Abstract
Objective
We sought to determine whether fibrochondrocytes from menisci express receptor activator of NF-κB (RANK), its ligand (RANKL), or osteoprotegerin (OPG) and, if so, whether their expression is modulated by dynamic mechanical loading under inflammatory and normal conditions.
Methods
Fibrochondrocytes from rat menisci were subjected to cyclic tensile strain (CTS) at various magnitudes and frequencies in the presence or absence of interleukin (IL)-1β for up to 24 h. In order to determine whether a possible regulatory effect of mechanical loading on RANKL and its receptors under inflamed conditions is sustained, cells were stimulated with IL-1β for 24 h while being subjected to CTS only for the initial 4 and 8 h, respectively. Regulation of RANKL, RANK, and OPG expression and synthesis were determined by semiquantitative and real-time PCR, Western blotting, and immunofluorescence.
Result
Fibrochondrocytes constitutively expressed low levels of RANKL and RANK but marked levels of OPG. IL-1β upregulated expression and synthesis of RANKL and RANK significantly (p<0:05), whereas expression of OPG was unaffected following 4 and 24 h. When fibrochondrocytes were simultaneously subjected to CTS and IL-1β, expression of RANKL and RANK was significantly (p<0:05) downregulated as compared to that of IL-1β-stimulated unstretched cells. The inhibitory effect of CTS on the IL-1β-induced upregulation of RANKL and RANK was sustained as well as magnitude and frequency dependent.
Conclusions
Our study provides evidence that RANKL and its receptors are expressed in fibrochondrocytes from meniscus. These data also demonstrate that dynamic mechanical loading can modify the expression of RANKL and RANK in inflammatory conditions.
Keywords: Mechanical strain, Fibrocartilage, RANKL, RANK, OPG
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is characterized by destruction and deformity of synovial joints. The articular lesion starts with inflamed synovium, focal erosion of cartilage, and proceeds to destruction of subarticular bone (Gravallese and Goldring, 2000). Matrix metalloproteinases that are released from inflammatory but also resident tissue cells mediate matrix degradation in cartilage and bone (Martel-Pelletier et al., 2001).
Unlike cartilage degradation, resorption of subchondral bone also requires sufficient osteoclastic activity. In experimentally induced arthritis and human RA, it has been demonstrated that receptor activator of nuclear factor (NF-κB) ligand (RANKL), its cellular receptor, receptor activator of NF-κB (RANK), and the decoy receptor osteoprotegerin (OPG) are key regulators in arthritis-associated bone loss (Jones et al., 2002; Kong et al., 1999; Romas et al., 2000).
RANKL, when bound to RANK, enhances bone resorption by promoting differentiation and fusion of osteoclast precursors as well as activation and survival of mature osteoclasts (Walsh and Choi, 2003). The effects of RANKL are counteracted by OPG that acts as a soluble neutralizing receptor. When rats with adjuvant arthritis were treated with OPG, bone loss was efficiently prevented. Interestingly, OPG application also reduced loss of cartilage matrix proteoglycans, most likely as an indirect consequence of protecting the subchondral bone (Campagnuolo et al., 2002). Furthermore, in RANKL knockout mice, the degree of bone erosion in arthritis was dramatically reduced compared with that seen in arthritic control mice. Although cartilage damage was present in both the arthritic RANKL knockout mice and in arthritic control littermates, there was a trend toward milder cartilage damage in the RANKL knockout mice (Gravallese, 2002). Therefore, it is obvious that the RANKL/RANK/OPG system regulates bone and at least indirectly cartilage loss in arthritis.
