Abstract
Introduction
Generic health-related quality of life (HRQOL) patient-reported outcome measures have been used in patients with chronic immune-mediated polyneuropathies. We have created a disease-specific HRQOL instrument.
Methods and Results
The 15-item chronic acquired polyneuropathy patient-reported index (CAP-PRI) was developed and validated in multiple steps. Items were initially generated through patient and specialist input. The performance of the preliminary 20 items was analyzed from a prospective, 5-center study involving chronic immune-mediated polyneuropathy patients. Data analysis suggested modification to a 15-item scale with 3 response categories, rather than 5. The final CAP-PRI was then validated in another prospective, 5-center study. The CAP-PRI appeared to be a unidimensional outcome measure that fits the Rasch Partial Credit Model in our multicenter cohort. It correlated appropriately with the outcome measures commonly used in this patient population.
Discussion
The CAP-PRI is a simple, easy, disease-specific HRQOL measure that appears to be useful for clinical care and possibly also for clinical trials.
Keywords: Quality of life, immune-mediated polyneuropathy, chronic inflammatory demyelinating polyneuropathy, patient-reported outcome measure, CAP-PRI
INTRODUCTION
A patient-reported outcome measure (PROM) may be used to estimate symptoms, function, and health-related quality of life (HRQOL). PROMs can estimate the severity and tolerability of dysfunction and symptoms. PROMs may be used to 1) estimate the patient’s perspective in the clinical setting; 2) monitor a patient’s clinical status; 3) study a population with a particular disease; and 4) serve as an outcome measure in a clinical trial. The FDA published a Guidance for industry on the development and use of PROMs in medical product development to support labeling claims.1
HRQOL, unlike global QOL, is viewed from the medical perspective and assesses self-perceived well-being related to or affected by the disease and treatment. PROMs, including HRQOL measures, are well-suited for immune-mediated polyneuropathies because: 1) the manifestations are evident to the patient; 2) changes in clinical status often occur rapidly; and 3) there are treatments available. To date, mostly generic instruments, particularly the Medical Outcomes Study 36-item Short Form Health Survey (SF-36), have been used to measure HRQOL in patients with immune-mediated polyneuropathies.2–8 In this paper, we discuss our attempt to create, modify and validate a disease-specific HRQOL scale for patients with chronic immune-mediated demyelinating polyneuropathies.
MATERIALS AND METHODS
Phase I: Construction of the preliminary 20-item, 5-response-category instrument with subsequent modification and creation of a 15-item scale with 3 response categories
Twenty items were initially generated through interviews of 30 chronic, immune-mediated polyneuropathy patients and 4 neuromuscular specialists at the University of Virginia with experience caring for patients with immune-mediated polyneuropathy. The specialists presented to the patient a list of 19 items and asked each patient the following open-ended questions: “Are there any questions that are particularly important to ask? Which items would you remove from this list because they were not worth asking? Are there items that you wish we had included? To what degree are you impaired by your neuropathy? What else bothers you about your neuropathy?” Twenty-nine items were generated following patient interviews. Each item was carefully evaluated for its disease-specificity and patient opinion of its appropriateness. Based on face validity, construct validity and our consensus expert opinion, 9 items were removed to yield the preliminary 20-item, 5-category-response instrument. The initial 20-item scale had 5 response categories: “not at all,” “a little bit,” “somewhat,” “quite a bit” and “very much.”
Following local Institutional Review Board (IRB) approval, neuropathy specialists from 5 academic centers (University of Virginia, University of Toronto, Columbia University, University of Vermont, and Massachusetts General Hospital) participated in the first prospective phase of the study. Consecutive subjects were enrolled at each center from 2011 to 2013. Inclusion criteria included: (1) a diagnosis of chronic immune-mediated polyneuropathy including chronic inflammatory demyelinating polyneuropathy (CIDP,) multifocal motor neuropathy (MMN), distal acquired demyelinating symmetric neuropathy (DADS), paraproteinemic (demyelinating or axonal) polyneuropathy, Sjögren’s associated neuronopathy, sensory neuronopathy, and vasculitic neuropathy, (2) age 18–80, (3) English-speaking, and (4) willing and able to give informed consent. At each of the two consecutive clinic visits, the following data were collected: demographic information (age, sex, and duration of symptoms), RAND-36 survey results, Rasch-built Overall Disability Scale (R-ODS) results, grip strength using a Jamar hand dynamometer, Neuropathy Impairment Score (NIS) scores, and Inflammatory Neuropathy Cause and Treatment Overall Disability Sumscores (INCAT ODSS).
