Abstract
Left ventricular (LV) dysfunction in conjunction with postcapillary pulmonary hypertension (PH) is frequently associated with right ventricular (RV) dysfunction, determining the patient prognosis. Compensatory mechanisms for RV dysfunction have not been previously evaluated in detail. Since calcium dependent right atrial (RA) dynamics are a surrogate for RA contractile properties, the present study examined the calcium dependency of RA tissue obtained from patients with or without postcapillary PH. In total, 15 patients with PH (PH group; mean age, 70.7±7.2 years) and 10 patients without postcapillary PH (non-PH group; mean age, 55.7±11.8 years) who were scheduled to undergo elective left heart valve surgery were included in the current study. Calcium concentration (pCa; shown as the negative log10) against force curves were generated, while LV and RV function was evaluated by echocardiography. Echocardiography data revealed a significantly reduced LV function in the PH group, while the RV function was preserved in the two groups, precluding overt RV dysfunction. In the PH group, significantly reduced force values were detected at high pCa values when compared with the non-PH group force, indicating impaired RA function. Furthermore, reduced calcium sensitivity was observed (which was determined as the pCa at half maximal activation) in the PH group, and the presence of a compensatory mechanism for reduced force capacity was hypothesized. In conclusion, the preliminary results of the current study showed impaired RA contractile properties in postcapillary hypertension with preserved RV function. The diminished RA compensatory mechanisms may lead to accelerated RV dysfunction in the clinical course of postcapillary PH.
Keywords: postcapillary pulmonary artery hypertension, skinned fibers, calcium sensitivity
Introduction
Pulmonary hypertension (PH) is a pathophysiological disorder that may involve multiple clinical conditions and can complicate numerous cardiovascular and respiratory diseases (1). The incidence of PH is 97 cases per million with a female-to-male ratio of 1.8 in the UK (1). Left-sided heart failure is considered to be the most common cause of PH. Furthermore, PH may have clinical consequences on the right-side heart function; however, severe PH in these cases is relatively uncommon (1).
Postcapillary PH in patients with left ventricular (LV) dysfunction is a well-defined risk factor for right ventricular (RV) heart failure and subsequent increased morbidity and mortality (2,3). Compensatory mechanisms to overcome RV dysfunction are not well defined for these patients, and only limited data are available in the literature about dealing with the effect of postcapillary PH on right atrial (RA) dynamics and contractile features (4,5).
For precapillary PH, RA compensatory mechanisms have been described in animal models with inconclusive results (6,7). In general, precapillary PH RV pressure overload leads to myocardial hypertrophy (7), with the detrimental effect of RV diastolic dysfunction (6,8,9). Thus, RA function is the critical factor for RV filling, and any RA dysfunction may initiate right heart failure in patients with PH (6,8). The compensatory mechanism for RV diastolic dysfunction involves increased RA contractility, thus maintaining the RV filling (6). The importance of RA function is underlined in a study by Shiina et al (10), which described a strong correlation between right heart failure and reduced RA contractility in patients with chronic precapillary PH.
The aim of the present study was to evaluate RA function in postcapillary PH in patients with LV dysfunction due to left heart valve pathology. Since RA calcium-dependent skinned fiber dynamics are a surrogate for RA contractile properties, the calcium-dependent RA contractile dynamics were investigated in patients scheduled to undergo elective left heart valve surgery who presented with or without postcapillary PH.
Patients and methods
Ethics
The study was approved by the Ethics Committee of the Medical Association Rheinhessen. All patients provided written informed consent for the use of intraoperative resected tissue in further research examination. The study was performed according to the Declaration of Helsinki (11).
Patients
A retrospective study was performed using prospectively collected data from patients scheduled to undergo elective left heart valve surgery due to aortic and mitral valve disease at the Department of Cardiothoracic and Vascular Surgery of the University of Mainz (Mainz, Germany) between January 2011 and December 2014. In total, 25 patients undergoing cardiac surgery were included in the present study. The patients were divided into two groups, as follows: The postcapillary PH group (PH group), consisting of 15 patients, and the non-postcapillary PH group (non-PH group), consisting of 10 patients. The patients were classified according to the New York Heart Association (NYHA) classification system (12). Patient demographics and characteristics are presented in Table I.
Table I.
