stimulation of β-oxidation through genetic manipulations or the use of small molecules results in improved insulin sensitivity and reduced blood glucose in models of obesity-induced diabetes [31-34]. On the other hand, the phenotype of the dKO mice clearly shows that two important aspects of the diabetic state, hyperglycemia and hyperinsulinemia, are similarly improved by inhibition of the β-oxidation capacity of the liver.
Concluding remarks
CoA is emerging as a global regulator of energy metabolism whose levels are carefully controlled and modulated to support metabolic flexibility. Indeed, adaptation of the liver to the fasted state is dependent upon a significant increase in CoA levels to sustain the high rates of fatty acid oxidation and glucose production required to maintain whole-body glucose homeostasis. Conversely, metabolic flexibility and glucose regulation are lost in diabetes, and this state is associated with abnormally high and deregulated CoA levels in both liver and muscle. Importantly, reduction of CoA synthesis primarily in the liver is sufficient to correct the hyperglycemia and hyperinsulinemia of diabetic mice in spite of persistent insulin resistance. Thus, CoA levels can regulate the output of pathways like fatty acid β-oxidation and gluconeogenesis independent of insulin signaling, transcription factors and metabolic enzymes.
Acknowledgments
Funding
Research was supported by National Institutes of Health grant GM062896 and the American Lebanese Syrian Associated Charities.
References
- 1.Leonardi R, Zhang YM, Rock CO, Jackowski S. Coenzyme A: back in action. Prog. Lipid Res. 2005;44:125–153. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder F, Huang H, Hostetler HA, Petrescu AD, Hertz R, Bar-Tana J, Kier AB. Stability of fatty acyl-coenzyme A thioester ligands of hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Lipids. 2005;40:559–568. doi: 10.1007/s11745-005-1416-y. [DOI] [PubMed] [Google Scholar]
- 3.Foster DW. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J. Clin. Invest. 2012;122:1958–1959. doi: 10.1172/JCI63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai L, Tu BP. On acetyl-CoA as a gauge of cellular metabolic state. Cold Spring Harb. Symp. Quant. Biol. 2011;76:195–202. doi: 10.1101/sqb.2011.76.010769. [DOI] [PubMed] [Google Scholar]
- 5.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Vaitheesvaran B, Hartil K, Robinson AJ, Hoopmann MR, Eng JK, Kurland IJ, Bruce JE. The fasted/fed mouse metabolic acetylome: N6-acetylation differences suggest acetylation coordinates organ-specific fuel switching. J. Proteome Res. 2011;10:4134–4149. doi: 10.1021/pr200313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siudeja K, Srinivasan B, Xu L, Rana A, de Jong J, Nollen EA, Jackowski S, Sanford L, Hayflick S, Sibon OC. Impaired Coenzyme A metabolism affects histone and tubulin acetylation in Drosophila and human cell models of pantothenate kinase associated neurodegeneration. EMBO Mol. Med. 2011;3:755–766. doi: 10.1002/emmm.201100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock CO, Calder RB, Karim MA, Jackowski S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J Biol Chem. 2000;275:1377–1383. doi: 10.1074/jbc.275.2.1377. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YM, Rock CO, Jackowski S. Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters. J. Biol. Chem. 2005;280:32594–32601. doi: 10.1074/jbc.M506275200. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 11.Klover PJ, Mooney RA. Hepatocytes: critical for glucose homeostasis. Int. J. Biochem. Cell. Biol. 2004;36:753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Reibel DK, Wyse BW, Berkich DA, Palko WM, Neely JR. Effects of diabetes and fasting on pantothenic acid metabolism in rats. Am J Physiol. 1981;240:E597–601. doi: 10.1152/ajpendo.1981.240.6.E597. [DOI] [PubMed] [Google Scholar]