We used conventional statistical analyses, including, when appropriate, Rasch Partial Credit Model (RPCM), to analyze the results. Statistical analyses, including RPCM, informed us when deciding on modifications to the preliminary instrument. The specialists from all 5 centers discussed the results, in person, by phone and by email. Based on the analyses, we made decisions about whether or not to delete or modify items and how many response categories (3, 4 or 5) would be optimal. Below is more detail on statistical methodologies. Please see the Results section for both traditional statistical analyses and RPCM results for phase I, as well as subsequent modifications to the scale. We tabulated item frequencies, and computed summary statistics, including the mean and standard deviation, for the modified scale and the RAND-36, R-ODS, grip strength and INCAT ODSS scores. Spearman rank correlations were used to assess the association between responses to specific items and the total score on the CAP-PRI with subscales on the RAND-36, the R-ODS and the INCAT ODSS scores.
Rasch Partial Credit Model
The RPCM was conducted using Winsteps software (version 3.70.0.5) to explore data for item-person targeting, item fitting, dependency, dimensionality, category response functioning (thresholds) and differential item functioning (DIF). Item and Person separation indexes represent item and person hierarchy; low person separation (< 2) implies poor sensitivity to distinguish between high and low performers and low item separation (<3) implies that person sample is not large enough.
Item-person Targeting
RPCM compares the probabilistic expectations of “item difficulty,” relative ability of each individual test item to differentiate between patient disability levels and “person ability,” relative patient disability rank measured by the outcome measure, on a common logarithmic scale. A more difficult item is abnormal in more disabled patients but not in less disabled patients. This would allow measuring how sensitive items are to pick up differences between different disability levels and whether items are covering an appropriate range of disability.
Item Fitting, Dependency & Dimensionality
Fit statistics examine data in comparison with expectations of RPCM. Item fitting is calculated using chi-square statistics and may be reported as mean square (MNSQ), unstandardized average value of squared differences between the RPCM’s expected and actual values for an item. This value should ideally fall between 0.50 and 1.70 for clinical tools. Item dependencies represent correlation between item difficulties, identifying items potentially measuring same concept potentially forming sub-dimension, affecting overall unidimensionality of the test. Principal component analysis (PCA) of the differences between observed and expected scores or residuals can reveal contrasting items breeching unidimensionality of outcome measure.
Category Response Functioning
RPCM compares the probability of a category response to other category responses of the same item as well as category responses from other items. The more difficult category responses are expected to have higher probabilities in more disabled patients.
Differential Item Functioning
DIF statistics measures different response probability of different subgroups. Winstep software reports Mantel method which is log-odds estimators of DIF size and significance from cross-tabs of observations of the two groups using t-test.
Phase II: Validation of a 15-item instrument, 3-response-category scale, called CAP-PRI
As discussed in the results section, the preliminary 20-item instrument was modified to the current 15-item scale with 3 response categories (“not at all,” “a little bit” and “a lot”), the chronic acquired polyneuropathy patient-reported index (CAP-PRI) (Figure 1). In phase II of the study, neuropathy specialists from 5 academic centers (University of Virginia, University of Toronto, Columbia University, Duke University, and Massachusetts General Hospital) enrolled consecutive patients. Inclusion criteria included: (1) a diagnosis of chronic immune-mediated polyneuropathy (CIDP, paraproteinemic demyelinating polyneuropathy, DADS neuropathy [anti-MAG associated and non-anti-MAG associated], and MMN), (2) age 18–80, (3) English-speaking, and (4) willing and able to give informed consent. At each of the two consecutive scheduled visits (3–6 months apart), investigators recorded the following data: demographic, RODS, RAND-36, INCAT ODSS and CAP-PRI scores.
Figure 1.