Interestingly, there are a few reports demonstrating expression of RANKL and its receptors in articular and costal cartilage (Hui et al., 2005; Komuro et al., 2001; Takamoto et al., 2003). Meniscal fibrocartilage is another tissue strongly affected by arthritis. Meniscal cells are capable of synthesizing proinflammatory cytokines and catabolic enzymes, which underlines their ability to participate in arthritis-associated joint destruction (Bluteau et al., 2001; Upton et al., 2003). However, if RANKL and its receptors are expressed in meniscus has yet to be investigated. Meniscal fibrochondrocytes are subjected to dynamic mechanical loading. Recently, it has been demonstrated that mechanical strain applied to murine marrow cultures reduced the expression of 1,25(OH)2D3-stimulated RANKL (Rubin et al., 2000). The data suggest that mechanical load can prevent osteoclast recruitment locally by modulating the level of the paracrine RANKL necessary for osteoclast differentiation. We sought to determine whether fibrochondrocytes from menisci express RANKL and its receptors, and if so, whether their expression is modulated by dynamic mechanical loading under inflammatory and normal conditions.
2. Material and methods
2.1. Cell culture
Menisci were harvested from knees of 10–12-week-old Sprague–Dawley rats (Harlan, Indianapolis, IN). After cleaning, the menisci were minced and transferred onto macroporous filters (Spectra/Mesh, Spectrum LA, CA) placed in a digestion chamber. Following incubation with 0.2% trypsin for 10 min and 0.2% collagenase I (Worthington® 10 gm Lakewood, NJ) for 1 h, the meniscal cells were centrifuged and the pellet was resuspended in DMEM/F12 (Cellgro® by Mediatech, Herndon, VA) supplemented with 10% FBS (Hyclone®, Logan, UT), 1% penicillin/streptomycin (Cellgro® by Mediatech, Herndon, VA), and 1% L-glutamine (Gibco by Invitrogen, Grand Island, NY). Cells were seeded into a T-25 vented flask (Becton-Dickinson®, Franklin Lakes, NJ), grown to 80–90% confluence, and used between 3rd and 5th passage. During these passages, the cell morphology and the mRNA expression for phenotypic markers (aggrecan, biglycan, versican as well as collagen type I) were unchanged.
2.2. Biomechanical loading
Fibrochondrocytes between 3rd and 5th passage (5 × 105 per well) were grown on collagen I-coated BioFlex® 6-well culture plates (Flexcell® International Corp., Hillsborough, NC) to 80% confluence (7–8 days) in 5% CO2 and at 37 1C. The FBS concentration was decreased to 1% one day prior to the experiments.
Cells grown on BioFlex plates were subjected to cyclic tensile strain (CTS) at 20% and 50 mHz in the presence or absence of rhIL-1β (1 ng/ml; Calbiochem, CA) with a FX-4000T™ Flexercell® System (Flexcell International Corp., Hillsborough, NC) for 4 and 24 h. To study if the effects of CTS are magnitude dependent, CTS at various magnitudes (5%, 10%, 15%, and 20%) and 50 mHz was applied to the cells in the presence or absence of rhIL-1β for 4 and 24 h. To analyze the frequency dependence, cells were subjected to CTS at 15% and various frequencies (4, 25, 50, 100, and 250 mHz) in the presence or absence of rhIL-1β for 4 h. In order to determine whether a possible regulatory effect of mechanical loading on RANKL and its receptors under inflamed conditions is sustained, cells were stimulated with IL-1β for 24 h while being subjected to dynamic tensile strain only for the initial 4 and 8 h, respectively. Unstretched cells in the presence or absence of IL-1β were used as controls. Cells of the meniscus experience a wide range of compressive, tensile, and shear stresses and strains. The magnitudes used in our study correspond to levels predicted by finite element analyses and are similar or identical to those used in studies on meniscal cells by other investigators (Fermor et al., 2004; LeRoux et al., 2001; Schreppers et al., 1990; Spilker et al., 1992; Zhang et al., 1999). No differences in the number of attached cells and cell viability after stretching with different regimens (magnitudes and frequencies) were observed.
