Abstract
Insulin receptor substrate (IRS) proteins play important roles by acting as a platform in transducing signals from transmembrane receptors upon growth factor stimulation. Although tyrosine phosphorylation on IRS proteins plays critical roles in signal transduction, phosphorylation of IRS proteins on serine/threonine residues are believed to play various regulatory roles on IRS protein function. However, studies on serine/threonine phosphorylation of IRS proteins are very limited, especially for insulin receptor substrate 2 (IRS2), one member of the IRS protein family. In this study, we identify Polo-like kinase 1 (Plk1) as the responsible kinase for phosphorylation of IRS2 on two serine residues, Ser 556 and Ser 1098. Phosphorylation of IRS2 on these two serine residues by Plk1 prevents the activation of the PI3K pathway upon growth factor stimulation by inhibiting the binding between IRS2 and the PI3K pathway components and increasing IRS2 protein degradation. Of significance, we show that IRS2 phosphorylation is cell cycle regulated and that Plk1 phosphorylation of IRS2 prevents premature mitotic exit via AKT inactivation.
Keywords: IRS2, Plk1, PI3K pathway, AKT, Cell cycle
INTRODUCTION
Growth factors like insulin and insulin-like growth factor (IGF)-1 mediate cellular metabolism and mitogenesis through activation of the PI3K and Ras/MAPK pathways 1-4. Upon stimulation, insulin/IGF-1 receptors are auto-phosphorylated on tyrosine residues, leading to recruitment of IRS proteins to the cell membrane, where activated insulin/IGF-1 receptors further phosphorylate IRS proteins on tyrosine residues. In turn, tyrosine phosphorylated IRS proteins act as docking molecules for downstream signaling molecules, such as PI3-kinase and Ras. Once being recruited to the cell membrane, PI3 kinase phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-bisphosphate (PIP3), which recruits both AKT and phosphoinositide-dependent protein kinase 1 (PDK1) to the cell membrane, where PDK1 phosphorylates AKT on threonine 308 (T308), leading to further AKT activation via phosphorylation on serine 473 (S473) 5. Although tyrosine phosphorylation plays a major role in mediating the growth factor-stimulated activation of downstream signaling, phosphorylation on serine/threonine residues seems to play regulatory roles on tyrosine phosphorylation thus mediating the activity of IRS proteins 6. Many serine/threonine residues exist at the tail region of IRS proteins to mediate the growth factor sensitivity 7. IRS1 and IRS2 belong to the IRS family with similar and non-redundant roles upon growth factor stimulation. Of note, phosphorylation of IRS1 on S/T residues have been shown to have both positive and negative effects on insulin sensitivity 7-10. However, much less is known about kinases responsible for the S/T phosphorylation of IRS2 proteins and their roles in the regulation of IRS2 function.
Polo-like kinase 1 (Plk1), a master regulator of cell cycle, plays multiple roles in different aspects of mitosis, including mitotic entry, kinetochore-microtubule attachment and cytokinesis 11, 12. Plk1 is overexpressed in various cancers, such as pancreatic cancer, prostate cancer etc, implicating its potential role in cancer progression. Plk1 as a potential target for cancer treatment has been proposed and small molecules inhibiting the functions of Plk1 are under various clinical trials 13-16. In addition to its well-studied roles in cell cycle, Plk1 is involved in regulation of other signaling pathways, such as DNA damage response and the PI3K pathway, which plays pivotal roles in regulating cell growth and survival 17, 18. Dysregulation of the PI3K pathway is often associated with different kinds of diseases, such as cancer 18, 19. Thus, identification of novel factors involved in regulation of the PI3K pathway would have a great impact in understanding diseases associated with its dysregulation. Crosstalk between Plk1 and the PI3K pathway has been reported recently20, 21. However, detailed mechanism of how Plk1 is involved in the regulation of the PI3K pathway still remains largely unknown. In this study, we identify Plk1 as the kinase responsible for phosphorylation of IRS2 on two serine residues. Phosphorylation of IRS2 by Plk1 prevents the binding between IRS2 and the PI3K pathway components and increases its protein degradation, thus inhibiting the activation of the PI3K pathway upon growth factor stimulation. Of significance, we also show that the phosphorylation of IRS2 by Plk1 prevents premature mitotic exit via AKT inactivation.