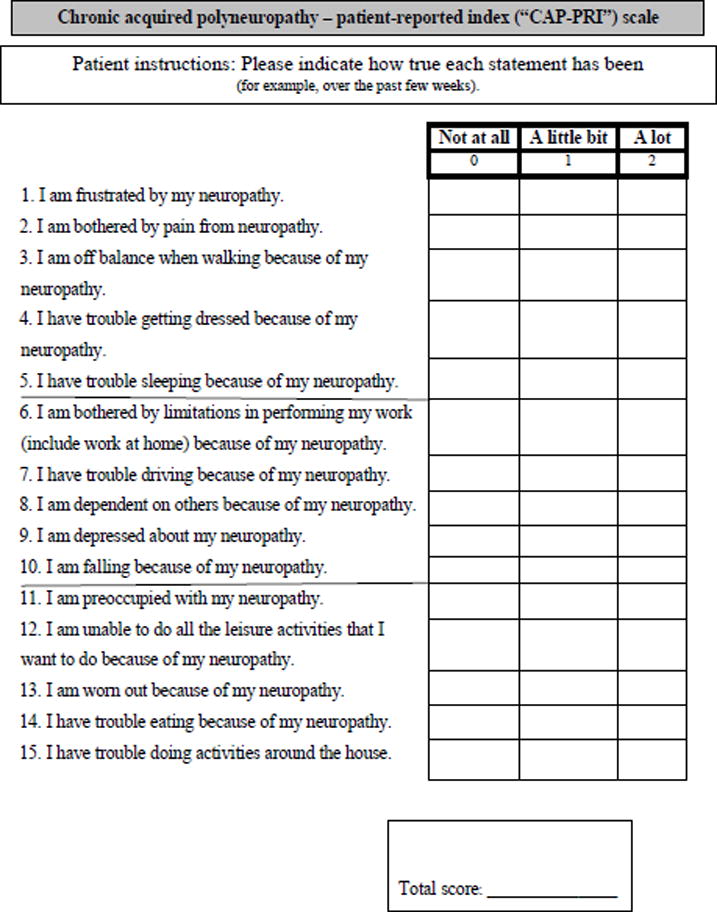
The final 15-item, 3-response-category instrument, the chronic acquired polyneuropathy patient-reported index (CAP-PRI).
RESULTS
Phase I: The preliminary 20-item instrument, with subsequent modification to a 15-item, 3-response-category scale
Seventy-three subjects (18 women and 46 men) were enrolled in phase I of the study and administered the preliminary 20-item instrument as well as the other instruments as outlined in the Methods section. Forty-six subjects were seen in follow-up for a total of 119 assessments.
The preliminary 20-item instrument correlated with the R-ODS, RAND-36, INCAT ODSS let score as well as NIS. RPCM was performed on the preliminary 20-item instrument (Figure 2A). Comparison of item and person distribution on a common logarithmic scale (Item-Person Targeting) showed a significant floor effect, suggesting that items were likely more suitable for moderate to severe forms of disease without many items suitable for less disabled patients (Figure 3, vertical axis). Most misfitting items included “Treatment,” “Hands,” and to a lesser extent “Sleeping” and “Pain” (Figure 3, horizontal axis). Item dependency testing showed strong correlations between items “Dependence”, “Social”, “Hands” and “Dressing” as well as “Balance”, “Dressing” and “Job.”
Figure 2.
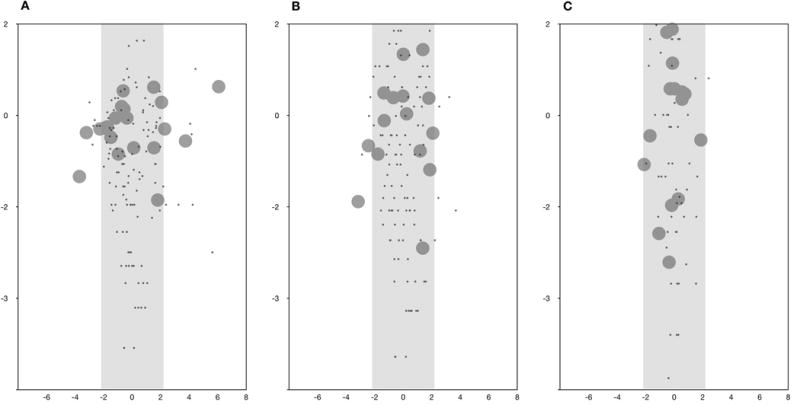
Developmental pathway maps. A) Preliminary 20-item, 5-category-response instrument; B) Modified 15-item, 3-category-response instrument (upon analysis of the phase I dataset); C) Final 15-item, 3-category-response instrument, the CAP-PRI (analyzed from the phase II dataset). Item difficulty-person ability probability map is on the vertical (y-axis) logarithmic scale (logits) and more difficult items (large circles placed higher on the vertical access) are more appropriate for more disabled patients (black dots). These Figure (A, B, C) shows that coverage of severity status of the scale improved with each modification. Item fitting is on the horizontal (x-axis) scale. Mis-fitting items are outside the shaded box (2A) and were subsequently removed or modified, as discussed in the Methods and Results. Figure 2C demonstrates that none of the 15 items in the final scale were misfitting. Z-score| > 2).