Data presented as mean ± standard deviation, or as % (n) unless otherwise indicated.
| Variable | PH group (n=15) | Non-PH group (n=10) | P-value |
|---|---|---|---|
| Age, years | 70.70±7.20 | 55.70±11.80 | 0.008 |
| Female, n (%) | 9 (60) | 4 (6) | 0.900 |
| Height, cm | 168.00±9.60 | 171.00±13.00 | 0.900 |
| Weight, kg | 68.00±12.00 | 76.00±17.00 | 0.700 |
| Body surface area, m2 | 1.78±0.90 | 1.90±0.70 | 0.900 |
| Body-mass index | 23.00±3.80 | 25.40±3.80 | 0.800 |
| COPD, n (%) | 0 (0) | 0 (0) | |
| Diabetes mellitus, n (%) | 3 (20) | 0 (0) | 0.050 |
| Creatinine (mg/dl) | 1.01±0.80 | 0.67±0.40 | 0.700 |
| Chronic atrial fibrillation, n (%) | 8 (53) | 2 (20) | 0.020 |
| PAVD, n (%) | 1 (6.6) | 0 (0) | 0.800 |
| Stroke incident, n (%) | 0 (0) | 0 (0) | |
| Arterial hypertension, n (%) | 8 (53) | 5 (50) | 0.900 |
| NYHA class III–IV, n (%) | 5 (38) | 0 (0) | 0.030 |
| LVEF, % | 47.00±0.14 | 60.00±0.50 | 0.007 |
PH, pulmonary hypertension; COPD, chronic obstructive pulmonary disease, PAVD, peripheral arterial venous disease; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction.
Inclusion and exclusion criteria
Postcapillary PH was defined by a mean pulmonary artery pressure (mPAP) of ≥25 mmHg (iE33 xMATRIX Echocardiography System; Philips Healthcare, Amsterdam, The Netherlands) in the presence of left-sided valvular heart disease and the absence of any primary pulmonary artery hypertension (PAH), according to previously reported guidelines (13). Preserved RV function was defined when the tricuspid annular plane systolic excursion (TAPSE) was ≥16 mm (14).
Patients with precapillary arterial hypertension, idiopathic, heritable forms of PAH, PAH associated with infectious disease, connective tissue diseases, congenital diseases, pulmonary veno-occlusive diseases, other pulmonary diseases and chronic thromboembolic PH were excluded from the current study. Furthermore, patients presenting as emergency cases, patients requiring cardiac procedures with the exception of aortic and mitral valve surgery, endocarditis cases, and patients with clinical and echocardiographic signs of chronic, acute right or acute left heart failure were also excluded.
Clinical setting
Hemodynamic data from all patients were obtained preoperatively by means of standard right heart catheterization, as described previously (1). The mean RA pressure (mRAP), mPAP and pulmonary artery wedge pressure (PAWP) were recorded (iE33 xMATRIX echocardiography system).
Preoperative echocardiography was performed in all patients (iE33 xMATRIX echocardiography system). Standard echocardiographic measurements were performed in expiration. The following parameters were measured: The LV ejection fraction (LVEF) was determined according to the biplane Simpson's method (14). In addition, dilatation of the right and left atria and ventricles was assessed by transthoracic echocardiography (2D and M-mode; iE33 xMATRIX echocardiography system). LV dilatation was defined when the LV end-diastolic diameter (LVEDD) was >55 mm and RV dilation was determined when the RV end-diastolic diameter was >30 mm. Similarly, RA dilatation was defined when the minor RA axis was >4.4 cm and the major axis was >5.3 cm, whereas left atrial dilatation was defined when the end-systolic diameter was >40 mm (M-mode).
TAPSE was measured with two-dimensional M-mode echocardiography. However, the RV diastolic function in presence of tricuspid regurgitation (TR) and atrial fibrillation was not assessed. Echocardiographic views, measurements, as well as calculations, were performed according to recent guidelines (14,15).
Skinned fiber preparation
Skinned fiber preparation was performed as previously described (16,17). Briefly, the fibers were collected during surgery following resection of the right auricle. Using a non-touch-technique, the auricle was transferred in an ice-cooled vial (4°C) containing a modified cardioplegic solution (Krebs-Henseleit solution, which included: 118.07 mmol/l NaCl, 11.1 mmol/l C6H12O6.H2O, 4.7 mmol/l KCl, 25 mmol/l NaHCO3, 1.2 mmol/l KH2PO4, 1.2 mmol/l MgSO4.7H2O, 1.8 mmol/l CaCl2.2H2O) and 30 mmol/l 2,3-butanedione monoxime (C4H7NO2) as an ATP-sensitive potassium channel inhibitor. For examination, the tissue was transferred to a dish containing an ice-cooled (4°C) preparation solution, with the following contents: 68.08 mM C3H4N2, 65.01 mM NaN3, 380.4 mM C14H24N2O10, 154.3 mM C4H10O2S2, 203.3 mM MgCl2.6H2O, and 605.2 mM C10H14N5O13P3Na2. The muscle bundles were then resected out of the auricle and transferred to a test tube containing the modified preparation solution with 1% Triton X-100 in order to permeabilize the membrane of the fibers, with incubation for 24 h at 4°C on a shaking device. The purpose of these preparatory steps was to remove all membrane-dependent properties (also known as ‘skinning’). Following this skinning process, the RA muscle bundles were transferred to a separate ice-cooled (4°C) dish containing preparation solution, in order to prepare single muscle stripes (size, 2–2,5×0,3 mm) under the microscope (magnification, ×10; Leica DM1000; Leica Microsystems GmbH, Wetzlar, Germany).