EXPERIMENTAL PROCEDURES
Cell culture, RNA interference (RNAi), constructs, and transfection
HEK 293T cells and HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) (Sigma), supplemented with 10% fetal bovine serum (Atlanta), L-glutamine (Sigma) and 100 units/ml penicillin, 100 units/ml streptomycin at 37 in 5% CO2. To specifically deplete endogenous IRS2, shRNA was constructed targeting IRS2 sequence CCGGCTTCCAGAATGGTCTCAACTA. Plk1 shRNA was designed as described targeting AAGGGCGGCTTTGCCAAGTGCTT 22 and cloned into pLKO vector. For RNAi, cells were transfected with indicated shRNA constructs using Lipo2000 (Life Technologies). Two days after transfection, cells were treated with puromycin to select transfection-positive cells. Plasmid DNA was transfected with Lipo2000 (Life Technologies). IRS2-Myc was constructed by cloning IRS2-Myc from pBABE-puro-IRS2-myc into pQCXIP vector. pBABE-puro-IRS2-myc, GFP-AKT-K179M and Myr-AKT-delta 4-129 were purchased from Addgene.
Protein Purification and in vitro kinase assay
After various domains of murine IRS2 were PCR amplified and subcloned into pGEX-KG, glutathione-S-transferasae (GST)-tagged IRS2 were expressed in Escherichia coli and purified using GST agarose beads. Point mutations were made by using the QuickChange Site-Directed Mutagenesis Kit (Agilent Technologies). Purified recombinant IRS2 was incubated with purified Plk1 in the presence of [γ-32P] ATP at 30°C for 30 min. The reaction mixtures were resolved by SDS-PAGE, stained with Coomassie brilliant blue, dried and subjected to autoradiography.
Antibodies
Two phospho-specific antibodies against IRS2-S556 and S1098 were generated by Proteintech (Chicago, IL). After two peptides containing phospho-Ser556 and phospho-Ser1098 were synthesized and immunized into rabbits, polyclonal antibodies were affinity purified followed by control experiments to confirm the specificity of the antibodies. We also purchased antibodies against Plk1 (sc-17783) from Santa Cruz Biotech., β-actin (A-5441) from Sigma), and other antibodies from Cell Signaling.
Western blot and Immunoprecipitation (IP)
Cells were collected from culture plates and harvested by centrifugation at 2000rpm for 2 min. After wash with PBS, cells were re-suspended in TBSN buffer with protease inhibitors, followed by sonication and centrifugation at 14,000rpm for 15 min. Cell lysates were incubated with indicated antibodies overnight at 4°C, followed by 1h of incubation with protein A/G plus-Agarose beads. After supernatants are removed, immunocomplexes were loaded onto SDS-PAGE, and the proteins of interest were detected using indicated antibodies.
Immunofluorescence
Cells were grown on coverslips under normal culture conditions, fixed with 4% formaldehyde, and blocked with 5% bovine serum albumin (BSA) for 1 h. Primary and secondary antibodies were dissolved in 5% BSA and incubated on coverslips for 2 h and 1 h, respectively.
RESULTS
IRS2 interacts with Plk1 in cells and phosphorylates IRS2 at Ser 556 and Ser 1098
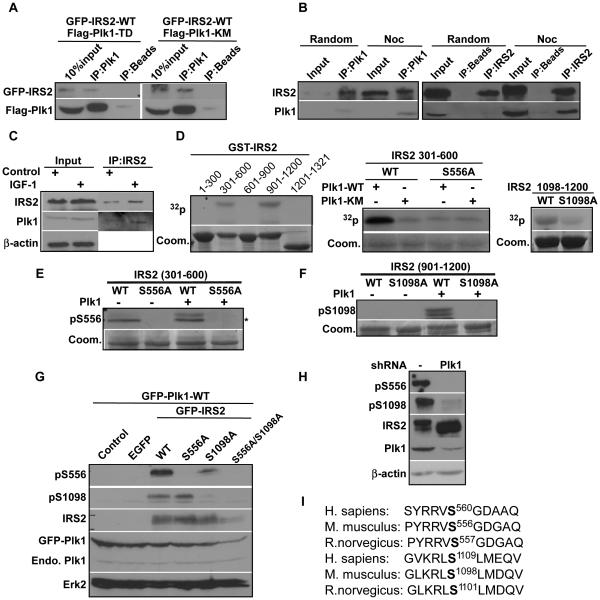
Mass spectrometry has identified IRS2 as a potential Plk1 substrate 23. To test whether IRS2 interacts with Plk1, we overexpressed Flag-tagged Plk1 along with green fluorescent protein tagged mouse IRS2 (GFP-IRS2) to detect binding between ectopically expressed IRS2 and Plk1. As indicated, overexpressed IRS2 was able to co-immunoprecipitate with overexpressed Plk1 (Fig 1A). To test the interaction of endogenous proteins, exponentially growing or mitosis-enriched cells were collected for anti-IRS2/Plk1 IP, and endogenous Plk1/IRS2 was detected in the IP pellets (Fig 1B). To test whether the Plk1/IRS2 interaction depends on growth factor, we treated exponentially growing cells with IGF-1 for 5 min, followed by anti-IRS2 IP/Western blot. Our data