- 13.Horie S, Isobe M, Suga T. Changes in CoA pools in hepatic peroxisomes of the rat under various conditions. J. Biochem. 1986;99:1345–1352. doi: 10.1093/oxfordjournals.jbchem.a135602. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi R, Rehg JE, Rock CO, Jackowski S. Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PLoS One. 2010;5:e11107. doi: 10.1371/journal.pone.0011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YM, Chohnan S, Virga KG, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Newgard CB, Lee RE, Rock CO, Jackowski S. Chemical knockout of pantothenate kinase reveals the metabolic and genetic program responsible for hepatic coenzyme A homeostasis. Chem. Biol. 2007;14:291–302. doi: 10.1016/j.chembiol.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia M, Leonardi R, Zhang YM, Rehg JE, Jackowski S. Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS One. 2012;7:e40871. doi: 10.1371/journal.pone.0040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S, Brodsky J, Jones CG, Zaitlen NA, Varilo T, Kaakinen M, Sovio U, Ruokonen A, Laitinen J, Jakkula E, Coin L, Hoggart C, Collins A, Turunen H, Gabriel S, Elliot P, McCarthy MI, Daly MJ, Jarvelin MR, Freimer NB, Peltonen L. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U S A. 2002;99:419–424. doi: 10.1073/pnas.012581799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura K, Yamashita T, Fujiki H, Aoyagi T, Yamauchi J, Mori T, Tanoue A. Enhanced glucose tolerance in the Brattleboro rat. Biochem. Biophys. Res. Commun. 2011;405:64–67. doi: 10.1016/j.bbrc.2010.12.126. [DOI] [PubMed] [Google Scholar]
- 20.Neschen S, Katterle Y, Richter J, Augustin R, Scherneck S, Mirhashemi F, Schurmann A, Joost HG, Klaus S. Uncoupling protein 1 expression in murine skeletal muscle increases AMPK activation, glucose turnover, and insulin sensitivity in vivo. Physiol. Genomics. 2008;33:333–340. doi: 10.1152/physiolgenomics.00226.2007. [DOI] [PubMed] [Google Scholar]
- 21.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1–11. doi: 10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardi R, Rock CO, Jackowski S. Pank1 deletion in leptin-deficient mice reduces hyperglycaemia and hyperinsulinaemia and modifies global metabolism without affecting insulin resistance. Diabetologia. 2014 doi: 10.1007/s00125-014-3245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasmi L, McLennan AG. The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochem. J. 2001;357:33–38. doi: 10.1042/0264-6021:3570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschbaum N, Clemons R, Marino KA, Sheedy G, Nguyen ML, Smith CM. Pantothenate kinase activity in livers of genetically diabetic mice (db/db) and hormonally treated cultured rat hepatocytes. J. Nutr. 1990;120:1376–1386. doi: 10.1093/jn/120.11.1376. [DOI] [PubMed] [Google Scholar]
- 27.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009;50(Suppl):S74–79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monsenego J, Mansouri A, Akkaoui M, Lenoir V, Esnous C, Fauveau V, Tavernier V, Girard J, Prip-Buus C. Enhancing liver mitochondrial fatty acid oxidation capacity in obese mice improves insulin sensitivity independently of hepatic steatosis. J. Hepatol. 2012;56:632–639. doi: 10.1016/j.jhep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr., Hevener AL, Farese R.V., Jr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell. Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Vikramadithyan RK, Hiriyan J, Suresh J, Gershome C, Babu RK, Misra P, Rajagopalan R, Chakrabarti R. DRF 2655: a unique molecule that reduces body weight and ameliorates metabolic abnormalities. Obes. Res. 2003;11:292–303. doi: 10.1038/oby.2003.44. [DOI] [PubMed] [Google Scholar]
- 32.Chou CJ, Haluzik M, Gregory C, Dietz KR, Vinson C, Gavrilova O, Reitman ML. WY14,643, a peroxisome proliferator-activated receptor alpha (PPARalpha ) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J. Biol. Chem. 2002;277:24484–24489. doi: 10.1074/jbc.M202449200. [DOI] [PubMed] [Google Scholar]
- 33.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orellana-Gavalda JM, Herrero L, Malandrino MI, Paneda A, Sol Rodriguez-Pena M, Petry H, Asins G, Van Deventer S, Hegardt FG, Serra D. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology. 2011;53:821–832. doi: 10.1002/hep.24140. [DOI] [PubMed] [Google Scholar]