Log10 calcium concentration (pCa) against force measurements in RA tissue
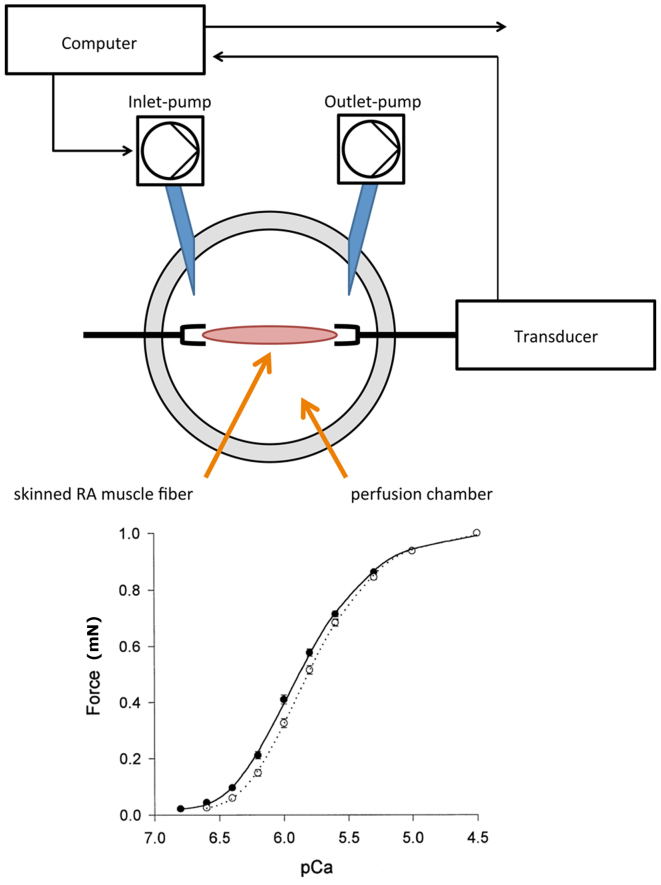
A muscle investigation system (Gradient Program; Scientific Instruments, Heidelberg, Germany) was used to expose the RA fibers to gradual increase of pCa for force measurements. The experimental set-up is depicted in Fig. 1. The RA fibers were fixed in the perfusion chamber and incubated with a relaxation solution, containing the following: 68.08 mM C3H4N2, 327.2 mM C4H8N3O5PNa2.4H2O, 65.01 mM NaN3, 380.4 mM C14H24N2O10, 203.3 mM MgCl2, 154.2 mM C4H10O2S2, 605.2 mM C10H14N5O13P3Na2, and 400 U/ml creatine kinase. By adding 147.02 mM CaCl2 to the relaxation solution, a pCa-force curve was created. pCa was shown as the negative log10 of the calcium concentration. A specifically designed software (Gradient Program; Scientific Instruments) was used to calculate the amount of calcium required to achieve a stepwise increase in pCa according to the equation described by Morano et al (16) and Fabiato and Fabiato (17). The pCa included concentrations between 6.5 and 4.0, in 0.5 increments. For each patient, a set of three RA fibers underwent calcium-induced force measurements. Thus, a total of 45 samples were evaluated in the PH group and 30 samples in the non-PH group.
Figure 1.
Experimental set-up of the ‘muscle investigation system’ used in the present study. The master pump transports a specific amount of calcium solution into the perfusion chamber, and the slave pump withdraws the same amount out of the perfusion chamber. Thus, the calcium concentration increases in the perfusion chamber and the pCa-force curve is recorded by the attached computer system. pCa, calcium concentration (given as the negative log10).
Statistical analysis
Statistical analysis was performed using SPSS software (version 23; IBM Corp., Armonk, NY, USA). Patient continuous demographics are presented as the mean ± standard deviation, while categorical data are presented as percentages. Normal distribution was tested with Shapiro-Wilk test. Continuous variables were statistically analyzed with Welch's t-test, and categorical data were compared with Wilcoxon signed-rank test and χ2 test. Two-sided P-values of <0.05 were considered to indicate statistically significant differences.
