Abstract
A 49-year-old female patient presented with a massive left renal tumor, recurrent left flank pain and gross hematuria. The tumor was accompanied by a renal vein tumor thrombus, pancreatic infiltration and a solitary adrenal metastasis. Radical nephrectomy, distal pancreatectomy, ipsilateral adrenalectomy and splenectomy were performed. Histopathological examination suggested high-grade urothelial carcinoma (UC); however, tumor recurrence and multiple metastases were detected only 3 months after the surgery, and the patient succumbed during follow-up 1 month later. To the best of our knowledge, this is the first case of renal UC of such advanced stage with renal vein tumor thrombus, pancreatic infiltration and a solitary adrenal metastasis.
Keywords: renal urothelial carcinoma, tumor thrombus, pancreas infiltration, adrenal metastasis
Introduction
Urothelial carcinoma (UC) arising from the renal pelvis or the ureter is uncommon in Western countries (1), accounting for ~7% of all kidney tumors and ~5% of all urothelial tumors (2). Despite the frequency of microscopic vascular invasion in UC, tumor thrombus into the renal vein or inferior vena cava (IVC) is a rare occurrence (3), with no more than 30 cases reported in the literature to date (1). Even more limited are reports of renal UC with pancreatic infiltration, with only one case reported (4). Isolated adrenal metastasis from UC is also rare (5).
The present study reports an rare case of synchronous massive renal UC with renal vein tumor thrombus, pancreatic infiltration and a solitary adrenal metastasis. This study was approved by Ethics Committee of West China Hospital, Sichuan University.
Case report
A 49-year-old female patient presented to the West China Hospital, Sichuan University (Chengdu, China) in March 2014 with a 9-month history of repeated left flank pain and gross hematuria. Symptoms were controlled by the administration of antibiotics and a weight loss of ~4 kg was observed during this 9-month period. Upon admission in March 2014, physical examination results were normal. Laboratory test results indicated anemia (hemoglobin, 66 g/l; normal range, 115–150 g/l) and leukocytosis [white blood cell (WBC) count, 13.95×109/l; normal range, 3.5–9.5×109/l], and urinalysis revealed hematuria [red blood cells, 52/high power field (HP); normal range, 0–3/HP], leukocyturia (WBCs, 39/HP; normal range, 0–5/HP) and pyuria (pyocytes, 5/HP; normal value, 0/HP); however, blood and urine cultures were sterile, and urinary cytology showed no malignant cells. All other laboratory test results were within the normal ranges.
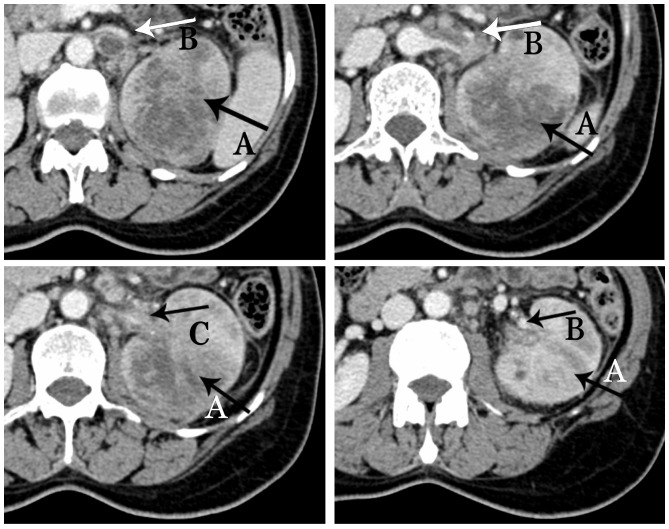
Contrast-enhanced computed tomography (CT) scans revealed a mixed-density mass (6.9×5.9×7.7 cm) in the upper pole of the left kidney with left renal vein invasion (Figs. 1 and 2) and possible infiltration of the pancreatic tail (Fig. 3). The left adrenal gland was thickened with a clear boundary, and a low-density nodule in the adrenal bifurcation was detected (Fig. 3). Dynamic radionuclide renal imaging showed severely impaired left renal function. The preliminary diagnosis was left-sided renal cell carcinoma (RCC) combined with renal vein thrombus with suspected pancreatic infiltration and left adrenal metastasis.
Figure 1.
Contrast-enhanced computed tomography scan of the abdomen showing a mixed density mass measuring 6.9×5.9×7.7 cm, with inhomogeneous enhancement, and large areas of necrosis and liquefaction (arrow A). Visualized (arrow B) and nonvisualized (arrow C) renal vein invasion by the tumor thrombus.
Figure 2.
