Abstract
Adipose tissue inflammation links obesity and metabolic disease. Both exercise and estrogen improve metabolic health, enhance mitochondrial function, and have anti-inflammatory effects. We hypothesize that there is an inverse relationship between mitochondrial function and inflammation in adipose tissue and that exercise acts as an estrogen “mimetic”. Explicitly, exercise may improve adipose tissue “immunometabolism” by improving mitochondrial function and reducing inflammation.
Summary
Exercise improves adipose tissue metabolic health by reducing inflammation and improving mitochondrial function.
Keywords: adipose tissue, inflammation, mitochondria, estrogen, exercise, immunometabolism
Introduction
Obesity and its co-morbidities continue to increase in the United States and abroad, in large part due to an increasingly sedentary lifestyle combined with excess energy intake. Work conducted over the past two decades has illustrated the important role played by the adipose tissue in mechanistically relating obesity to disease. Beginning with the discoveries of the hormone leptin (12) and the cytokine tumor necrosis factor alpha (TNF-α) (14), both secreted by adipose tissue, the realization that the adipose tissue is much more than an inert storage depot evolved. Now, the adipose tissue is known to produce and secrete a rapidly expanding list of hormones and immune factors collectively referred to as “adipokines.” Moreover, the adipose tissue becomes infiltrated with recruited immune cells such as macrophages (Mϕs) and T lymphocytes which cross-activate one another and perform various immuno-modulatory and metabolic functions (37). The study of these interrelationships in adipose tissue has been referred to as “immunometabolism.” Moreover, the recent appreciation of the relevance in adult humans of brown adipose tissue (BAT), an adipose tissue depot present in small amounts whose major role is in thermoregulation rather than energy storage, highlights the metabolic importance of the adipose organ. A rapidly evolving body of research in this new area has demonstrated that BAT may play an important role in metabolic health and has the potential to be “activated” by various stimuli including cold exposure and exercise. In addition, positive relationships exist between energy expenditure and BAT across species, while an inverse relationship has been documented in humans between BAT mass and obesity (1).
White adipose tissue (WAT) becomes inflamed with the progression of obesity and the inflammatory profile in WAT associates strongly with increased adipocyte size and systemic insulin resistance. In fact, animal studies have illustrated that WAT inflammation per se, even in the absence of obesity, contributes to system metabolic dysfunction. Early studies elucidated a mechanistic role of specific immune factors released from WAT in whole body insulin resistance. The most notable example is the pathway by which TNF-α inhibits insulin signaling by directly interfering with phosphorylation of the insulin receptor (14). However, at the present time, dozens of such examples exist. It is now understood that much of the inflammatory processes that occur in WAT are the result of the resident and infiltrating Mϕs. Classical obesity-associated WAT inflammation that correlates strongly with insulin resistance is predominated by M1 “inflammatory” Mϕs which secrete pro-inflammatory cytokines such as TNF-α. However, lean, healthy, insulin-sensitive adipose tissue is classified by a predominance of alternatively-activated “M2” Mϕs. However, it is important to emphasize that this model of Mϕ polarization is over-simplified and it is now known that Mϕs lie on a wide continuum and may display characteristics of both M1 and M2. While the mechanisms underlying WAT inflammation in obesity are not fully elucidated, one current view is that WAT expansion per se results in adipocyte stress which triggers inflammation (37). Emerging evidence indicates that specific behavioral factors may impact adipose tissue immunometabolism. Exercise training is perhaps the most important among these factors.
A growing body of work performed across species and with only few exceptions has illustrated an anti-inflammatory role of exercise training in the WAT. Prior to the realization of such an exercise-mediated effect in WAT, it was established that exercise training results in a reduction in circulating inflammatory markers (33). This effect has been shown in obese populations as well as other populations with elevated inflammation, such as older sedentary individuals. Since there is a strong link between WAT inflammation, particularly that in the intra-abdominal area (i.e., visceral fat), and systemic inflammation, it is reasonable to hypothesize that exercise may directly affect the inflammatory state of WAT.