2.3. Semiquantitative RT-PCR
The mRNA expression for RANKL, RANK, OPG, and GAPDH was analyzed by semiquantitative RT-PCR during its linear phase. RNA was extracted according to the manufacturer’s recommended protocols by an RNA extraction kit (Qiagen Inc., Valencia, CA). A total of 1.0 μg of RNA was reverse transcribed with 200 U of M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) at 42 1C for 25 min followed by 65 1C for 5 min. The cDNA was amplified with 0.1 °g of specific primers in a reaction mixture (PCR supermix, Invitrogen, Carlsbad, CA) containing Taq DNA polymerase, Tris-HCl, KCl, MgCl2, and dNTPs. Amplification was carried out for 30 cycles of 45 s at 94 °C, 45 s at 59 °C, and 60 s at 72 °C by Mastercycler Gradient (Eppendorf, Hamburg, Germany). The sequence of sense and anti-sense rat primers was as follows: GAPDH (323 bp): sense 5′-AGACAGCCGCATCTTCTTGT-3′ and anti-sense 5′-TACTCAGCACCAGCATCACC-3′ (Accession number: X02231); RANKL (224 bp): sense 5′-TCGGG-TTCCCATAAAGTCAG-3′ and anti-sense 5′-CTTGG-GATTTTGATGCTGGT-3′ (AF187319); RANK (365 bp): sense 5′-CGAGAAGCTGTCCACATTGA-3′ and anti-sense 5′-GACAAGCTCCGTTTTTCAGC-3′ (XM_222503); OPG (318 bp): sense 5′-CACTGCACAGTCAGGAGGAA-3′ and anti-sense 5′-TGCTTTCGATGACGTCTCAC-3′ (U94330).
2.4. Real-time PCR
Real-time PCR was performed in a Biorad iCycler iQ (Biorad, Hercules, CA) using SYBR Green Supermix (Biorad Hercules, CA), according to the manufacturer’s protocol. 2 μl of cDNA as a template was amplified with SYBR Green Supermix (Biorad Hercules, CA) in a 25 μl reaction containing 1x SYBR Green Supermix, 0.3 μM of each primer, and de-ionized water. The mixture was heated initially at 95 °C for 3 min and then followed by 40 cycles with denaturation at 95 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 30 s. Each amplification was done in triplicate. GAPDH was used as a house-keeping gene. Following amplification, melt curve protocols were performed to ensure that primer dimers or non-specific products had been eliminated or minimized. The sequence of sense and anti-sense rat primers used for real-time PCR was as follows: GAPDH (81 bp): sense 5′-CTCAACTACATGGTC-TACATGTTCCA-3′ and anti-sense 5′-CTTCCCATT-CTCAGCCTTGACT-3′; RANKL (85 bp): sense 5′-CGTGCAAAGGGAATTACAACAC-3′ and anti-sense 5′-CACATCGAGCCACGAACCT-3′; RANK (96 bp): sense 5′-GTCTGAGAATTGCCCAGTTAA-TATCC-3′ and anti-sense 5′-CCTGGGTTATGGA-CAGGAATCA-3′. To quantify the results obtained by real-time PCR, the comparative threshold method was used (Giulietti et al., 2001).
2.5. Western blot analysis
Synthesis of RANKL and RANK proteins was determined by Western blot analysis. RANKL and RANK were analyzed from cytoplasmic extracts of cells subjected to the regimens described above, and resolved on SDS-10%-PAGE under reducing conditions. After electrophoresis, the proteins were electrotransferred to Immun-Blot™ PVDF membranes (Bio-Rad Laboratories, Hercules, CA), blocked with 5% non-fat milk, probed with a goat anti-RANKL polyclonal antibody (Santa Cruz Biotechnology, CA) and a rabbit anti-RANK polyclonal antibody (Santa Cruz Biotechnology, CA). HRP-conjugated donkey anti-goat IgG and HRP-conjugated goat anti-rabbit IgG were applied for detection (Chemicon International, CA). Lightening chemiluminescence reagent (Perkin-Elmer Life Sciences, MA) was used as substrate for HRP. The bands were semiquantitatively assessed by densitometric analysis using the Kodak Image Station 1000 (Rochester, NY) and the KODAK 1D Image Analysis software.