Figure 3.
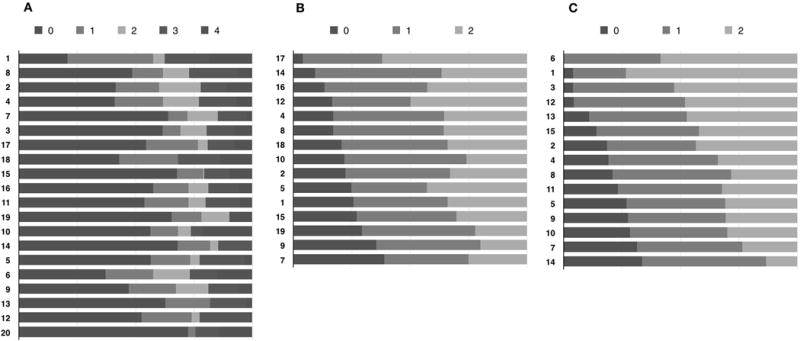
Category response threshold maps. A) Preliminary 20-item, 5-category-response instrument; B) Modified 15-item, 3-category-response instrument (phase I dataset); C) Final 15-item, 3-category-response instrument, the CAP-PRI. For Figure A, see methods for the descriptors of the 5 response categories. For Figures B and C, 0 = “not at all,” 1 = “a little bit,” and 2= “a lot.”
Average measures did not significantly ascend with category responses and hence thresholds were disordered and disorganized for most items of the 20-item preliminary scale. In other words, patients with less disability scored higher than more disabled patients. Category responses with similar or disordered thresholds were subsequently amalgamated (1 instead of 1 and 2, 2 instead of 3 and 4), reducing number of category responses from 5 (0= “not at all,” 1= “a little bit,” 2= “somewhat,” 3=”quite a bit,” 4=”very much”) to 3 (0= “not at all,” 1= “a little bit,” 2= “a lot”). Considering content validity of the scale, item dependency and fit statistics, we removed items “Balance (Standing),” “Family,” “Social,” “Hands” and “Treatment.” Two further items were reworded: “My occupational skills and job status have been negatively affected” was modified to become “I am bothered by limitations in performing my work (include work at home) because of my neuropathy.” “My neuropathy prevents me from doing what I want to do” was modified to “I am unable to do all the leisure activities that I want to do because of my neuropathy.” Following these changes, the preliminary 20-item scale with 5 response categories was shortened to a 15-item scale with 3 response categories (Figure 1).
Applying RPCM on the modified 15 item-scale (phase I dataset) showed improved psychometric properties, resulting in modest improvement in item-person targeting (Figure 2B, vertical axis), fit statistics (Figure 2B, horizontal axis) and no disordered thresholds were found (Figure 3).
Phase II: The 15-item, 3-response-category scale, the CAP-PRI
For the prospective evaluation of the 15-item, 3-response category scale, the CAP-PRI, a total of 39 subjects were enrolled at 5 academic institutions. Twenty-seven patients returned for routine follow up within 3–6 months and visit 2 data was collected. Twenty-five patients were male (65.8%). The average age was 57.8 years (SD 12.2, range 35–90). The mean disease duration was 8.9 years (SD = 6.3, range 0.1–22). Nine (23.1%) had MMN, 26 (66.7%) had CIDP, 2 (5.1%) had DADS, 1 (2.6%) had chronic immune sensory polyradiculopathy (CISP)-form of CIDP and 1 (2.6%) had an unspecified “peripheral neuropathy.”
CAP-PRI, R-ODS, and INCAT-ODSS data from both phase II visits was combined for analysis and is presented in Table 1. Please refer to Table 2 for the RAND-36 values. The distributions of scores for each category response for each CAP-PRI item are shown in Table 3.
Table 1.
CAP-PRI, R-ODS, and INCAT ODSS Data Combined from Visit 1 and 2 of Phase I
| N | Mean | SD | Median | Score Range | |
|---|---|---|---|---|---|
| CAP-PRI | 65 | 11.3 | 8.0 | 11.0 | 0.0–26.0 |
| R-ODS | 66 | 36.1 | 11.0 | 38.5 | 0.0–48.0 |
| INCAT ODSS arm | 49 | 1.4 | 1.2 | 1.0 | 0.0–4.0 |
| INCAT ODSS leg | 49 | 1.0 | 1.1 | 1.0 | 0.0–4.0 |
| INCAT ODSS total | 48 | 2.4 | 1.8 | 2.0 | 0.0–7.0 |
CAP-PRI=Chronic Acquired Polyneuropathy Patient-reported Index, R-ODS=Rasch-built Overall Disability Scale, INCAT ODSS=Inflammatory Neuropathy Cause and Treatment Overall Disability Sumscore, N=number, SD=standard deviation
Table 2.