Results
Demographics
The demographic information and clinical data of the patients are depicted in Table I. When compared with the non-PH group, patients in the PH group had a significantly higher age (P=0.008) and higher prevalence of atrial fibrillation (P=0.02). In addition, a higher proportion of PH patients presented class III–IV disease (according to the NYHA classification; P=0.03) and a reduced LVEF (P=0.007).
Indication for left heart surgery differed between the two groups, with mitral valve regurgitation surgery required in significantly more PH group patients, as the compared with the non-PH group (100% vs. 30%, respectively; P 0.001).
Echocardiography
The hemodynamic and echocardiographic data of the patients are shown in Table II. In the PH group, mean PAWP, mPAP and mRAP were significantly higher, as compared with the values in the non-PH group (all P<0.001; Figs. 2–4), whereas the LVEF was significantly reduced (P=0.007; Table II) as an expression of post capillary PH with impaired LV function. Although LV dilatation was more frequently observed in the PH group (P=0.03; Table II), the LVEDD revealed no differences between the two groups (60±11 mm in the PH group vs. 48±14 mm in the non-PH group; P=0.8; Table II). Similarly, TAPSE did not differ significantly between the two groups (18±4.4 mm in the PH group vs. 21.5±2.2 mm in the non-PH group; P=0.8; Fig. 5), although the presence of RA dilation (80 vs. 20%; P 0.001) and RV dilatation (20 vs. 10%; P 0.02) was significantly increased in the PH group compared with the non-PH group (Table II). Furthermore, significantly more patients in the PH group had a TR ≥II°, while TR was more frequently observed in the PH group (86 vs. 40%; P=0.04), when compared with the non-PH group.
Table II.
Patient hemodynamic parameters.
| Variable | PH group (n=15) | Non-PH group (n=10) | P-value |
|---|---|---|---|
| LV ejection fraction, % | 47.00±0.14 | 60.00±0.05 | 0.007 |
| Mean LVEDD, mm | 60.00±11.00 | 48.00±14.00 | 0.800 |
| TAPSE, mm | 18.00±4.40 | 21.50±2.20 | 0.800 |
| Tricuspid regurgitation ≥II°, n (%) | 10 (67) | 0 (0) | 0.030 |
| Mitral valve regurgitation, n (%) | 15 (100) | 5 (50) | 0.030 |
| Mitral valve regurgitation ≥II°, n (%) | 15 (100) | 3 (30) | 0.001 |
| Aortic valve stenosis, n (%) | 6 (40) | 3 (30) | 0.700 |
| Aortic regurgitation, n (%) | 0 (0) | 2 (20) | 0.050 |
| mRAP, mmHg | 10.00±2.70 | 4±2.4 | <0.001 |
| mPAP, mmHg | 52.6±17 | 21.30±1.30 | <0.001 |
| Mean PAWP, mmHg | 23.00±1.00 | 8.26±3.00 | <0.001 |
| RA dilatation, n (%) | 12 (80) | 2 (20) | 0.001 |
| RV dilatation, n (%) | 3 (20) | 1 (10) | 0.020 |
| LA dilatation, n (%) | 11 (73) | 2 (20) | 0.040 |
| LV dilatation, n (%) | 7 (47) | 1 (10) | 0.030 |
Data are presented as the mean ± standard deviation or as n (%), unless otherwise indicated. PH, pulmonary hypertension; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; TAPSE, tricuspid annular plane systolic excursion; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; RA, right atrial; RV, right ventricular; LA, left atrial.
Figure 2.
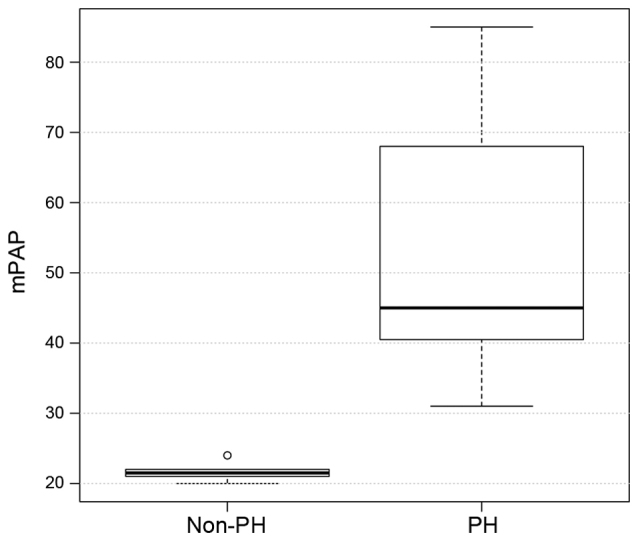
Distribution of the mPAP values in the PH and non-PH groups. PH, pulmonary hypertension; mPAP, mean pulmonary artery pressure.