2.6. Immunofluorescence
RANKL and RANK protein synthesis was also analyzed by immunofluorescence. Cells attached to the fiexible bottom of the BioFlex® 6-well culture plates were fixed with cold (−20 1C) 100% methanol for 5 min, washed with PBS, and blocked with protein blocking agent (Thermo Electron Corporation, Pittsburgh, PA) and normal serum (Santa Cruz Biotechnology, CA). A goat anti-RANKL polyclonal antibody (Santa Cruz Biotechnology, CA) in combination with FITC-conjugated donkey anti-goat IgG (Jackson Immuno Research, West Grove, PA) and a rabbit anti-RANK polyclonal antibody (Santa Cruz Biotechnology, CA) in combination with CY3-conjugated goat anti-rabbit IgG (Jackson Immuno Research, West Grove, PA) were applied.
For nuclear staining, 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO) was used. Cells were observed under 20 × or 40 × objectives, with an Axioplan 2 imaging microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY). The images were captured with an AxioCam HR camera and Axiovision 4.1 capturing software (both from Carl Zeiss MicroImaging Inc., Thornwood, NY).
2.7. Statistical analysis
The SPSS 13.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Each experiment was performed at least three times. For quantitative analysis, means+S.E.M. were calculated. To determine whether significant differences exist between groups, one-way ANOVA and the post-hoc multiple comparison Tukey test were applied (n = 6). To identify differences between IL-1β-treated cells in the absence or presence of CTS at various magnitudes and frequencies, one-way ANOVA and the post-hoc multiple comparison Dunnett test were used (n = 6). Differences were regarded as statistically significant at values of p<0:05.
3. Results
3.1. Fibrochondrocytes express RANKL and its receptors
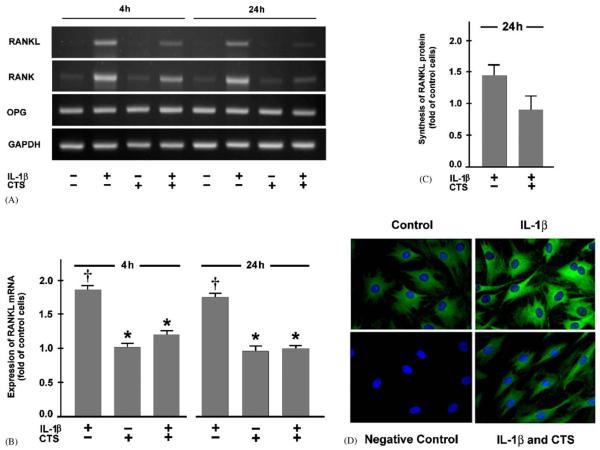
Fibrochondrocytes constitutively expressed low levels of mRNA for RANKL and RANK but higher levels for OPG. IL-1β used to mimic an inflammatory cell environment, significantly (po0:05) upregulated the expression of both RANKL and RANK but not OPG at 4 and 24 h. When fibrochondrocytes were simultaneously subjected to CTS and IL-1β treatment, the expression of RANKL and RANK mRNA was significantly (p<0:05) downregulated as compared to that of IL-1β-stimulated unstretched cells. The inhibitory effect of CTS on the upregulation of RANKL and RANK by IL-1β was evident at 4 h and even more pronounced at 24 h. Interestingly, CTS had no effect on the spontaneous mRNA expression of RANKL and its receptors (Fig. 1A and B). The regulatory effects of IL-1β and CTS on RANKL and RANK were also observed at protein level, as evidenced by Western blot analysis and immunofluorescence microscopy (Fig. 1C and D).
Fig. 1.
Fibrochondrocytes from meniscus were subjected to cyclic tensile strain (CTS) at a magnitude of 20% and 0.05 Hz in the presence and absence of interleukin (IL)-1β (1 ng/ml). (A) mRNA expression for RANKL, RANK, and OPG at 4 and 24 h, as determined by semiquantitative RT-PCR. Representative gels from one of three experiments are presented. (B) Quantitative assessment of RANKL mRNA at 4 and 24 h, as determined by real-time PCR. Results are shown as means±S.E.M., n = 6, † significantly (p<0:05) different from control cells and stretched cells in the presence or absence of IL-1β. * significantly (p<0:05) different from unstretched IL-1β-treated cells. (C) Protein synthesis of RANKL at 24 h, as analyzed by Western blot and subsequent semiquantitative densitometry. Results are shown as means±S.E.M., n = 3. (D) Protein synthesis of RANK at 24 h, as analyzed by immunofluorescence. Representative pictures from one of three experiments are presented.