RAND-36 Subscale Data Combined from Visit 1 and 2 of Phase II
| RAND-36 Subscale | N | Mean | SD | Median | Score Range |
|---|---|---|---|---|---|
| Physical functioning | 66 | 57.1 | 32.5 | 52.5 | 5.0–100.0 |
| Role limitations due to physical health | 66 | 49.2 | 45.1 | 37.5 | 0.0–100.0 |
| Role limitations due to emotional problems | 66 | 66.7 | 44.1 | 100.0 | 0.0–100.0 |
| Energy/fatigue | 66 | 50.0 | 24.3 | 50.0 | 5.0–100.0 |
| Emotional well-being | 66 | 73.6 | 19.3 | 80.0 | 24.0–100.0 |
| Social functioning | 65 | 67.7 | 29.1 | 75.0 | 0.0–100.0 |
| Pain | 66 | 70.9 | 22.8 | 67.5 | 20.0–100.0 |
| General health | 66 | 52.0 | 22.3 | 50.0 | 10.0–100.0 |
| Health change | 66 | 53.8 | 26.8 | 50.0 | 0.0–100.0 |
N=number, SD=standard deviation
Table 3.
Item Fit and Measure Summary for the CAP-PRI; Item Measure, Mean of Squared Residuals (MNSQ), Outfit Z-score
| Description | Measure | Score Frequencies by Item | Fit statistics | ||||
|---|---|---|---|---|---|---|---|
| Items in order of “easiest” to most “difficult” | Missing Items | Item difficulty | 0= “None” | 1= “A little” | 2= “A lot” | Infit | Outfit |
| 1. Frustration | 1 | −2.56 | 12 (18.5%) | 18 (27.7%) | 35 (53.8%) | 1.1(0.6) | 0.8(−0.4) |
| 6. Job | 0 | −2.01 | 16 (24.2%) | 17 (25.8%) | 33 (50%) | 0.7(−1.6) | 0.6(−1.1) |
| 3. Balance (Walking) | 0 | −1.47 | 16 (24.2%) | 27 (40.9%) | 23 (34.8%) | 1.0(0.2) | 1.0(−0.2) |
| 12. Do what I want | 0 | −1.35 | 18 (27.3%) | 24 (36.4%) | 24 (36.4%) | 1.0(0) | 1.1(0.3) |
| 13. Worn out | 0 | −0.69 | 23 (34.8%) | 24 (36.4%) | 19 (28.8%) | 0.7(−2.1) | 0.6(−2.1) |
| 2. Pain | 0 | −0.22 | 24 (36.4%) | 29 (43.9%) | 13 (19.7%) | 1.3(1.7) | 1.4(1.9) |
| 15. Activities around house | 1 | −0.14 | 22 (33.8%) | 32 (49.2%) | 11 (16.9%) | 0.8(−1.5) | 0.7(−1.7) |
| 9. Depression | 0 | 0.56 | 39 (59.1%) | 13 (19.7%) | 14 (21.2%) | 1.1(0.6) | 1.2(0.6) |
| 11. Preoccupation | 0 | 0.66 | 32 (48.5%) | 26 (39.4%) | 8 (12.1%) | 1.2(1.0) | 1.2(0.8) |
| 4. Dressing | 0 | 0.7 | 27 (40.9%) | 34 (51.5%) | 5 (7.6%) | 1.2(1.0) | 1.1(0.5) |
| 5. Sleeping | 0 | 0.76 | 37 (56.1%) | 19 (28.8%) | 10 (15.2%) | 1.2(1.1) | 1.0(0) |
| 8. Dependence | 0 | 0.76 | 32 (48.5%) | 27 (40.9%) | 7 (10.6%) | 1.1(0.3) | 0.9(−0.3) |
| 10. Falling | 0 | 1.25 | 39 (59.1%) | 21 (31.8%) | 6 (9.1%) | 0.8(−0.8) | 0.9(−0.1) |
| 14. Eating | 0 | 1.84 | 48 (72.7%) | 13 (19.7%) | 5 (7.6%) | 0.9(−0.2) | 0.5(−0.5) |
| 7. Driving | 0 | 1.9 | 43 (65.2%) | 20 (30.3%) | 3 (4.5%) | 0.9(−0.5) | 0.8(−0.1) |
CAP-PRI=Chronic Acquired Polyneuropathy Patient-reported Index
Mean of the squared residuals, which represents the unstandardized form of fit statistics
Standardized t-value residuals
We set out to demonstrate concurrent validity of the CAP-PRI by comparing it with the R-ODS, RAND-36, and INCAT ODSS scores by calculating Pearson correlation coefficients (Table 4). The CAP-PRI correlated well with the R-ODS, INCAT ODSS (total as well as arm and leg subscores), the RAND-36 physical functioning, role limitations due to physical health, emotional problems, energy/fatigue, social functioning, general health, and health change subscales. The total CAP-PRI scores correlated poorly with the RAND-36 emotional well-being and pain subscales (r = −0.24 [p=0.095] and r = −0.32 [p=0.024] respectively). The average correlation between CAP-PRI items was 0.54. The first PCA explained 81.0% of the variation in the scale.