Figure 4.
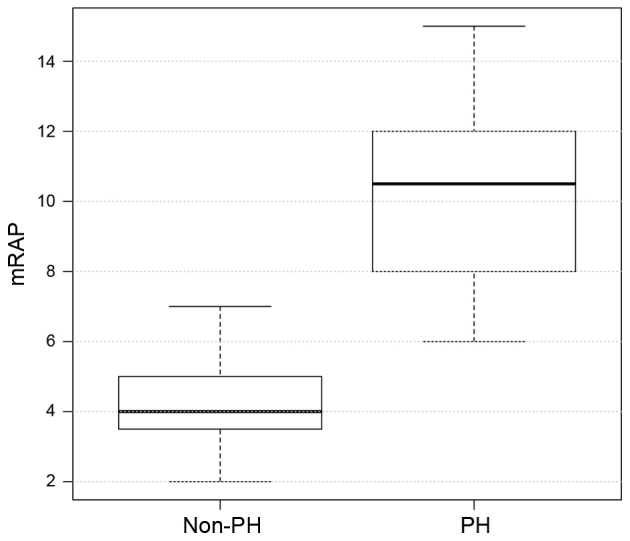
Distribution of the mRAP values in the PH and non-PH groups. PH, pulmonary hypertension; mRAP, mean right atrial pressure.
Figure 5.
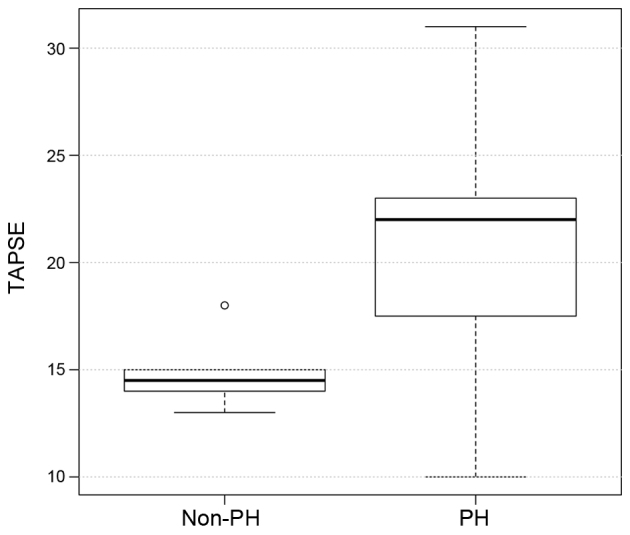
Distribution of the TAPSE values in the PH and non-PH groups. PH, pulmonary hypertension; TAPSE, tricuspid annular plane systolic excursion.
pCa-force measurements
The pCa-force values of the two groups are demonstrated in Table III. Higher force values were observed at the three highest concentrations of calcium, with force values in the non-PH group being significantly increased when compared with the PH group (at pCa 4.0: 4.1±0.5 vs. 2.9±0.3 mN, P=0.001; at pCa 4.5: 2.9±0.2 vs. 2.2±0.3 mN, P=0.01; at pCa 5.0: 2.2±0.3 vs. 1.7±0.2 mN, P=0.01). In addition, calcium sensitivity, which was defined as the pCa at half maximal force (shown as pCa2+50) was different among groups. The PH group achieved half maximal force at a pCa of 5.5, whereas the non-PH group reached half maximal force at a pCa of 5.0. These findings suggest that patients with PH develop half maximal force at a lower concentration of calcium, when compared with non-PH patients; therefore, the affinity to calcium is higher in the PH group.
Table III.
pCa force values (mean ± standard deviation).
| Variable | PH group (n=15) | Non-PH group (n=10) | P-value |
|---|---|---|---|
| pCa, mN | |||
| 4.0 | 2.90±0.30 | 4.1±0.50 | 0.001 |
| 4.5 | 2.20±0.30 | 2.9±0.20 | 0.010 |
| 5.0 | 1.70±0.20 | 2.2±0.30 | 0.010 |
| 5.5 | 1.40±0.04 | 1.2±0.03 | 0.800 |
| 6.0 | 1.00±0.04 | 0.8±0.03 | 0.900 |
| 6.5 | 0.60±0.05 | 0.4±0.03 | 0.800 |
| pCa2+50 | 5.5 | 5.0 | 0.010 |
PH, pulmonary hypertension; pCa2+50, calcium concentration at which half maximal activation was achieved; pCa, calcium concentration (given as the negative log10).