showed that IGF-1 treatment enhanced the interaction between Plk1 and IRS2 (Fig 1C). Next, we asked whether IRS2 is a Plk1 substrate. Accordingly, different regions of murine IRS2 were subcloned into a GST vector, expressed in bacteria and purified with GST tag. In vitro kinase assay showed that two regions of IRS2, amino acids (aa) 301-600 and aa 901-1200, were phosphorylated by Plk1 (Fig 1D, the left panel). Similar experiments were performed to further narrow down the Plk1 phosphorylation sites within aa 454-600 and aa 1098-1200 (data not shown). Then, virtually every single serine and threonine residues of aa 454-600 and aa 1098-1200 was mutated into alanine to identify Ser556 and Ser1098 as two Plk1 phosphorylation sites (Fig 1D, right two panels). To further characterize the two sites we mapped, two polyclonal antibodies specifically targeting phospho-Ser 556 and phospho-Ser 1098 were generated. Only WT IRS2 but neither IRS2-S556A nor IRS2-S1098A mutant was recognized by respective phospho-specific antibodies upon incubation with Plk1, confirming that 1) both Ser556 and Ser1098 were directly phosphorylated by Plk1 in vitro and 2) the two antibodies we generated specifically recognized the phosphorylated IRS2 in vitro (Fig 1E, 1F). In vivo, GFP-IRS2 (WT, S556A or S1098A) were expressed in 293T cells. As indicated in Fig 1G, both pS556 and pS1098 were detected in cells expressing GFP-IRS2-WT, but not in cells expressing IRS2-S556A or -S1098A, respectively, indicating that these two phosphorylation events do occur in cells and the two phospho-specific antibodies generated can specifically detect phosphorylation events on the targeted sites in cells (Fig 1G). Most importantly, pS556 and pS1098 antibodies were able to detect phosphorylation signals on human IRS2 from nocodazole-treated HeLa cells but not from cells depleted of Plk1 using RNAi, suggesting that phosphorylation of endogenous human IRS2 at serine 556 and serine 1098 in cells is Plk1 dependent (Fig 1H). Since both serines are highly conserved between mouse and human (Fig 1I), we used murine IRS2 to generate different constructs for further experiments.
Figure 1.
Plk1 interacts with IRS2 and phosphorylates IRS2 at Ser 556 and Ser 1098. A, Binding between overexpressed IRS2 and Plk1. HEK293T cells were transfected with Flag-Plk1 (T210D, constitutively active or K82M, kinase dead), along with GFP-IRS2 wild type (WT), harvested for Plk1 immunoprecipitation (IP), followed by immunoblotting (IB) analysis. B, Binding between endogenous Plk1 and IRS2. HeLa cells were treated with nocodazole for 12 h and harvested for Plk1 and IRS2 IP, followed by IB. C, Growth factor treatment enhanced interaction between Plk1 and IRS2. HeLa cells were treated with IGF-1 for 5 min and harvested for IRS2 IP, followed by IB. D, Plk1 phosphorylates IRS2 in vitro. Recombinant Plk1 (WT or KM, kinase dead mutant) was incubated with various purified GST-IRS2 fragments (WT or mutants) in the presence of [γ-32P]ATP. In the left and the right panels, only WT Plk1 was used. E and F, Generation of two phospho-specific antibodies. Plk1 was incubated with indicated forms of GST-IRS2 fragments, followed by IB against pS556-IRS2 (where the upper band is pS556 signal and the lower band with * is unspecific signal) (E) or pS1098-IRS2 (F). G, S556 and S1098 of IRS2 are phosphorylated in vivo. 293T cells were transfected with GFP-IRS2 constructs and GFP-Plk1 WT, and subjected to IB with phospho-specific antibodies. H, Plk1 is responsible for phosphorylation of IRS2 at S556 and S1098 in vivo. HeLa cells were transfected with either control or Plk1 shRNA, treated with nocodazole and subjected to IB. I, Alignment of IRS2 protein sequences in homo sapiens, mus musculus and Rattus norvegicus containing the equivalents of S556 and S1098.