3.2. Regulation of RANKL and RANK by CTS is magnitude and frequency dependent
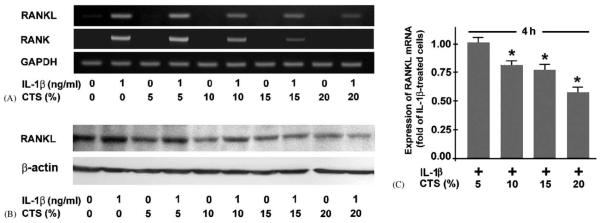
Fibrochondrocytes treated with IL-1β were simultaneously subjected to dynamic strain at various magnitudes. The inhibitory effect of CTS on the IL-1β-induced upregulation of RANKL and RANK mRNA expression was magnitude dependent. CTS at a magnitude of 5% had no effect, whereas CTS at 10–20% significantly (p<0:05) inhibited the IL-1β-induced mRNA expression for RANKL and RANK, with CTS at 20% being most effective (Fig. 2A and B). The magnitude-dependent effect of CTS on IL-1β-stimulated RANKL was also found at protein level (Fig. 2C). When IL-1β-stimulated cells were subjected to CTS at a suboptimal magnitude (15%), it was apparent that the inhibitory effect of CTS was also frequency dependent. CTS at 250 mHz did not change the mRNA expression for RANKL and RANK in IL-1β-treated cells. In contrast, CTS at 4, 25, 50, and 100 mHz significantly (p<0:05) suppressed the IL-1β-upregulated RANKL and RANK expression, with CTS at 25 mHz being most effective (Fig. 3A and B). For all magnitudes and frequencies used, the spontaneous expression of OPG was not affected by CTS (data not shown).
Fig. 2.
Fibrochondrocytes were subjected to CTS at various magnitudes (5%, 10%, 15%,and 20%) and 0.05 Hz in the presence and absence of interleukin (IL)-1β (1 ng/ml). (A) mRNA Expression for RANKL and RANK at 4 h, as determined by semiquantitative RT-PCR. Representative gels from one of three experiments are presented. (B) Quantitative assessment of RANKL mRNA at 4 h, as determined by real-time PCR. Results are shown as means±S.E.M., n = 6, * significantly (p<0:05) different from unstretched IL-1β-treated cells. (C) Protein synthesis of RANKL at 24 h, as analyzed by Western blotting. Representative gels from one of two experiments are presented.
Fig. 3.
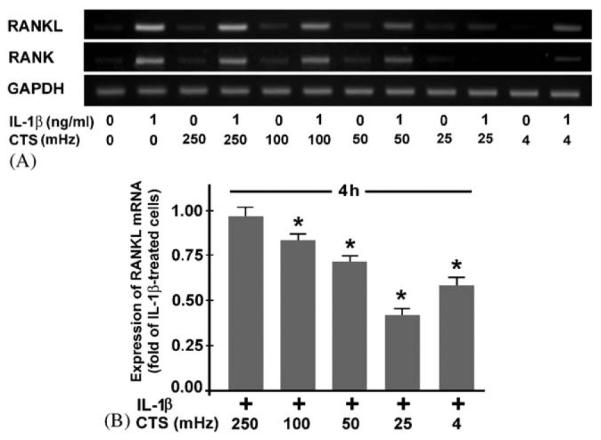
Fibrochondrocytes were subjected to CTS at various frequencies (4–250 mHz) and at a magnitude of 15% in the presence and absence of IL-1β (1 ng/ml). (A) mRNA expression for RANKL and RANK mRNA at 4 h, as determined by semiquantitative RT-PCR. Representative gels from one of three experiments are presented. (B) Quantitative assessment of RANKL mRNA at 4 h, as determined by real-time PCR. Results are shown as means+S.E.M., n = 6, * significantly (p<0:05) different from unstretched IL-1β-treated cells.