Table 4.
Correlations of CAP-PRI with R-ODS, RAND-36 subscales, and INCAT ODSS
| Correlation | 95% CI | P-value | |
|---|---|---|---|
| R-ODS | −0.51 | (−0.67, −0.30) | <0.001 |
| RAND-36 subscale | |||
| Physical functioning | −0.64 | (−0.78, −0.45) | <0.001 |
| Role limitations due to physical health | −0.66 | (−0.79, −0.46) | <0.001 |
| Role limitations due to emotional problems | −0.49 | (−0.68, −0.24) | <0.001 |
| Energy/fatigue | −0.59 | (−0.74, −0.37) | <0.001 |
| Emotional well-being | −0.24 | (−0.49, 0.04) | 0.095 |
| Social functioning | −0.66 | (−0.79, −0.46) | <0.001 |
| Pain | −0.32 | (−0.55, −0.05) | 0.024 |
| General health | −0.40 | (−0.61, −0.14) | 0.004 |
| Health change | −0.43 | (−0.64, −0.17) | 0.002 |
| INCAT ODSS | |||
| INCAT ODSS arm | 0.48 | (0.22, 0.67) | 0.001 |
| INCAT ODSS leg | 0.52 | (0.27, 0.70) | <0.001 |
| INCAT ODSS total | 0.65 | (0.44, 0.79) | <0.001 |
CAP-PRI=Chronic Acquired Polyneuropathy Patient-reported Index, R-ODS=Rasch-built Overall Disability Scale, INCAT ODSS=Inflammatory Neuropathy Cause and Treatment Overall Disability Sumscore, CI=confidence interval
RPCM was performed on the CAP-PRI. Overall, there was good item (4.6) and person separation (3.2) as well as person (0.91) and item (0.96) reliability. “Driving” (1.9 logit) and “Eating” (1.8 logit) were more appropriate for differentiating disease severity in more severely affected patients, while “Frustration” (−2.6 logit) and “Work” (−1.0 logit) were more suitable for patients with milder levels of disease severity. Comparison of item and person distribution on a common logarithmic scale showed significant improvement in large floor effect noted with previous version (Table 5, Figure 3, vertical axis). Most affected patients scored 26 (2.9 logit) and 5 patients scored 0 (−6.1 logit), mean item difficulty (0 logit) and mean person ability (−1.1 logit). In this cohort, almost all items fitted RPMC really well, with the exception of Item “Worn out” (MNSQ = 0.6, Z-Score = −2.1), which was very mildly misfitting (Table 3, Figure 2C). Item dependency analysis showed only mild to moderate correlations most notably between items “Depression” and “Preoccupation,” but also “Balance” and “Sleeping”. PCA analysis of residuals revealed that only 6.3% (2.5 eigenvalue) of 15% raw unexplained variance could be explained by variances in mood related items “Depression” and “Worn out” suggesting a very negligible dimensionality trait. Average measure ascended in an orderly fashion with the category scale of all items (Figure 3) and there were no disordered thresholds. We explored psychometric properties between visits, genders, diagnoses (CIDP versus non-CIDP) and found no significant intergroup differences.