Phosphorylation of IRS2 by Plk1 inhibits activation of PI3K pathway upon growth factor stimulation
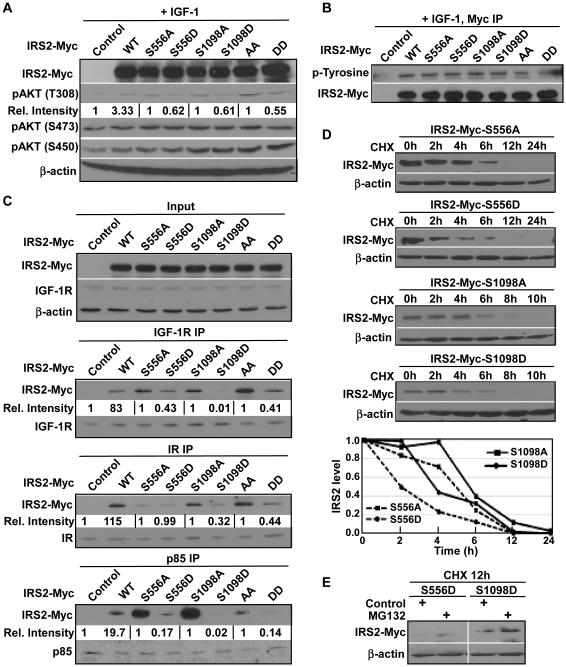
IRS2 is well known for its role as an adaptor protein in recruiting different signaling molecules to the cell membrane, including the PI3 kinase, upon growth factor stimulation 7, 24. Further, serine and threonine phosphorylation has been shown to regulate the function of IRS proteins 9, 10. Towards the end to understand the importance of Plk1-dependent phosphorylation of IRS2 on the potential regulation of the PI3K pathway, we transfected different IRS2 constructs into the cells, either the unphosphorylatable IRS2 alanine (A) mutant or the phosphorylation-mimicking aspartate (D) mutant, for either single sites or double sites. Cells transfected with IRS2-A mutants showed higher phosphorylation levels of AKT on threonine 308 (T308), compared with cells expressing IRS2-D mutants under IGF-1 stimulation. However, phosphorylation of other AKT sites did not show significant difference between cells expressing IRS2-A and -D mutants (Fig 2A). Since serine phosphorylation of IRS proteins is believed to regulate the phosphorylation of tyrosine residues on IRS2 7, 8, we also tested the total tyrosine phosphorylation on IRS2. Unfortunately, we did not find any significant difference among cells expressing different IRS2 constructs (Fig 2B). We might point out that it is possible that serine phosphorylation by Plk1 might not have a strong effect on the total tyrosine phosphorylation of IRS2, but rather affects the phosphorylation of tyrosine residues next to the two phospho-serine residues.
Figure 2.
Plk1-dependent phosphorylation of IRS2 antagonizes PI3K pathway activation upon growth factor stimulation. A, Cells expressing S556D, S1098D or S556D/S1098D mutants were less responsive to IGF-1 treatment than cells expressing the corresponding A mutants. HeLa cells were transfected with different IRS2 constructs, treated with IGF-1 for 5 min and subjected to IB. The relative intensities of pAKT-T308 were quantified by ImageJ with normalized signal intensities of paired control or IRS2 A mutants to be set as 1. B, Plk1 phosphorylation of IRS2 does not affect its IGF-1-induced tyrosine phosphorylation. 293T cells were transfected with different IRS2 constructs and harvested for anti-Myc IP, followed by anti-p-tyrosine IB. C, IRS2 phosphorylation at S556/S1098 inhibits its binding to other members of the PI3K pathway. 293T cells were transfected with different IRS2 constructs and harvested for IP using indicated antibodies, followed by IB. Relative intensities of co-IPed IRS2-Myc were quantified by using of ImageJ with normalized signal intensities of paired control or IRS2 A mutants to be set as 1. D, Plk1 phosphorylation of IRS2 promotes its degradation. 293T cells were transfected with different IRS2 constructs, treated with CHX (cycloheximide) for indicated times and harvested for IB. E, Degradation of IRS2 is proteasome dependent. 293T cells were transfected with IRS2-556D or -1098D mutants, treated with CHX for 6 h, incubated with or without proteasome inhibitor MG132 for an additional 1 h and harvested for IB.
To further understand the mechanism underlying the regulation of the PI3K pathway by Plk1-dependent phosphorylation of IRS2, we then asked whether phosphorylation of IRS2 by Plk1 affects the affinity of IRS2 binding to cell membrane receptors and PI3K subunits. Accordingly, cells expressing different IRS2 constructs were subjected to IP against either cell membrane receptors or PI3 kinase regulatory subunits p85, which interacts with IRS2 25. Consistence with the observed decreased phosphorylation of AKT-T308, we found that three IRS2-D mutants (S556D, S1098D, S556D/S1098D) show significantly less affinity with both upstream insulin/IGF-1 receptors and PI3K subunit p85, compared with the three respective IRS2-A mutants (S556A, S1098A, S556A/S1098A) (Fig 2C). Serine phosphorylation has also been shown to affect the stability of IRS2 protein level through a protease dependent pathway 26. In order to test whether Plk1-dependent phosphorylation of IRS2 affects its stability, we treated cells expressing different IRS2 constructs with cycloheximide to inhibit protein synthesis and analyzed the degradation rate of different IRS2 mutants. Phosphorylation of IRS2 on these two serine residues leads to faster degradation of IRS2 as indicated by the faster degradation of the D mutants (Fig 2D), thus likely contributing to the decreased phosphorylation levels of AKT-T308 as well. We also treated cells transfected with two IRS2 D mutants with proteasome inhibitor MG132 for 1 h after stopping protein translation using cycloheximide for 12 h and observed stabilization of both IRS2 D mutants, suggesting that Plk1 phosphorylation-associated IRS2 degradation is proteasome dependent (Fig 2E).