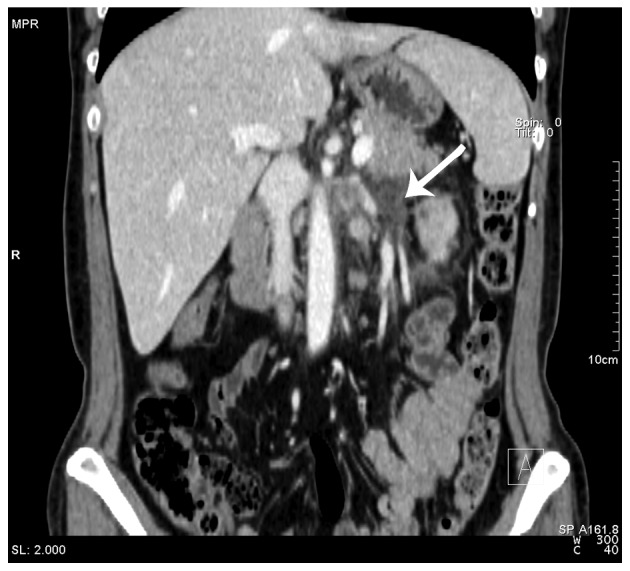
Contrast-enhanced computed tomography scan of the abdomen revealing tumor thrombus in the left renal vein (black arrow).
Figure 3.

Contrast-enhanced computed tomography scan of the abdomen showing an infiltrated pancreatic tail (arrow D) and a low-density nodule in the bifurcation (arrow E).
Left radical nephrectomy, distal pancreatectomy and ipsilateral adrenalectomy were performed in March 2014. In addition, the splenic hilum was observed to be surrounded by reactive fibrotic tissue. The two tissues were difficult to separate, therefore, splenectomy was also performed. Histopathological examination of the kidney, pancreatic tail and adrenal gland were conducted. Tissues were fixed with paraformaldehyde, paraffin-embedded, and cut into 5-mm sections. Immunohistochemical studies using the avidin-biotin-complex immunoperoxidase technique were performed. The following commercially available antibodies were used: Pan cytokeratin (CK) (cat. no. C2931; Sigma-Aldrich, St. Louis, MO, USA), P63 (cat. no. SRP5112; Sigma-Aldrich), CK5 (cat. no. ab52635; Abcam, Cambridge, MA, USA), CK6 (cat. no. ab124821; Abcam), CK7 (cat. no. ab82253; Abcam), CK20 (cat. no. ab76126; Abcam), CD10 (cat. no. ab126593; Abcam) and RCC1 (cat. no. ab109379; Abcam). Appropriate positive and negative controls were run concurrently for all the markers tested. The findings revealed poorly differentiated carcinoma, and immunohistochemical staining revealed positivity for panCK, P63, CK5, CK6, CK7 and CD10 and negativity for RCC1 CK20, which indicated high-grade UC. The thrombus arose from the UC, which had histologically invaded the wall of the renal vein.
The patient was excluded from chemotherapy due to personal reluctance and poor postoperative physical condition. Postoperative CT scans performed 3 months after the surgery detected tumor recurrence and metastasis in several sites, including in the lung, retroperitoneal space, pancreatic resection margin and psoas muscle. The patient succumbed during follow-up 1 month later.
Discussion
With the development of novel diagnostic modalities, the preoperative diagnosis of a renal tumor with renal vein or IVC tumor thrombus, as well as the assessment of the infiltrating range of the tumor, are relatively simple; however, distinguishing UC from RCC remains a challenge. Urinary cytological analysis was reported to have a diagnostic accuracy of 59%, but with a high ratio of false negatives for malignancy (5). A CT scan finding of preserved reniform kidney shape by an infiltrating neoplastic process supports the diagnosis of UC (2). In addition to CT and magnetic resonance urography, flexible ureteroscopy techniques, fluorescent in situ hybridization and narrow-band imaging may also facilitate diagnosis, and have thus been widely accepted (6). However, none of these methods is sufficiently accurate or effective for differentiating UC from RCC; therefore, intraoperative frozen section examination is recommended and additional attention must be paid to the renal neoplasm to determine the most appropriate surgical strategy, as surgical strategies for RCC and UC differ.
Therapeutic strategies for UC vary. Despite the associated toxicity, platinum-based chemotherapy is currently the primary treatment option for upper tract UC; however, certain studies have reported that it has no impact on survival (6) and resulted in repeated relapses, even following an initial response to systemic chemotherapy (7,8).
