Abstract
Cleidocranial dysplasia (CCD) is a rare autosomal dominant condition that affects ossification. The dental abnormalities associated with CCD present an obstacle to orthodontic treatment planning. Early diagnosis is crucial to provide the patient with different treatment modalities that will suit the particular patient. In the present case, combined surgical and orthodontic treatment were performed to guide multiple impacted teeth. A single nucleotide missense variation was identified in exon 3 of runt-related transcription factor 2 (RUNX2) in this patient. The current results suggest a correlation between dental alterations and mutations in the runt domain of RUNX2 in CCD patients. Further clinical and genetic studies may required to confirm the association between phenotypes and genotypes in CCD and to identify other factors that may influence the clinical features of this disease. Patients with cleidocranial dysplasia require a team approach which demands good communication and cooperation from the patient. Timing of the intervention is critical, and numerous surgeries may be required. The patient in the present case report was treated by a team of practitioners, which involved several dental specialties to achieve an optimal result.
Keywords: cleidocranial dysplasia, supernumerary teeth, RUNX2 mutation
Introduction
Cleidocranial dysplasia (CCD) is a rare developmental disease with skeletal dysplasia and is considered to be of autosomal dominant inheritance (1). Individuals with CCD are typically of short stature, with brachycephalic skull and bossing of the parietal and frontal bones (2). Hypoplasia of the mid-face in patients with CCD frequently presents as mandibular prognathism (3). The sutures and fontanelles of the skull exhibit delayed closure with the occurrence of secondary centers of ossification in these areas and the formation of Wormian bones (4). The skeletal relationship of the jaws tends to be in a Class III position due to the presence of a hypoplastic maxilla (5). Vertical facial growth is decreased due to poor development of the alveolar bone (3). Other oral manifestations include delayed exfoliation of deciduous teeth, delayed eruption of permanent dentition, multiple supernumerary teeth and predilection for cyst formation involving the unerupted teeth (6). Besides defects in the skull and clavicle, CCD patients may suffer from other bony anomalies such as scoliosis, knock-knees and flat feet (4). In addition, hearing loss and upper respiratory tract infections are not uncommon in patients with CCD (4). However, it is important to note that CCD does not affect the individual mentally or intellectually (2).
Mutations of the runt-related transcription factor 2 gene (RUNX2), located on chromosome 6p21, have been identified as the cause of CCD (7,8). RUNX2 controls the differentiation of precursor cells into osteoblasts and is essential for intramembranous bone formation, which is associated with delayed ossification of skull, clavicles, maxilla and teeth (9). The gene is also required for mesenchymal condensation, osteoblast differentiation from mesenchymal stem cells, chondrocyte hypertrophy and vascular invasion in the developing skeleton (10,11). The incidence of CCD is one per million, and may develop at any age, affecting males and females equally. Significant variability in the clinical expression of this syndrome reflects a degree of phenotypic polymorphism (12). The present case report describes the clinical presentation and management of dentofacial abnormalities in an adult Chinese male patient with CCD presenting with mutation of the RUNX2 gene.
Case report
The present study was approved by the Second Affiliated Hospital of Dalian Medical University (Dalian, China), and all patients provided their informed consent prior to inclusion in the study.
A 23 year old Chinese male complaining of failure of complete eruption of the upper permanent anterior teeth was admitted to The Second Affiliated Hospital of Dalian Medical University. The patient was not satisfied with his dental and facial appearance. The patient was of short stature and presented with a history of delayed growth. Craniofacial results included delayed closure of cranial sutures and fontanelles, brachycephalia, and the presence of Wormian bones. Clinical diagnosis of cleidocranial dysplasia was reached based on the presence of an enlarged skull, frontal bossing, depressed nasal bridge, hypoplasia of the clavicles, failure of tooth eruption, multiple supernumerary teeth and genetic test for mutation in the RUNX2 gene. In brief, the patients were tested for the RUNX2 gene (13) and 2 ml aliquots of the peripheral blood were collected in anticoagulant ethylenediaminetetraacetic acid tubes and used to extract genomic DNA. A total of 1 µl of the extract was subjected to 1.0% agarose gel electrophoresis. Eight pairs of primers (Table I) were used for the polymerase chain reaction (PCR) amplification of eight exons in the RUNX2 gene. Next, the PCR products were detected and separated on agarose gel electrophoresis, and the target band was recycled for sequencing. Medical history indicated no parental consanguinity and no similar signs of hereditary disorder on either side of the family.