IRS2 protein level and phosphorylation are cell cycle regulated
As Plk1 level peaks at mitosis, it is expected that Plk1-dependent phosphorylation of IRS2 also peaks at mitosis. Accordingly, we treated HeLa cells with nocodazole to arrest cells at mitosis (Fig 3A). Compared with randomly growing cells, phosphorylation of both Plk1 sites were higher when cells were arrested at mitosis. Unexpectedly, we found that IRS2 protein level was also elevated in nocodazole-treated cells (Fig 3A), raising the possibility that IRS2 protein level is also cell cycle regulated. To carefully examine this possibility, HeLa cells were synchronized at the G1/S boundary with the DTB protocol and released for different time to monitor the protein and serine phosphorylation levels of IRS2. As indicated in Fig 3B, both IRS2 protein level and its phosphorylation at S556/S1098 start to elevate at G2 phase and peak at mitosis, matching the expression profile of Plk1. To further examine the protein and phosphorylation levels of IRS2 during mitosis, HeLa cells were subjected to the mitotic shake off protocol and released into fresh medium for indicated time. Both IRS2 protein and its phosphorylation at S556/S1098 were detected at mitosis and decreased as the cells exit mitosis (Fig 3C). Therefore, we conclude that both IRS2 protein level and its phosphorylation at S556/S1098 are increased as cells enter mitosis and decreased as cells exit mitosis. We next determined the subcellular localization of both total IRS2 and phospho-IRS2 during mitosis. Although Plk1 shows clear distribution at various mitotic structures, we did not observe a clear localization of IRS2 at typical mitotic structures (Fig 3D). In contrast, the pS1098-IRS2 epitope was detected at spindle poles in metaphase, midzones in anaphase, and midbodies in telophase/cytokinesis, colocalizing with Plk1 (Fig 3E, 3F).
Figure 3.
IRS2 protein level and Plk1-dependent phosphorylation are cell cycle regulated. A, Mitotic specific phosphorylation of IRS2 at S556 and S1098. Exponentially growing or mitosis enriched (via nocodazole treatment) - HeLa cells were harvested for IB against two phospho-specific antibodies and IRS2. B, HeLa cells were arrested at the G1/S boundary using a double thymidine block (DTB) protocol, released for different times and harvested for IB. C, Dephosphorylation of S556 and S1098 of IRS2 during mitotic exit. HeLa cells were arrested at mitosis with nocodazole, released for different times and harvested. D-E, Co-localization of Plk1 with phospho-S1098-IRS2 at mitotic structures. HeLa cells were cultured on coverslips and subjected to immunofluorescence (IF) staining with antibodies against Plk1 and IRS2 (D) or pS1098-IRS2 (E). DNA was stained with DAPI. Arrows showing the co-localization of Plk1 and pS1098-IRS2 are indicated in each panel. F, Co-localization of Plk1 with phospho-S1098-IRS2 at spindle polea. Cells were pre-treated with 0.25% TritonX100 for 2 min, followed by IF as in E. G, Overexpression of IRS2-WT does not affect mitotic exit. HeLa cells were transfected with IRS2-myc, treated with nocodazole and released for different times for IB. H, Depletion of IRS2 does not slow down mitotic exit. HeLa cells were transfected with IRS2 shRNA and processed as in G.