Furthermore, surgical excision of metastatic UCs with curative intent has been introduced as a treatment strategy, and the results of several studies have proposed adrenalectomy for isolated or multiple adrenal metastases (4,7,8). Several case reports and small-scale studies have also favored pancreatic metastasectomy, particularly for primary RCC (9,10); however, studies regarding UC are lacking. According to Nikfarjam et al (3), right nephrectomy combined with pancreaticoduodenectomy was performed in a single case of adhesion of renal UC to the duodenal wall, resulting in a disease-free condition for 3 months. Therefore, distal pancreatectomy and adrenalectomy should be considered in the setting of pancreatic infiltration and adrenal metastasis arising from UC.
Notably, the present case posed an additional challenge due to the presence of a tumor thrombus. According to the literature, aggressive surgery for the removal of an IVC thrombus proven beneficial for patients with RCC; however, its value in the management of renal UC remains unclear, due to limited data and the aggressive nature of UC (1,2). Existing data indicates that patients who present with UC and an IVC tumor thrombus have a poor prognosis, despite aggressive radical nephrectomy or nephroureterectomy, as the majority of patients succumbed within 6 months of the initial diagnosis (2). Although no benefits of surgery have been reported in such advanced cases of UC (6), surgical intervention may serve as a palliative treatment option or as a means to decrease the risk of thrombus shedding, as in the present case.
The current case indicates that cases of late-stage aggressive renal UC, characterized by massive renal UCs with renal vein tumor thrombi, pancreatic infiltration and adrenal metastasis, may not benefit from surgery or chemotherapy; therefore, strategies aimed at the early identification and treatment of such cases are required.
In conclusion, it is crucial to distinguish UC from RCC in order to determine the appropriate surgical treatment strategy; however, differential diagnosis remains a challenge. Furthermore, the present case and those in the literature indicate that massive renal UC with tumor thrombus, organ infiltration and metastasis is associated with a poor prognosis, regardless of whether surgery or chemotherapy is used.
Acknowledgements
The present study was supported by the Science & Technology Pillar Program of Sichuan Province (grant no. 2012SZ0009), the 2013 Young Investigator Award of the Prostate Cancer Foundation, and the Project of Natural Science Foundation of China (grant no. 81300627). The authors would also like to thank Enago (New York, NY, USA) for their assistance in the revision of this manuscript.
Glossary
Abbreviations
- UC
urothelial carcinoma
- IVC
inferior vena cava
- CT
computed tomography
- RCC
renal cell carcinomas
References
- 1.Tseng YS, Chen KH, Chiu B, Chen Y, Chung SD. Renal urothelial carcinoma with extended venous thrombus. South Med J. 2010;103:813–814. doi: 10.1097/SMJ.0b013e3181e63393. [DOI] [PubMed] [Google Scholar]
- 2.Miyazato M, Yonou H, Sugaya K, Koyama Y, Hatano T, Ogawa Y. Transitional cell carcinoma of the renal pelvis forming tumor thrombus in the vena cava. Int J Urol. 2001;8:575–577. doi: 10.1046/j.1442-2042.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 3.Nikfarjam M, Gusani NJ, Kimchi ET, Mahraj RP, Staveley-O'Carroll KF. Combined right nephrectomy and pancreaticoduodenectomy. Indications and outcomes. JOP. 2008;9:449–455. [PubMed] [Google Scholar]
- 4.Washino S, Hirai M, Matsuzaki A, Kobayashi Y. Long-term survival after adrenalectomy for asynchronous metastasis of bladder cancer to the bilateral adrenal glands. Case Rep Urol. 2012;2012:425230. doi: 10.1155/2012/425230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungberg B, Stenling R, Osterdahl B, Farrelly E, Aberg T, Roos G. Vein invasion in renal cell carcinoma: Impact on metastatic behavior and survival. J Urol. 1995;154:1681–1684. doi: 10.1016/S0022-5347(01)66749-1. [DOI] [PubMed] [Google Scholar]
- 6.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Siefker-Radtke AO, Walsh GL, Pisters LL, Shen Y, Swanson DA, Logothetis CJ, Millikan RE. Is There a role for surgery in the management of metastatic urothelial cancer? The M. D. Anderson Experience. J Urol. 2004;171:145–148. doi: 10.1097/01.ju.0000099823.60465.e6. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann J, Suttmann H, Albers P, Volkmer B, Gschwend JE, Fechner G, Spahn M, Heidenreich A, Odenthal A, Seif C, et al. Surgery for metastatic urothelial carcinoma with curative intent: The German experience (AUO AB 30/05) Eur Urol. 2009;55:1293–1299. doi: 10.1016/j.eururo.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Machado NO, Chopra P. Pancreatic metastasis from renal carcinoma managed by Whipple resection. A case report and literature review of metastatic pattern, surgical management and outcome. JOP. 2009;10:413–418. [PubMed] [Google Scholar]
- 10.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–293. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]