Table I.
Sequences of the primers used for the eight exons of the RUNX2 gene.
| Exon | Primer sequences (3′-5′) | Annealing temperature °C |
|---|---|---|
| 0 | F: ATGGTTAATCTCCGCAGGTCA | 58.0 |
| R: GCTATTTGGAAAAGCTAGCAG | ||
| 1 | F: CCAAAGACTCCGGCAAAGAT | 56.5 |
| R: AAGGCAGGAGGTCTTGGAG | ||
| 2 | F: TGGCATCACAACCCATACAC | 59.0 |
| R: GTCTACATTTCATCAAAGGAGC | ||
| 3 | F: AATTTAGAAGAAGGAGTCCTG | 59.0 |
| R: AAATATATGCAGATAGCAAAG | ||
| 4 | F: ATTCCTTGGCTTAAACTCCCAG | 56.0 |
| R: GCCAGCTTCACAGCTCCAGG | ||
| 5 | F: AACGCTTTGTGCTATTTAAGGCC | 61.0 |
| R: CCAGTTGTCATTCCCTTGCCC | ||
| 6 | F: CTCTGGGAAATACTAATGAGGGA | 61.0 |
| R: AGTGCCATGATGTGCATTTGTAAT | ||
| 7 | F: GGCTTGCTGTTCCTTTATGG | 60.0 |
| R: GGCTGCAAGATCATGACTGA |
RUNX2, runt-related transcription factor 2; F, forward; R, reverse.
The patient appeared to have a concave facial pattern and a skeletal Class III malocclusion (5). Intraoral examination revealed a mixed dentition with Angle's Class III molar relationship. The patient's oral hygiene was poor and the upper incisors and the canine on the left side were missing. Oral examination revealed the following dental formula:
Radiological examination was conducted to assess the skeletal morphology of the cranium, face and the status of dental development. In brief, the radiological examination is conducted by the DOWN (5) and TWEED (14) analysis system. Initially, we mark the point nasion, point A, B and some other important points and then we get lines and planes for the film. Next, the distance and angle are measured and an analysis of the correlation among the maxillary, mandibular and cranium is undertaken. Finally, a skeletal class III correlation is identified.
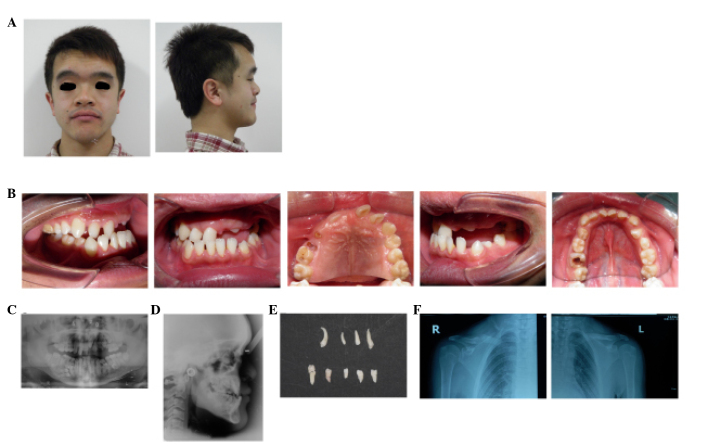
A panoramic radiograph revealed the presence of impacted maxillary and mandibular teeth along with supernumerary teeth in each quadrant (four in the maxilla and five in the mandible). Lateral radiography confirmed skeletal malocclusion. The various craniofacial angle values were as follows: SNA, 89.5°; SNB, 88°; and ANB, 1.5. The FMIA angle was 68°; FMA was 23°; IMPA was 89°; and the Z-angle was 93°. Nine supernumerary teeth were present in the patient's oral cavity. The patient was thus confirmed to have mandibular prognathism with multiple congenitally impacted and supernumerary teeth. In addition to craniofacial anomalies, chest radiographs revealed hypoplasia of the right clavicle (Fig. 1).
Figure 1.