Phosphorylation of IRS2 by Plk1 prevents premature mitotic exit
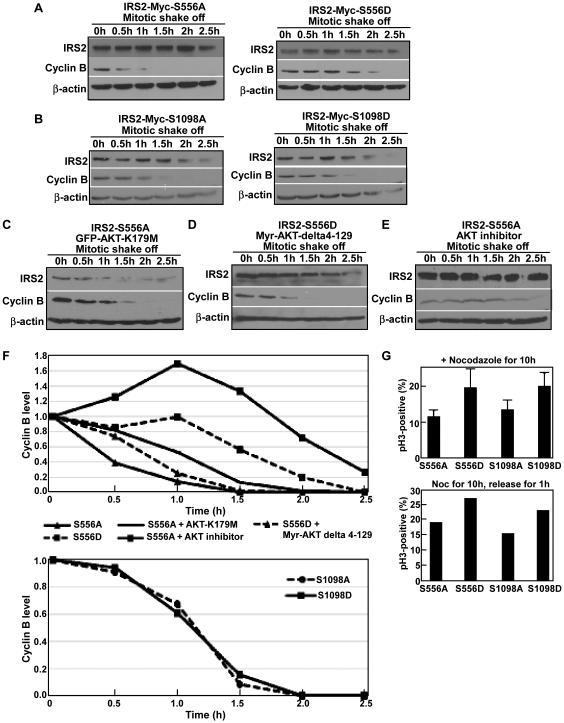
Since both the IRS2 protein and its serine phosphorylation are cell cycle regulated, we next asked whether IRS2 and its Plk1-dependent phosphorylation affect cell cycle progression. Accordingly, cells with different IRS2 status were arrested at mitosis using nocadazole, then released for different time to follow mitotic exit. Surprisingly, we did not observe significant differences in the rate of mitotic exit for cells either overexpressing WT IRS2 (Fig 3G) or depleting of IRS2 with RNAi (Fig 3H). Next, we compared mitotic exit of cells expressing different forms of IRS2 at Plk1 phosphorylation sites. In comparison to cells expressing IRS2-S556A, expression of IRS2-S556D apparently delayed mitotic exit (Fig 4A). However, expression of IRS2-S1098D only slightly delayed mitotic exit compared to cells expressing IRS2-S1098A (Fig 4B, 4F bottom). Considering that AKT is involved in cell cycle progression, especially mitotic exit 27, we asked whether the hindered mitotic exit of cells expressing IRS2-S556D is due to inhibited AKT activity. For that purpose, we transfected cells with IRS2-S556A along with dominant negative AKT (GFP-AKT-K179M). As indicated in Fig 4C, 4F, expression of the inactivated AKT indeed slowed down the mitotic exit of cells expressing IRS2-S556A. Furthermore, we also co-transfected cells with IRS2-S556D and myr-AKT-delta4-129, a constitutively active form of AKT due to lack of the PH domain. As expected, co-expression of the active AKT accelerated the mitotic exit of cells expressing IRS2-S556D (Fig 4D, 4F top), suggesting that inhibition of AKT activity is responsible for the hindered mitotic exit of cells expressing IRS2-S556D. Furthermore, we treated cells expressing IRS2-S556A with AKT inhibitor LY294002 during mitotic exit. Similar to cells expressing dominant negative AKT, cells treated with AKT inhibitor showed inhibited mitosis exit (Fig 4E, 4F top). Finally, cells expressing IRS2-S556D and -S1098D were apparently much more sensitive to nocodazole-induced mitotic arrest than cells expressing the corresponding alanine mutants (Fig 4G).
Figure 4.
Plk1-dependent phosphorylation of IRS2 prevents premature mitotic exit. A, IRS2-S556D expression slows down mitotic exit. HeLa cells were transfected with IRS2-Myc (S556A or S556D) constructs, treated with nocodazole and released for indicated times for IB. B, Phosphorylation of IRS2-S1098 does not affect mitotic exit. HeLa cells were transfected with IRS2-Myc (S1098A or S1098D) and processed as in A. C-D, Coexpression of inactive AKT delays mitotic exit in IRS2-S556A-expressing cells, whereas coexpression of constitutively active AKT accelerates mitotic exit in IRS2-S556D-expressing cells. HeLa cells were transfected with IRS2-Myc-S556A and GFP-AKT-K179M (C) or IRS2-Myc-S556D and Myr-AKT-delta 4-129 (D), and processed as in A. E, HeLa cells expressing IRS2-S556A were treated with AKT inhibitor LY294002 and processed as in A. F, Quantification of Cyclin B levels of A-E using ImageJ software. G, Cells expressing S556D or S1098D are more sensitive to nocodazole-induced mitotic arrest than the corresponding alanine mutants. HeLa cells were transfected with different IRS2-Myc constructs, treated with nocodazole, and subjected for anti-pH3 IF staining.