3.3. Inhibitory effect of CTS on RANKL and RANK is sustained
To determine whether the inhibitory effect of dynamic strain on the expression of RANKL and RANK in the presence of IL-1β is sustained, fibrochondrocytes were treated with IL-1β for 24 h while being subjected to mechanical strain only for the initial 4 and 8 h. As control, IL-1β-stimulated cells were also exposed to CTS for 24 h. Interestingly, application of CTS for only 4 and 8 h was sufficient to observe a significant (p<0:05) downregulation of the IL-1β-stimulated RANKL and RANK mRNA expression at 24 h, with 8 h of CTS exposure being more effective than 4 h (Fig. 4A and B).
Fig. 4.
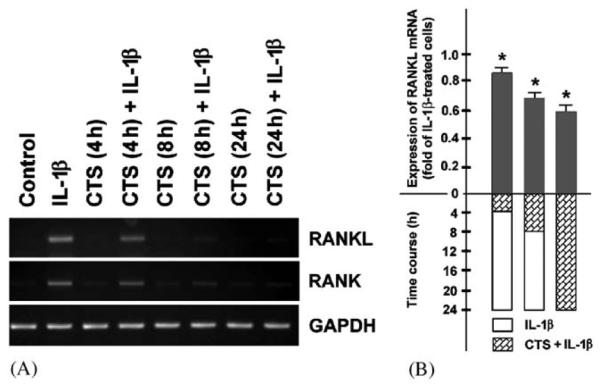
Fibrochondrocytes were stimulated with IL-1β for 24 h while being subjected to CTS (20%, 0.05 Hz) either only for the initial 4 and 8 h or for the entire 24-h-interval. (A) mRNA Expression for RANKL and RANK at 24 h, as determined by semiquantitative RT-PCR. Representative gels from one of three experiments are presented. (B) Quantitative assessment of RANKL mRNA at 24 h, as determined by real-time PCR. Results are shown as means±S.E.M., n = 6, * significantly (p<0:05) different from unstretched IL-1β-treated cells.
4. Discussion
Our study showed that RANKL and its receptors are expressed in fibrochondrocytes from meniscus. These data also demonstrate that dynamic mechanical loading can modulate the expression of RANKL and RANK in inflammatory conditions. Interestingly, OPG levels were not affected, whereas IL-1β-induced levels of RANKL and RANK were downregulated by CTS. This suggests that biomechanical forces increase the ratio of OPG to RANKL and, thereby, decrease the likelihood that RANKL will bind to RANK in an inflammatory environment. This may be another mechanism by which CTS acts as an anti-catabolic signal.
In our experiments, IL-1β was used to mimic an inflammatory cell environment because this cytokine is a key player in inflmmatory diseases and is increased in synovial fluid of arthritic joints (Kay and Calabrese, 2004). Fibrochondrocytes can participate in the inflammation-driven matrix degradation in arthritis by production and activation of MMPs (Bluteau et al., 2001).
Like intervertebral discs, meniscus consists of fibrocartilage and is of importance for load bearing, load distribution, and shock absorption (Benjamin and Ralphs, 2004). In intervertebral discs, static compression, abnormal hydrostatic pressure, and even immobilization have been shown to induce synthesis and activation of MMPs, indicating that mechanical loading modulates the expression of genes critically involved in joint homeostasis and remodeling (Handa et al., 1997; Hsieh and Lotz, 2003; MacLean et al., 2003). Therefore, it is not surprising that dynamic mechanical loading reduced the expression of RANKL and RANK that were upregulated in inflamed conditions. This finding might at least partly explain how exercise or motion-based therapies can protect against arthritic joint destruction.