Exercise reduces white adipose tissue inflammation
Several studies examining obese individuals with chronic low-grade inflammation demonstrate that exercise training lowers systemic inflammation. The mechanism by which exercise reduces systemic inflammation may involve exercise-mediated reductions in WAT inflammation. In support of this notion, we conducted a 10-month training intervention on previously sedentary older men and women and found that the reduction we observed in circulating C-reactive protein (CRP), an important clinical marker of systemic inflammation, correlated strongly with a reduction in visceral adipose tissue, measured indirectly via dual energy X-ray absorptiometry (DXA) (33). To more directly test this idea, human studies have examined inflammatory gene expression in subcutaneous WAT (SQAT) biopsy samples following exercise training. Some, but not all, of these studies have shown reductions in SQAT gene expression of common markers of WAT inflammation. In a study of obese but otherwise healthy premenopausal females, the 12-week exercise protocol resulted in significant weight loss, but no changes in SQAT inflammatory gene expression (23). Similarly, another study using a similar exercise intervention, also with a healthy female population, reported a significant reduction in adiposity and circulating CRP, but no improvements in SQAT inflammation (e.g., adiponectin, interleukin (IL)-β, IL-6, IL-10) (31). Interestingly, the anti-inflammatory marker, IL-10 was initially higher in those female participants and remained so post-exercise. It is probable that the absence of changes in SQAT in those subjects was attributed to their relatively healthy state (i.e., lack of WAT inflammation at the outset of the trial) and/or, the fact that those subjects were female. Interestingly, exercise intensity may also play a role in whether or not exercise reduces SQAT inflammation in humans because at least one study investigating both sexes and employing a high-intensity training protocol reported significant reductions in adiposity and circulating inflammatory markers, as well as increases in SQAT adiponectin; no other inflammatory genes were altered, however (8).
It is interesting to note that, of the human studies that do show reductions in SQAT inflammation with exercise, the vast majority were conducted on both men and women. Although most studies show concomitant effects of weight loss and improved WAT inflammation (6), some demonstrate that exercise may exert anti-inflammatory effects in WAT in the absence of weight loss (2, 18). Taken together, the human data show a modest anti-inflammatory effect of exercise on SQAT in some but not all studies. The effect does not appear to be exclusively dependent upon body weight reduction or even exercise intensity, but rather the metabolic state of the population. There are insufficient data comparing the effects of caloric restriction to exercise while controlling other important variables such as total fat loss, and/or comparing responses by men and women. However, the anti-inflammatory effects of exercise appear most notable in subject groups with inflamed WAT and/or preexistent metabolic conditions prior to the onset of the exercise intervention. Importantly, women appear to present with protection against WAT inflammation which may explain the lack of effect specifically in this population. Another important consideration is that the body of literature assessing the potential anti-inflammatory role of exercise on WAT in humans has only evaluated SQAT whereas a preponderance of research implicates the visceral WAT as the major source of inflammation. This is an important area of future investigation.
Our group and others have conducted animal studies to more comprehensively assess the effect of exercise on WAT inflammation by investigating inflammation across several depots and controlling the duration and intensity of exercise. Early work from our group ((33, 35) and others (4) demonstrated that exercise reduces WAT inflammation and this finding has been replicated several times using many different animal models, in many different laboratories. In the vast majority of cases, the anti-inflammatory effect of exercise is stronger in visceral WAT compared to SQAT, suggesting that exercise may have depot-specific effects on WAT immunometabolism. This contention is supported by depot comparisons in response to exercise training (9). We recently conducted a study comparing voluntary wheel running, which may be considered a model of habitual physical activity rather than structured exercise training, to caloric restriction (30% caloric restriction) in a male obesity-prone rat model, Otsuka Long-Evans Tokushima Fatty (OLETF). Part of what made that study unique was an in-depth adipose tissue depot comparison which included traditional SQAT and visceral WAT depots as well as periaortic adipose tissue (i.e., PAT) and BAT. While both interventions reduced most inflammatory markers across depots (with BAT being the one exception), the extent of the anti-inflammatory effects appeared most pronounced in visceral compared to the SQAT and PAT. And, in a recent study by Castellani et al. (2014) where trained versus untrained mice were exposed to an inflammatory stimulus, exercise mitigated the inflammatory response in trained animals in the perigonadal WAT (PGAT) only and not SQAT (7). The fact that exercise/enhanced physical activity has been demonstrated to primarily reduce visceral but not SQAT inflammation in animal studies supports the possibility that the negative findings reported in the human literature may be attributed to the lack of evaluation in the visceral WAT.