DISCUSSION
The CAP-PRI is a HRQOL measure specifically developed for the chronic, immune-mediated polyneuropathy patient population. Some of the positive attributes of the CAP-PRI include: 1) it is quick and easy to use; 2) it is quick and easy to interpret; 3) it is free and in the public domain; 4) it addresses various life domains, including physical and social functioning, pain and emotional well-being; 5) the scale appears to be unidimensional, allowing scores to be summed to offer a total score; 6) the items appear to cover well the various degrees of disease severity; 7) the selection of items was based on both physician and patient input; and 8) it can be considered a validated scale for this cohort of chronic immune-mediated polyneuropathy patients.
We think that the CAP-PRI is appropriate for use in everyday clinic for patients with chronic immune-mediated polyneuropathy. The CAP-PRI might also be useful in the clinical trial setting. Further study, for example of other cohorts, including cohorts in clinical trials, is necessary to better understand its value, including its strengths and limitations. For everyday clinical care, we think the CAP-PRI efficiently captures a patient perspective that complements the clinician’s impression of the patient’s clinical status. We think this partnership between the patient and clinician—one that considers both patient-reported and clinician-obtained clinical data—is optimal for patient care. Concerning its possible use in clinical trials, we have not yet studied the psychometric properties of the CAP-PRI in a clinical trial setting. It is worth pointing out that the many differences in clinical trial design and implementation can alter, for example, the mindset of the patient, which can impact perceived, patient-reported HRQOL. For example, patient hope generated from an experimental trial may be much different than hope found (or not found) in everyday clinical care. Also, in a randomized clinical trial, HRQOL can be influenced by the patient’s guess regarding treatment or placebo status. For example, if the patient understands the drug-to-placebo ratio to be 3:1, or is experiencing side effects felt to be caused by a treatment and not by placebo, the patient will likely experience hope for benefit from the experimental intervention, hope that may influence the patient’s HRQOL.9 Other factors unique to experimental trials may also influence the patient perspective and thus influence HRQOL scores. And thus, the CAP-PRI warrants ongoing study and psychometric scrutiny in future immune-mediated polyneuropathy clinical trial settings.
We think this study illustrates a few practical and key points about the creation and validation of an ordinal, patient-reported scale, including: 1) the importance of including patient input in the item generation phase1; and 2) how statistical analyses of prospectively-collected data can inform specialists about item performance, assisting in decisions about which items to include, which items merit rewording and the optimal number of response categories. Through this comprehensive analysis and informed decision-making process, we were able to modify our preliminary 20-item, 5-response category scale into a better-performing 15-item, 3-response category scale. We were then able to validate our 15-item CAP-PRI scale in another multicenter cohort of patients with chronic, immune-mediated polyneuropathy.
As we predicted, the CAP-PRI correlates well with 2 widely used outcome measures commonly used in immune-mediated polyneuropathies (INCAT ODSS and R-ODS). These scales are well-established for use in everyday clinical care and in the clinical trial setting. We did not create the CAP-PRI to compete with these scales, but instead to provide a different “lens” to look through when gauging disease status. R-ODS, for example, focuses on disability, whereas our scale also includes other domains, such as psychological well-being. On the other hand, in comparison to the generic HRQOL scale, the RAND-36, the CAP-PRI may offer the advantages of being disease-specific, shorter, easier-to-administer and interpret, and the advantage of containing only relevant items.
It is noteworthy that while the total CAP-PRI scores did not correlate with the RAND-36 emotional well-being subscale scores (Table 4), the RAND-36 emotional well-being subscale did in fact correlated with each of the 3 CAP-PRI emotional well-being items: “Frustrated,” “Depressed,” and “Preoccupied.” Also, the “Pain” item from the CAP-PRI correlated highly with the RAND-36 pain subscale, despite total CAP-PRI scores not correlating with the RAND-36 pain subscale scores. These item-subscale correlations provide further validity for the inclusion on these items in the disease-specific CAP-PRI.
Our initial work with the CAP-PRI also teaches us more about the HRQOL of immune-mediated polyneuropathy patients. For example, > one-third of patients scored “a lot” on 4 items, “I am frustrated…,” “I am off balance…,” “I am bothered by limitations in performing my work…” and “I am unable to do all the leisure activities that that I want.” (Table 3) These findings illustrate that chronic immune-mediated polyneuropathy patients are frustrated and most severely impacted by concerns about safety (“off balance”), some aspects of everyday function (“working”) and performing desirable “leisure activities.” In contrast, and as expected, about two-thirds of patients reported no trouble with sleeping, driving, eating or feeling depressed about the polyneuropathy. These items, while only infrequently abnormal, work best in patients with more severe polyneuropathy, including those with significant upper extremity involvement. Further study needs to look at how our various treatments impact the scoring of these items. For example, are there differential effects of intravenous immunoglobulin compared to corticosteroids? And how might symptomatic pain medications impact item scores?