Discussion
The present study investigated an RA skinned fiber model in patients with postcapillary PH due to valvular LV dysfunction, and the results showed significantly reduced contractile forces when compared with those in patients without PH. These data may be interpreted as signs of impaired RA compensatory mechanism in postcapillary PH. Notably, patients in the PH group had absence of clinical and echocardiographic signs of overt RV impairment, as indicated by the TAPSE values. According to previous studies (18–20), TAPSE is a reliable echocardiographic parameter for the assessment of RV function in the presence of postcapillary PH. TAPSE has been recently reported to be equal or even superior to other RV-echo-Doppler indices (18,19). Guazzi et al (21) showed that TAPSE and systolic PAP reflect the contractile state of the RV in clinical settings; in this echocardiographic study about RV contractile function, TAPSE and systolic PAP used as in vivo indices for RV length and developed force were found to better reflect the contractile state of RV. However, it was not possible to exclude latent RV dysfunction with the methods used in the present study, since TAPSE was at the lower limit of the established values, RA and RV dilatation was present, and a higher incidence of TR was detected. Furthermore, diastolic dysfunction was not evaluated in the present study population due to the high incidence of atrial fibrillation and TR, factors that make determination of RV diastolic impairment difficult. Thus, based on the observations, RV dysfunction can be suspected.
In precapillary PH, impaired RA contractility is associated with signs of right heart failure (10). Since no clinical signs of right heart failure were observed in the present study, but signs of chronic LV dysfunction were detected, it was hypothesized that the patients had chronic LV dysfunction involving the right ventricle at the level of the contractile apparatus albeit with the RV function clinically preserved. Due to the interventricular interaction between LV and RV function, the RV function can be impaired in patients with LV dysfunction. This physiological interaction has to be considered, since up to one third of right-sided stroke occurrence is due to LV septal contraction (22). This interaction has been proven in the clinical setting of chronic volume overload in mitral valve regurgitation. For instance, Le Tourneau et al (23) showed that, in patients with impaired LV systolic function, the RV function is mainly dependent upon LV remodeling and septal function, but only weakly dependent on pulmonary systolic pressure. In precapillary PH, a compensatory mechanism for RV functional impairment involves increased RA contractility, which maintains RV filling over a long period of time (6,9). Considering the importance of interventricular interaction and RA function for RV performance, dysfunction of both components may be detrimental in PH patients. Reduced absolute force values in combination with increased calcium sensitivity in the PH group indicate that RA compensatory mechanisms are limited in postcapillary PH due to chronic LV dysfunction, which may lead to an accelerated development of RV failure. Only limited clinical data are available on the RA compensatory mechanism for RV-functional adaptation in precapillary and postcapillary PH (5,22,24).
The results of animal studies investigating the effect of precapillary hypertension on the RA compensatory mechanism are inconsistent (9,25). For instance, Gaynor et al (6) demonstrated the increase of RA contractility and distensibility as a result of increased RV strain; however, methodological limitations exist, since these data were calculated and not measured. By contrast, studies in rodent models demonstrated that induced PH resulted in marked reduction of Ca2+-activated force and in reduced maximal Ca2+-dependent force values (9,25). To the best of our knowledge, no animal studies showing the effect of postcapillary hypertension on the RA compensatory mechanism are available. Due to the inconsistent results from animal studies, it can only be speculated whether these data are applicable for the clinical setting of chronic precapillary or postcapillary PH. The majority of clinical studies investigated patients subsequent to heart lung transplantation for severe precapillary PH, and did not focus on cellular RA compensatory mechanisms (2,5). Rain et al (5,26) demonstrated increased stiffness of cardiomyocytes and cardiomyocyte sarcomeres as a result of RV diastolic dysfunction, and increased calcium sensitivity as a compensatory mechanism in RV-myocardial specimens in patients with precapillary PH and impaired RV function. Upon investigation of the left ventricle, it was demonstrated that reduced force capacity can be compensated with increased affinity to calcium (27). This compensatory mechanism cannot utilize full force capacity, since increased calcium sensitivity leads to incomplete actin-myosin detachment; therefore, this mechanism compensates only in part for RV myocardial dysfunction (26).