DISCUSSION
IRS2 acts as an important adaptor to active the PI3K pathway in response to growth factor stimulation 1, 2, 25. Although tyrosine phosphorylation of IRS2 and its function have been studied, very few studies on serine/threonine phosphorylation have been reported. Serine/threonine phosphorylation sites on IRS2 and their responsible kinases are mostly unknown, leaving the function of serine/threonine phosphorylation of IRS2 and regulatory mechanisms on IRS2 function largely undiscovered. In this study we show that 1) IRS2 is a novel substrate of serine/threonine kinase Plk1 both in vitro and in cells and further mapped the phosphorylation sites to be serine 556 and serine 1098; 2) Plk1 phosphorylation of IRS2 abolishes its interaction with several members of the PI3K pathway; and 3) Plk1 phosphorylation of IRS2-S556 prevents premature mitotic exit via AKT inactivation. Serine/threonine phosphorylation on IRS proteins has both positive and negative effects on the PI3K pathway activation, depending on phosphorylation sites and responsible kinases 7, 9, 10. Considering that we recently showed that Plk1 phosphorylation of PTEN leads to activation of the PI3K pathway20, it is somewhat surprising that Plk1 phosphorylation of IRS2 results in partial inhibition of AKT phosphorylation at T308, but not on other sites, such as serine 473 and serine 450, which are phosphorylated after T308 phosphorylation. It has been accepted that regulation of the PI3K pathway is exceedingly complex, supported by existence of many positive and negative feedback mechanisms 24, 28. It is possible that phosphorylation of IRS2 by Plk1 on serine residues is only involved in the regulation of the very first step of the activation of the PI3K pathway, thus only affecting AKT-T308 phosphorylation without affecting other AKT sites. Further mechanistic studies indicated that phosphorylation of IRS2 by Plk1 disrupts the binding between IRS2 and other members of the PI3K pathway, including both upstream growth factor receptors and downstream PI3 kinase subunits (Fig 2C). We noticed that transfection of various Plk1 unphosphorylatable IRS2 mutants leads to higher binding between IRS2 and IGF-1R, IR or p85, which is probably due to constitutive phosphorylation of the endogenous IRS2 by Plk1. Plk1 dependent phosphorylation also affects the stability of IRS2, leading to a higher degradation rate (Fig 2D). However, serine phosphorylation of IRS2 by Plk1 does not seem to have an significant impact on total tyrosine phosphorylation of IRS2, which may explain why we did not see very significant difference in the AKT phosphorylation levels between various A and D mutants upon growth factor stimulation. Plk1-dependent phosphorylation of IRS2 seems to be involved in a negative regulation that disrupts the further activation of the PI3K pathway upon growth factor stimulation, but might not have a significant effect on already active PI3K and AKT, as we observed abolished binding between IRS2 and p85 but only observed a 50% decrease in AKT activation status. We acknowledge that whether Plk1-dependent phosphorylation of IRS2 affects phosphorylation of specific tyrosine residues close to S556/S1098 needs further experimentation.
Plk1 plays important roles in different steps of cell cycle through its kinase activity towards various substrates 12, 29. Plk1 promotes proper mitotic progression by interacting with and phosphorylating distinct substrates during different stages of cell cycle. Plk1 protein level is strictly cell cycle regulated, rising from S phase and peaking in mitosis 12, 30. Interestingly, we found that both IRS2 protein level and its phosphorylation at S556/S1098 are cell cycle regulated, peaking at mitosis and decreasing as cells exit mitosis. AKT activity has been shown to regulate mitosis exit 27, 31. While activation of AKT promotes mitotic exit, AKT inhibition leads to hindered mitotic exit. We show in the study that Plk1-dependent phosphorylation of IRS2-S556 inhibits mitotic exit, partially through reduced AKT activity. Strikingly, transfection of phospho-mimic IRS2 mutant (S556D) delays mitotic exit compared with the unphosphorylatable IRS2 mutant (S556A), and this phenotype can be reversed by co-expression of a constitutively active form of AKT. Our data provide additional evidence to support that the PI3K pathway is involved in mitosis and that Plk1-associated phosphorylation of IRS2 is one mechanism to regulate this function of the PI3K pathway. A more detailed mechanism of how expression of IRS2-S556D inhibits mitotic exit needs further experimentation.
ACKNOWLEDGEMENT
L. Chen was financially supported by China Scholarship Council (CSC).
FUNDING
This work was supported by NIH grants R01CA157429 (X.L.), R01 AR059130 (N.A.), R01 CA176748 (N.A.) and ACS grant RSG-13-073 (X.L.).