Extraoral and intraoral photographs of a patient with cleidocranial dysplasia (CCD). (A) Extraoral facial pretreatment photographs of a 23 year old Chinese male with CDD exhibit a concave facial profile (skeletal Class III malocclusion) with an enlarged skull, frontal bossing and a depressed nasal bridge. (B) Intraoral examination of a 23 year old Chinese male with CCD reveals a mixed dentition with an Angle's Class III molar relationship. (B) The oral hygiene was poor and the upper incisors and the canine on the left side were missing. Radiographic assessment of pretreatment radiographs of a 23 year old Chinese male with CCD indicate several impacted teeth and skeletal malocclusion in (C) panoramic and (D) lateral radiographs. Nine supernumerary teeth that were extracted from the patient prior to the onset of orthodontic treatment, including four from the maxilla and five from the mandible. Analysis of clavicles using chest radiographs of a patient with CCD reveals hypoplasia of the (E) right clavicles while not observed in the (F) left side.
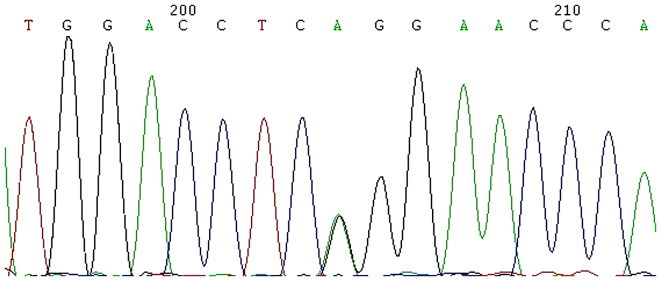
Genetic testing to investigate the presence of mutation(s) in RUNX2 gene was performed in this patient. A missense single nucleotide variation was detected in cDNA site 674 G>A in exon 3, as shown in Fig. 2. The sequencing consisted of all the RUNX2 exons. The amino acid was changed from arginine encoded by CGG, to glutamine encoded by CAG. The gene test was supported by Beijing Genomics Institute (http://www.genomics.cn/; Beijing, China) and the sample number was LA13001-220.
Figure 2.
Mutation analysis of the RUNX2 gene in a 23 year old patient with cleidocranial dysplasia indicates a single nucleotide variation in cDNA site 674 G>A in exon 3.
Treatment
The orthodontic goals that were set to manage the dentofacial anomalies in this patient were as follows: i) Facilitating the completion of dental eruption by surgical exposure of impacted teeth; ii) correction of anterior crossbite; and iii) increasing the vertical dimension of the face. Due to the advanced age of the patient in this report, treatment was provided in separate stages. The first phase of the treatment included the surgical extraction of all the primary and supernumerary teeth. This was followed by the orthodontic procedures as described below. An impacted permanent tooth (#23) was surgically exposed under local anesthesia with articaine (Produits Dentairs Pierre Rolland, Merignac, France) and >1.7 ml was injected at a time, following which a small lingual button was bonded immediately, and it was then ligated to the archwire [nickel-titanium (Ni-Ti) round] for immediate activation. Due to the flexibility of the Ni-Ti archwire, orthodontic traction was not required at this stage. As only one impacted permanent tooth was exposed each time, we did not require the use of an extra device for anchorage. The same method was used to guide tooth #25. Taking the anterior esthetics into consideration, closed-eruption surgical technique was used for tooth #21, while other impacted permanent teeth (#23,#25) were guided via the open-eruption technique.