A limitation of this study is the relatively small sample size of our phase II validation study. It is also worth remembering that while our results indicate that the CAP-PRI fits well the Rasch model standards, this analysis is only for 1 cohort of patients (from 5 centers, in the everyday clinical care setting,) and, thus, as stated above, further analyses are indicated for future cohorts and especially any clinical trials. Also, the patients enrolled in this study may or may not be representative of all immune-mediated polyneuropathy patients, as our cohort of patients were followed at academic centers and thus may be slightly different than those followed at community clinics. Another limitation of our study is that we have not yet performed test-retest testing or yet attempted to evaluate our scale for responsiveness to clinical change. As outlined in the COSMIN (Consensus-Based Standards for the Selection of Health Measurement Instruments) guidelines,10 these properties, along with other analyses such as cross-cultural analyses, are desirable and should be performed on future cohorts, if possible. Lastly, we are interested in studying the performance of the CAP-PRI for other non-immune-mediated polyneuropathy cohorts, such as other chronic, acquired polyneuropathies (diabetic, uremic, chemotherapy, etc.) and these studies will soon begin.
Abbreviations
- CAP-PRI
chronic acquired polyneuropathy patient-reported index
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CISP
chronic inflammatory sensory polyradiculopathy
- COSMIN
Consensus-Based Standards for the Selection of Health Measurement Instruments
- DADS
distal acquired demyelinating symmetric
- DIF
differential item functioning
- HRQOL
health-related quality of life
- INCAT ODSS
Inflammatory Neuropathy Cause and Treatment Overall Disability Sumscore
- IRB
Institutional Review Board
- MMN
multifocal motor neuropathy
- MNSQ
mean square
- NIS
Neuropathy Impairment Score
- PCA
principal component analysis
- PROM
patient-reported outcome measure
- QOL
quality of life
- R-ODS
Rasch-built Overall Disability Scale
- RPCM
Rasch Partial Credit Analysis
- SF-36
Medical Outcomes Study 36-item Short Form Survey
References
- 1.U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns TM, Graham CD, Rose MR, Simmons Z. Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle Nerve. 2012;46:9–25. doi: 10.1002/mus.23245. [DOI] [PubMed] [Google Scholar]
- 3.Harbo T, Andersen H, Overgaard K, Jakobsen J. Muscle performance relates to physical function and quality of life in long-term chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2008;13:208–217. doi: 10.1111/j.1529-8027.2008.00179.x. [DOI] [PubMed] [Google Scholar]
- 4.Hughes R, Bensa S, Willison H, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50:195–201. doi: 10.1002/ana.1088. [DOI] [PubMed] [Google Scholar]
- 5.Merkies ISJ, Bril V, Dalakas MC, et al. Health-related quality-of-life improvements in CIDP with immune globulin IV 10%: the ICE Study. Neurology. 2009;72:1337–1344. doi: 10.1212/WNL.0b013e3181a0fd80. [DOI] [PubMed] [Google Scholar]
- 6.Merkies ISJ, Schmitz PIM, van der Meché FGA, Samijn JPA, van Doorn PA. Quality of life complements traditional outcome measures in immune-mediated polyneuropathies. Neurology. 2002;59:84–91. doi: 10.1212/wnl.59.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Merkies ISJ, Hughes RAC, Donofrio P, et al. Understanding the consequences of chronic inflammatory demyelinating polyradiculoneuropathy from impairments to activity and participation restrictions and reduced quality of life: the ICE study. J Peripher Nerv Syst. 2010;15:208–215. doi: 10.1111/j.1529-8027.2010.00274.x. [DOI] [PubMed] [Google Scholar]
- 8.van Nes SI, Faber CG, Merkies ISJ. Outcome measures in immune-mediated neuropathies: the need to standardize their use and to understand the clinimetric essentials. doi: 10.1111/j.1529-8027.2008.00169.x. [DOI] [PubMed] [Google Scholar]
- 9.Lou J-S, Moore D, Gordon PH, Miller R. Correlates of quality of life in ALS: Lessons from the minocycline study. Amyotroph Lateral Scler. 2010;11:116–121. doi: 10.3109/17482960902918719. [DOI] [PubMed] [Google Scholar]
- 10.Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10:22. doi: 10.1186/1471-2288-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