ABBREVIATIONS
- Plk1
polo-like kinase 1
- IRS2
Insulin receptor substrate 2
- GIF-1
Insulin-like Growth Factor-1
- IGF-1R
Insulin-like growth factor-1 receptor
- IR
Insulin receptor
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PIP3
phosphatidylinositol-3,4,5-bisphosphate
- RNAi
RNA interference
- GST
glutathione S-transferase
- GFP
green fluorescence protein
- WT
wild type
- DAPI
4’,6-diamidino-2-phenylindole
- aa
amino acids
- IP
immunoprecipitation
- IF
immunofluorescence
- IB
immunoblotting
- DTB
double thymidine block
REFERENCES
- 1.Lizcano JM, Alessi DR. The insulin signalling pathway. Current Biology. 2002;12:R236–R238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- 2.Taha C, Klip A. The Insulin Signaling Pathway. J. Membrane Biol. 1999;169:1–12. doi: 10.1007/pl00005896. [DOI] [PubMed] [Google Scholar]
- 3.Braccini L, Ciraolo E, Martini M, Pirali T, Germena G, Rolfo K, Hirsch E. PI3K keeps the balance between metabolism and cancer. Advances in biological regulation. 2012;52:389–405. doi: 10.1016/j.jbior.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Vottero A, Guzzetti C, Loche S. New aspects of the physiology of the GH-IGF-1 axis. Endocrine development. 2013;24:96–105. doi: 10.1159/000342573. [DOI] [PubMed] [Google Scholar]
- 5.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Micro. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological reviews. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 9.Neukamm SS, Ott J, Dammeier S, Lehmann R, Haring HU, Schleicher E, Weigert C. Phosphorylation of serine 1137/1138 of mouse insulin receptor substrate (IRS) 2 regulates cAMP-dependent binding to 14-3.3 proteins and IRS2 protein degradation. J Biol Chem. 2013;288:16403–16415. doi: 10.1074/jbc.M113.474593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsche L, Neukamm SS, Lehmann R, Kremmer E, Hennige AM, Hunder-Gugel A, Schenk M, Haring HU, Schleicher ED, Weigert C. Insulin-induced serine phosphorylation of IRS-2 via ERK1/2 and mTOR: studies on the function of Ser675 and Ser907. American journal of physiology. Endocrinology and metabolism. 2011:E824–836. doi: 10.1152/ajpendo.00409.2010. 2010/11/26 ed. [DOI] [PubMed] [Google Scholar]
- 11.Petronczki M, Lenart P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nature reviews. Drug discovery. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 13.de Cárcer G, Manning G, Malumbres M. From Plk1 to Plk5: Functional evolution of polo-like kinases. Cell Cycle. 2011;10:2255–2262. doi: 10.4161/cc.10.14.16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medema RH, Lin CC, Yang JC. Polo-like kinase 1 inhibitors and their potential role in anticancer therapy, with a focus on NSCLC. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6459–6466. doi: 10.1158/1078-0432.CCR-11-0541. [DOI] [PubMed] [Google Scholar]
- 15.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 16.Yim H, Erikson RL. Plk1-targeted therapies in TP53- or RAS-mutated cancer. Mutation Research/Reviews in Mutation Research. 2014;761:31–39. doi: 10.1016/j.mrrev.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annual Review of Pathology: Mechanisms of Disease. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Reviews Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 19.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K//Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Li J, Bi P, Lu Y, Burcham G, Elzey BD, Ratliff T, Konieczny SF, Ahmad N, Kuang S, Liu X. Plk1 phosphorylation of PTEN causes a tumor-promoting metabolic state. Molecular and cellular biology. 2014;34:3642–3661. doi: 10.1128/MCB.00814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi BH, Pagano M, Dai W. Plk1 protein phosphorylates phosphatase and tensin homolog (PTEN) and regulates its mitotic activity during the cell cycle. J Biol Chem. 2014;289:14066–14074. doi: 10.1074/jbc.M114.558155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosstessner-Hain K, Hegemann B, Novatchkova M, Rameseder J, Joughin BA, Hudecz O, Roitinger E, Pichler P, Kraut N, Yaffe MB, Peters JM, Mechtler K. Quantitative phospho-proteomics to investigate the polo-like kinase 1-dependent phospho-proteome. Molecular & cellular proteomics : MCP. 2011;10 doi: 10.1074/mcp.M111.008540. M111.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valverde AM, Gonzalez-Rodriguez A. IRS2 and PTP1B: Two opposite modulators of hepatic insulin signalling. Archives of physiology and biochemistry. 2011;117:105–115. doi: 10.3109/13813455.2011.557386. [DOI] [PubMed] [Google Scholar]
- 25.White M. The IRS-signalling system: A network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 26.Briaud I, Dickson LM, Lingohr MK, McCuaig JF, Lawrence JC, Rhodes CJ. Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in beta-cells. J Biol Chem. 2005;280:2282–2293. doi: 10.1074/jbc.M412179200. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara K, Goto H, Izawa I, Kiyono T, Watanabe N, Elowe S, Nigg EA, Inagaki M. PI 3-kinase-dependent phosphorylation of Plk1-Ser99 promotes association with 14-3.3gamma and is required for metaphase-anaphase transition. Nature communications. 2013;4:1882. doi: 10.1038/ncomms2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annual review of pathology. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vugt MATM, Medema RH. Getting in and out of mitosis with Polo-like kinase-1. Oncogene. 2005;24:2844–2859. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 30.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Current biology : CB. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]