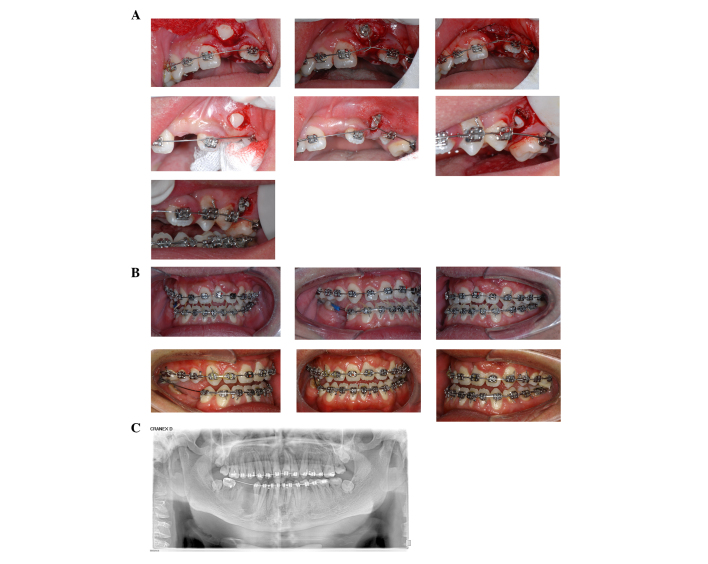
After the upper anterior teeth had erupted and were brought into alignment, orthodontic traction was applied. Intraoral elastic traction was used to correct for anterior crossbite which demanded the patient to wear the elastics 24 h a day for a period of 2 months. The final stage of the orthodontic treatment involved the establishment of a stable occlusal relationship by intramaxillary triangle traction. The patient was advised to receive an implant supported prosthesis in the lower right posterior region. Furthermore, the patient was referred to the department of periodontics for the management of the periodontal health of the maxillary anterior teeth following orthodontic treatment. Post-treatment clinical and panoramic images (Fig. 3B and C) showed successful alignment of the upper left incisor in the arch as well as the correction of the root direction.
Figure 3.
Intraoral clinical photographs showing orthodontic treatment for the guided eruption of impacted permanent teeth. The figure shows: i) Exposure; ii) bonding and immediate activation of the left maxillary central (A) incisor, canine and second premolar in a patient with cleidocranial dysplasia. The intraoral photograph was captured between 17 and 24 months following treatment. (B) Intraoral clinical photographs and (C) panoramic radiograph at the end of orthodontic treatment. The left upper incisor was nearly aligned within the arch and the direction of the root was corrected.
Discussion
The molecular defect in CCD is situated at the chromosomal locus of 6p21 (7,8). Although CCD is relatively uncommon, it has a wide geographic distribution probably due to the ongoing random occurrence of new mutations in the determinant gene. In this scenario, there is no biological pressure against autosomal dominant transmission from generation to generation and a chance mutation or a founder effect can be perpetuated in a particular population (15).
Dental anomalies and certain degrees of clavicular hypoplasia appear to be consistent features of this disorder. CCD is of clinical significance to dentistry due to the involvement of the facial bones, a prolonged retention of deciduous teeth, impacted permanent successors and supernumerary elements, occasionally accompanied by follicular cysts and eruptive pseudocysts (16). The highest number of supernumerary teeth in a patient with cleidocranial dysplasia reported in literature is 63 (13). For these reasons, dental management is a significant aspect of the health care of affected persons.
A patient with CCD presents with many treatment challenges for the dental team. The most challenging part of treatment involving the patient in the present report was the occurrence of unerupted permanent teeth, which were uncovered and guided into the arch using the flexible Ni-Ti round archwire. A number of supernumerary teeth had to be extracted, and the mandibular right permanent premolars were located so deep and so close to the inferior alveolar nerve that a decision was made to remove and replace them with implants subsequent to orthodontic treatment.
In the present study, surgical exposure of the unerupted permanent teeth with orthodontically guided eruption was the method of choice. This allowed the patient to retain his own teeth and avoided the need for a prosthesis that would have to be maintained or replaced several times during his lifetime. The patient was satisfied with the esthetic and functional aspects of the treatment. In the case of the orthodontic and surgical methods used in the present study, the procedure was based on the Jerusalem approach presented by Becker et al in 1997 (12,17). This Jerusalem approach exposed the permanent teeth in two stages, allowing the patient to retain their permanent incisors while the posterior permanent teeth are extracted at an appropriate time.
RUNX2 haploinsufficiency may have led to abnormal alveolar bone remodeling due to defects in the osteoblasts that compromises osteoblast-osteoclast interactions (18). Evidence from RUNX2 heterozygous knockout mice supports an increased impact of this factor in cancellous bone formation with advanced age (19). In addition to the genotype, environmental factors and a complex system involving epigenetics and copy number variations may regulate the formation of supernumerary teeth in patients with CCD.