Our observation that biomechanical factors interact with inflammatory and catabolic signals in meniscus is in accordance with findings reported by other investigators. For example, tensile strain has been shown to be anabolic, as assessed by increased proteoglycan synthesis in meniscal cells. Interestingly, the mechanical stimulation of the proteoglycan synthesis was prevented by TNF-α (Fermor et al., 2004). Similarly, IL-1 inhibited the stimulatory effects induced by dynamic compression on the proteoglycan synthesis in meniscal explants (Shin et al., 2003). In another study, static compression decreased levels for collagens, while upregulating the mRNA expression for collagenase, suggesting that the homeostatic balance between collagen synthesis and degradation is altered by mechanical signals (Upton et al., 2003). Collectively, these studies support our findings that interactions between mechanical stress and catabolic cytokines exist and that mechanical signals modify gene expression for proteins that are important in controlling homeostasis in the joint.
The inhibiting effect of dynamic strain on the expression of RANKL and RANK was magnitude dependent, with higher magnitudes being more effective. The data suggest that a minimal loading is required for the maintenance of the joint structures. This is in accordance with studies in which immobilization has been shown to induce catabolic effects (Jortikka et al., 1997; Ochi et al., 1997). It has been reported that murine bone marrow cells stimulated with 1,25(OH)2D3 express RANKL (Rubin et al., 2000). Like in our study, dynamic strain abolished the induced upregulation of RANKL. Nevertheless, in contrast to our findings, tensile strain of much lower magnitude was sufficient to inhibit the RANKL expression in these murine stromal cells. The different cell sensitivity to biophysical strain can be due to the use of distinct species as cell sources (Sweigart et al., 2004).
In addition to magnitude, frequency also seems to be an important parameter of CTS with regard to the inhibition of RANKL and RANK under inflammatory conditions. This has to be considered when mechanical load is applied in terms of motion-based therapies.
Intriguingly, the inhibition of RANKL and RANK by CTS could be observed after 24 h even if CTS was only applied for the initial 4 h, suggesting a therapeutic potential of mechanical loading for a clinical setting. Surprisingly, RANKL and RANK were similarly affected by IL-1β and CTS. By which mechanisms CTS regulates the IL-1β-stimulated RANKL and RANK synthesis has yet to be elucidated. Several transcription factors, e.g. indian hedgehog, Runx-2, p38, PPAR-γ, can increase expression of both genes. We have recently shown that CTS exploits the NF-κB pathway for anti-inflammatory and anti-catabolic actions (Agarwal et al., 2004; Deschner et al., 2003). Therefore, future research should focus on whether CTS regulates the expression of RANKL and RANK by interfering with these or other signaling pathways.
Our findings pose the important question about the role of the RANKL/RANK/OPG system in fibrocartilage. So far it is not known if RANKL and its receptors exert any direct effects on any type of cartilage. In one study, human articular chondrocytes were incubated with RANKL that did not cause activation of these cells (Komuro et al., 2001). On the other hand, treatment with OPG reduced loss of cartilage matrix proteoglycans in experimental arthritis, and arthritic RANKL knockout mice showed a trend toward milder cartilage damage when compared to their arthritic control littermates (Campagnuolo et al., 2002; Gravallese, 2002). However, these effects were most likely an indirect consequence of protecting the subchondral bone. Interestingly, since RANKL as well as RANK mutant mice exhibit significant changes in the columnar alignment of chondrocytes at the growth plate, it is possible that these molecules play a direct part in cartilage growth and homeostasis (Walsh and Choi, 2003).
In summary, fibrochondrocytes from meniscus express RANKL, RANK, and OPG. More importantly, biophysical signals modulate the expression of RANKL and RANK in meniscal cells under inflammatory conditions, suggesting a need for a further understanding of the role of the RANKL/RANK/OPG system in fibrocartilage.
Acknowledgements
This study was supported by grants from National Institute of Health: DE15399, DE13799, and AT000646.