To the best of our knowledge, this is the first study in humans to examine RA tissue exposed to chronic volume overload. However, the present study presented various limitations. Firstly, the number of patients examined in current study was limited. To overcome this drawback, a larger sample size is required to support the current results. In addition, RA tissue was examined, which may not be representative for RV contractile dynamics. However, Vannier et al (28) demonstrated that the myofibrils contractile force of atrial and ventricular fibers show the same contractile and properties, which may support the assumption that these results are representative for the right heart. Another limitation of the present study is that the groups differed concerning the indication for surgery. In the PH group, mitral valve regurgitation with chronic volume overload and eccentric hypertrophy was the main indication for surgery, whereas in the non-PH group, aortic stenosis with pressure overload and concentric hypertrophy was the predominant indication for surgery. Myocardial hypertrophy is a strong factor influencing maximal calcium activated force and calcium sensitivity in left heart failure in rodents (29), although it remains unclear whether this is also the case in humans. Differences in the manifestation of hypertrophy may have an impact on RA calcium activation, thus influencing the results of the present study. We hypothesize that, although RV systolic function was preserved (TAPSE, >16 mm), the higher prevalence of TR may be a sign of a latent RV impairment that is not yet clinically apparent. This hypothesis requires further investigation with magnet resonance imaging (30).
In conclusion, the present preliminary results in patients with postcapillary PH demonstrated reduced RA contractile forces and increased calcium sensitivity. The present clinical study is the first to show evidence that the RA compensatory mechanism is already impaired at a point in time when no clinical and overt echocardiographic signs of RV dysfunction are present in postcapillary PH. This may have a clinical impact on the timing of surgical intervention, since occurrence of RV dysfunction in left heart disease is a powerful predictor of cardiovascular and overall survival (23,31). The current results may support the policy of early surgical or interventional treatment in patients presenting with left heart valvular pathology. Nevertheless, it has to be considered that these results are only preliminary, and further clinical studies with larger patient cohorts are mandatory to determine the clinical importance of these findings.
Figure 3.
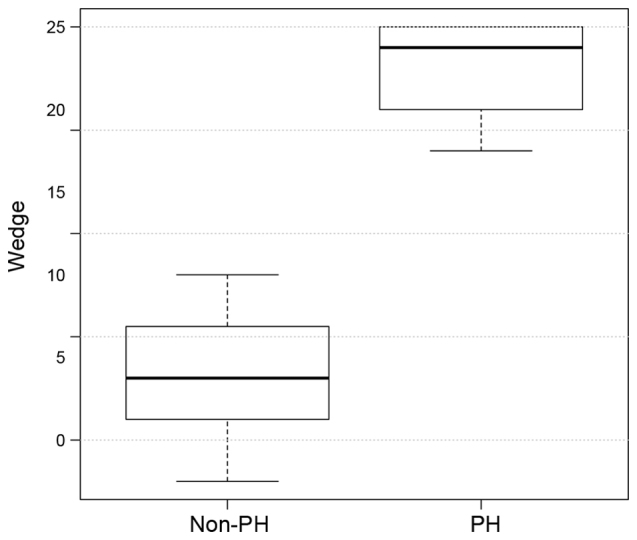
Distribution of the PAWP values in the PH and non-PH groups. PH, pulmonary hypertension; PAWP, pulmonary artery wedge pressure.
Acknowledgements
The authors would like to thank Mr. Alexander Graf at the Department of Cardiothoracic and Vascular Surgery of the University of Mainz for statistical support.
References
- 1.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 2.Trammell AW, Pugh ME, Newman JH, Hemnes AR, Robbins IM. Use of pulmonary arterial hypertension-approved therapy in the treatment of non-group 1 pulmonary hypertension at US referral centers. Pulm Circ. 2015;5:356–363. doi: 10.1086/681264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: The role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–1723. doi: 10.1161/01.CIR.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 4.Murch SD, La Gerche A, Roberts TJ, Prior DL, MacIsaac AI, Burns AT. Abnormal right ventricular relaxation in pulmonary hypertension. Pulm Circ. 2015;5:370–375. doi: 10.1086/681268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmüller P, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128:2016–2025. doi: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]
- 6.Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: Impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol. 2005;288:H2140–H2145. doi: 10.1152/ajpheart.00566.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation. 2005;112(Suppl 9):S1212–S1218. doi: 10.1161/CIRCULATIONAHA.104.517789. [DOI] [PubMed] [Google Scholar]
- 8.Leeuwenburgh BP, Helbing WA, Steendijk P, Schoof PH, Baan J. Biventricular systolic function in young lambs subject to chronic systemic right ventricular pressure overload. Am J Physiol Heart Circ Physiol. 2001;281:H2697–H2704. doi: 10.1152/ajpheart.2001.281.6.H2697. [DOI] [PubMed] [Google Scholar]