The present case demonstrated numerous characteristics routinely observed in a patient with CCD. Guidelines for the treatment of the CCD case are sparse, due to the rarity of the disease. Patients with CCD require a team approach with good communication and cooperation from the patient. Timing of the intervention is critical, and numerous surgeries may be required. It is also important to collaborate with geneticists to ensure early diagnosis.
References
- 1.Gorlin RJ, Cohen MM, Hennekam RC. Gorlin's Syndromes of the Head and Neck. Oxford University Press, Inc.; New York, NY: 1990. [Google Scholar]
- 2.Becker A. The Orthodontic Treatment of Impacted Teeth. Martin Dunitz; London: 1998. [Google Scholar]
- 3.Richardson A, Deussen FF. Facial and dental anomalies in cleidocranial dysplasia: A study of 17 cases. Int J Paediatr Dent. 1994;4:225–231. doi: 10.1111/j.1365-263X.1994.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper SC, Flaitz CM, Johnston DA, Lee B, Hecht JT. A natural history of cleidocranial dysplasia. Am J Med Genet. 2001;104:1–6. doi: 10.1002/ajmg.10024. [DOI] [PubMed] [Google Scholar]
- 5.Hitchin AD, Fairley JM. Dental management in cleido-cranial dysostosis. Br J Oral Surg. 1974;12:46–55. doi: 10.1016/0007-117X(74)90060-2. [DOI] [PubMed] [Google Scholar]
- 6.Proffit W R. Malocclusion and dentofacial deformity in contemporary society. 4th. Elsevier Ltd.; St. Louis, Missouri, USA: 2007. Contemporary Orthodontics. [Google Scholar]
- 7.Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 8.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/S0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 9.Mundlos S. Cleidocranial dysplasia: Clinical and molecular genetics. J Med Genet. 1999;36:177–182. [PMC free article] [PubMed] [Google Scholar]
- 10.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 11.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 12.Becker A, Lustmann J, Shteyer A. Cleidocranial dysplasia: Part 1-General principles of the orthodontic and surgical treatment modality. Am J Orthod Dentofacial Orthop. 1997;111:28–33. doi: 10.1016/S0889-5406(97)70298-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto H, Sakae T, Davies JE. Cleidocranial dysplasia: A light microscope, electron microscope and crystallographic study. Oral Surg Oral Med Oral Pathol. 1989;68:195–200. doi: 10.1016/0030-4220(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 14.Tweed CH. The frankfort-mandibular plane angle in orthodontic diagnosis, classification, treatment planning, and prognosis. Am J Orthodontics and Oral Surg. 1946;32:175–230. doi: 10.1016/0096-6347(46)90001-4. [DOI] [PubMed] [Google Scholar]
- 15.Tina R, Lawrence S, Peter B. Cleidocranial dysplasia: A review of the dental, historical, and practical implications with an overview of the South African experience. Oral Surg Oral Med Oral Pathol and Oral Radiol. 2013;115:46–55. doi: 10.1016/j.oooo.2012.07.435. [DOI] [PubMed] [Google Scholar]
- 16.Farronato G, Maspero C, Farronato D, Gioventù S. Orthodontic treatment in a patient with cleidocranial dysostosis. Angle Orthod. 2009;79:178–185. doi: 10.2319/111307-393.1. [DOI] [PubMed] [Google Scholar]
- 17.Becker A, Shteyer A, Bimstein E, Lustmann J. Cleidocranial dysplasia: Part 2-Treatment protocol for the orthodontic and surgical modality. Am J Orthod Dentofacial Orthop. 1997;111:173–183. doi: 10.1016/S0889-5406(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 18.Dorotheou D, Gkantidis N, Karamolegkou M, Kalyvas D, Kiliaridis S, Kitraki E. Tooth eruption: Altered gene expression in the dental follicle of patients with cleidocranial dysplasia. Orthod Craniofac Res. 2013;16:20–27. doi: 10.1111/ocr.12000. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji K, Komori T, Noda M. Aged mice require full transcription factor, Runx2/Cbfa1, gene dosage for cancellous bone regeneration after bone marrow ablation. J Bone Miner Res. 2004;19:1481–1489. doi: 10.1359/JBMR.040601. [DOI] [PubMed] [Google Scholar]