References
- Agarwal S, Deschner J, Long P, Verma A, Hofman C, Evans CH, Piesco N. Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis and Rheumatism. 2004;50:3541–3548. doi: 10.1002/art.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Biology of fibrocartilage cells. International Review of Cytology. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochimica et Biophysica Acta. 2001;1526:147–158. doi: 10.1016/s0304-4165(01)00122-2. [DOI] [PubMed] [Google Scholar]
- Campagnuolo G, Bolon B, Feige U. Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis and Rheumatism. 2002;46:1926–1936. doi: 10.1002/art.10369. [DOI] [PubMed] [Google Scholar]
- Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes. Current Opinion in Clinical Nutrition and Metabolic Care. 2003;6:289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis and Cartilage. 2004;12:956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Gravallese EM. Bone destruction in arthritis. Annals of the Rheumatic Diseases. 2002;61(Suppl 2):ii84–86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis and Rheumatism. 2000;43:2143–2151. doi: 10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–1091. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine. 2003;28:1781–1788. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- Hui W, Cawston TE, Richards CD, Rowan AD. A model of inflammatory arthritis highlights a role for oncostatin M in pro-inflammatory cytokine-induced bone destruction via RANK/RANKL. Arthritis Research Therapy. 2005;7:R57–64. doi: 10.1186/ar1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Kong YY, Penninger JM. Role of RANKL and RANK in bone loss and arthritis. Annals of the Rheumatic Diseases. 2002;61(Suppl 2):ii32–39. doi: 10.1136/ard.61.suppl_2.ii32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortikka MO, Inkinen RI, Tammi MI, Parkkinen JJ, Haapala J, Kiviranta I, Helminen HJ, Lammi MJ. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Annals of the Rheumatic Diseases. 1997;56:255–261. doi: 10.1136/ard.56.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology. 2004;43(Suppl 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M. The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis and Rheumatism. 2001;44:2768–2776. doi: 10.1002/1529-0131(200112)44:12<2768::aid-art464>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- LeRoux MA, Upton ML, Laursen TA, Setton LA. Biphasic finite element modeling of tear effects on the mechanics of the meniscus. ASME Summer Bioengineering Conference. 2001;50:851–852. [Google Scholar]
- MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Practice and Research in Clinical Rheumatology. 2001;15:805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- Ochi M, Kanda T, Sumen Y, Ikuta Y. Changes in the permeability and histologic findings of rabbit menisci after immobilization. Clinical Orthopaedics and Related Research. 1997;334:305–315. [PubMed] [Google Scholar]
- Romas E, Bakharevski O, Hards DK, Kartsogiannis V, Quinn JM, Ryan PF, Martin TJ, Gillespie MT. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis and Rheumatism. 2000;43:821–826. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. American Journal of Physiology-Cell Physiology. 2000;278:1126–1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- Schreppers GJ, Sauren AA, Huson A. A numerical model of the load transmission in the tibio-femoral contact area. Proceedings of the Institution of Mechanical Engineers [H] 1990;204:53–59. doi: 10.1243/PIME_PROC_1990_204_228_02. [DOI] [PubMed] [Google Scholar]
- Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. Journal of Applied Physiology. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. Journal of Biomechanics. 1992;25:1027–1045. doi: 10.1016/0021-9290(92)90038-3. [DOI] [PubMed] [Google Scholar]
- Sweigart MA, Zhu CF, Burt DM, DeHoll PD, Agrawal CM, Clanton TO, Athanasiou KA. Intraspecies and inter-species comparison of the compressive properties of the medial meniscus. Annual Biomedical Engineering. 2004;32:1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- Takamoto M, Tsuji K, Yamashita T, Sasaki H, Yano T, Taketani Y, Komori T, Nifuji A, Noda M. Hedgehog signaling enhances core-binding factor a1 and receptor activator of nuclear factor-kappaB ligand (RANKL) gene expression in chondrocytes. Journal of Endocrinology. 2003;177:413–421. doi: 10.1677/joe.0.1770413. [DOI] [PubMed] [Google Scholar]
- Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. Journal of Orthopaedic Research. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine and Growth Factor Reviews. 2003;14:251–263. doi: 10.1016/s1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Totterman SMS, Perucchio R, Lerner AL. Magnetic resonance image based 3D poroelastic finite element model of tibio-menisco-femoral contact. 23rd Annual Meeting of the American Society of Biomechanics. 1999 [Google Scholar]