- 9.Fan D, Wannenburg T, de Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation. 1997;95:2312–2317. doi: 10.1161/01.CIR.95.9.2312. [DOI] [PubMed] [Google Scholar]
- 10.Shiina Y, Funabashi N, Lee K, Daimon M, Sekine T, Kawakubo M, Takahashi M, Yajima R, Tanabe N, Kuriyama T, Komuro I. Right atrium contractility and right ventricular diastolic function assessed by pulsed tissue Doppler imaging can predict brain natriuretic peptide in adults with acquired pulmonary hypertension. Int J Cardiol. 2009;135:53–59. doi: 10.1016/j.ijcard.2008.03.090. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association: World Medical Association Declaration of Helsinki: Ethical Principles for Medial Research Involving Human Subjects. JAMA. 2000;284:3043–3045. doi: 10.1001/jama.284.23.3043. [DOI] [PubMed] [Google Scholar]
- 12.Kossman CE. The Criteria of the New York Heart Association: Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th. Little, Brown and Co.; Boston: 1964. pp. 110–114. [Google Scholar]
- 13.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl 25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Morano I, Arndt H, Gärtner C, Rüegg JC. Skinned fibers of human atrium and ventricle: Myosin isoenzymes and contractility. Circ Res. 1988;62:632–639. doi: 10.1161/01.RES.62.3.632. [DOI] [PubMed] [Google Scholar]
- 17.Fabiato A, Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972;31:293–307. doi: 10.1161/01.RES.31.3.293. [DOI] [PubMed] [Google Scholar]
- 18.Zakaria D, Sachdeva R, Gossett JM, Tang X, O'Connor MJ. Tricuspid annular plane systolic excursion is reduced in infants with pulmonary hypertension. Echocardiography. 2015;32:834–838. doi: 10.1111/echo.12797. [DOI] [PubMed] [Google Scholar]
- 19.Bano M, Kanaan UB, Ehrlich AC, McCracken C, Morrow G, Oster ME, Sachdeva R. Improvement in tricuspid annular plane systolic excursion with pulmonary hypertension therapy in pediatric patients. Echocardiography. 2015;32:1228–1232. doi: 10.1111/echo.12835. [DOI] [PubMed] [Google Scholar]
- 20.Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Randé JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 22.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: Noninvasive monitoring using MRI. Chest. 2001;119:1761–1765. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 23.Le Tourneau T, Deswarte G, Lamblin N, Foucher-Hossin C, Fayad G, Richardson M, Polge AS, Vannesson C, Topilsky Y, Juthier F, et al. Right ventricular systolic function in organic mitral regurgitation: Impact of biventricular impairment. Circulation. 2013;127:1597–1608. doi: 10.1161/CIRCULATIONAHA.112.000999. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari R, Böhm M, Cleland JG, Paulus WJ, Pieske B, Rapezzi C, Tavazzi L. Heart failure with preserved ejection fraction: Uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665–671. doi: 10.1002/ejhf.304. [DOI] [PubMed] [Google Scholar]
- 25.Pérez NG, Hashimoto K, McCune S, Altschuld RA, Marbán E. Origin of contractile dysfunction in heart failure: Calcium cycling versus myofilaments. Circulation. 1999;99:1077–1083. doi: 10.1161/01.CIR.99.8.1077. [DOI] [PubMed] [Google Scholar]
- 26.Rain S, Bos Dda S, Handoko ML, Westerhof N, Stienen G, Ottenheijm C, Goebel M, Dorfmüller P, Guignabert C, Humbert M, et al. Protein changes contributing to right ventricular cardiomyocyte diastolic dysfunction in pulmonary arterial hypertension. J Am Heart Assoc. 2014;3:e000716. doi: 10.1161/JAHA.113.000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wankerl M, Böhm M, Morani I, Rüegg JC, Eichhorn M, Erdmann E. Calcium sensitivity and myosin light chain pattern of atrial and ventricular skinned cardiac fibers from patients with various kinds of cardiac disease. J Moll Cell Cardiol. 1990;22:1425–1438. doi: 10.1016/0022-2828(90)90986-C. [DOI] [PubMed] [Google Scholar]
- 28.Vannier C, Veksler V, Mekhfi H, Mateo P, Ventura-Clapier R. Functional tissue and development specifities of myofibrils and mitochondria in cardiac muscle. Can J Physiol Pharmacol. 1996;74:23–31. doi: 10.1139/y95-223. [DOI] [PubMed] [Google Scholar]
- 29.Perreault CL, Bin OH, Brooks WW, Ransil BJ, Morgan JP. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ Res. 1990;67:707–712. doi: 10.1161/01.RES.67.3.707. [DOI] [PubMed] [Google Scholar]
- 30.Naito H, Arisawa J, Harada K, Yamagami H, Kozuka T, Tamura S. Assessment of right ventricular regional contraction and comparison with the left ventricle in normal humans: A cine magnetic resonance study with presaturation myocardial tagging. Br Heart J. 1995;74:186–191. doi: 10.1136/hrt.74.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]