Interestingly, in animal studies which examined SQAT inflammation (e.g., TNF-α, IL-6, monocyte chemoattractant-1 (MCP-1), leptin) following either voluntary wheel running (9) or treadmill training (5), reductions in inflammatory gene expression have been reported. Still, in cases where SQAT and a visceral depot were investigated, the inflammatory changes were more robust in visceral WAT (9). The most common species used to assess the anti-inflammatory effect of exercise in WAT has been the mouse. Of the training studies using mice, most show reductions in at least one or more inflammatory markers, independent of the type of exercise or chronicity. Importantly, the vast majority of animal studies have used only males and virtually all used C57BL/6 mice, the strain most commonly used in diet-induced obesity studies. We conducted a study using a different strain, Balb/c (36), and did show exercise-mediated reduction in WAT inflammation, hence indicating that the anti-inflammatory effect of exercise is not specific to the C57BL/6 mouse. Only one rat study used females (using a model of polycystic ovary syndrome, PCOS) and demonstrated exercise-related improvements in adiposity and reductions in mesenteric WAT (MES) inflammatory adipokines including IL-6 and leptin (19); it is important to note that PCOS associates with dysregulated female ovarian hormones and insulin resistance.
Another factor that may affect whether or not exercise training reduces WAT inflammation in animal studies is the dietary means by which obesity was initiated. For example, two studies using very similar protocols of moderate intensity treadmill training both reported no exercise-mediated weight loss yet only one found a reduction in WAT inflammation. Baynard et al. (3) found a significant reduction of F4/80, a non-specific Mϕ marker, in PGAT but no reductions in proinflammatory adipokines such as TNF-α, MCP-1, and leptin whereas Kawanishi et al. (15) showed significant reductions in TNF-α, IL-6, and MCP-1. The only difference between those two studies was the fat composition of the diet, 45% vs. 60% respectively. Commercial high fat diets (HFDs), such as those used in the above mentioned studies, are particularly high is saturated fatty acids, which are known to activate inflammatory pathways directly, thus potently triggering WAT inflammation. Similarly, rodent HFD studies often use a commercial low-fat diet (LFD) control which is very low in fat; this may amplify the between-group differences assessed via gene expression. That is, the difference between groups in a study using 60% HFD and the standard LFD would be more robust than one using a 45% HFD and the same standard LFD. Thus, similar to the human studies, the effect of exercise on reducing WAT inflammation appears largely dependent upon the initial inflammatory state of the tissue. In summary, human and animal studies support the contention that there is an anti-inflammatory effect of exercise training in WAT. The effect may be most pronounced in visceral WAT but appears not to be exclusively dependent upon fat loss and is conserved across species.
Potential mechanisms behind the anti-inflammatory effect of exercise
While the role of adipose tissue as an endocrine organ is only recently appreciated, it has long been known that exercise, especially endurance exercise, profoundly affects WAT metabolism. As blood glucose levels become limited during exercise, catecholamine-mediated lipolysis increases to provide free fatty acids (FFAs) for utilization by skeletal muscle cells. Indeed, adipocyte lipolysis and skeletal muscle FFA oxidation are tightly linked during exercise. Together, these events create a negative energy balance with exercise training to facilitate fat loss, presumably due to reduced adipocyte size. Smaller adipocytes are more insulin sensitive; this likely contributes to the powerful insulin sensitizing effects of exercise on WAT. On the other hand, a hallmark feature of adipocyte insulin resistance, which occurs in metabolically disturbed states such as obesity and diabetes, is impaired insulin-mediated suppression of lipolysis and larger, insulin resistant adipocytes. Moreover, dysfunctional adipocyte lipolysis is associated with inflammation due to the inflammatory nature of the mobilized FFA. In fact, in the study by Castellani et al. described above, the drug used to trigger WAT inflammation was a known stimulator of lipolysis; the trained mice in that study had a lessened inflammatory response to the increase in lipolysis (7). Thus, one mechanism by which exercise may reduce WAT inflammation is via an increase in adipocyte insulin sensitivity and a corresponding decrease in dysregulated lipolysis. Figure 1 summarizes this hypothesis. This idea is supported by another recent study in mice which demonstrated enhanced stimulation of lipolysis and insulin sensitivity following exercise training, which correlated with increased FFA oxidation and lower lipid storage in WAT (13). That study examined male diet-induced obese C57BL/6 mice and found that exercise attenuated gains in adiposity, prevented insulin resistance, reduced expression of glucose 6-phosphate dehydrogenase, an enzyme involved in fatty acid synthesis, and increased citrate synthase activity, a marker of mitochondrial function, in WAT (13).
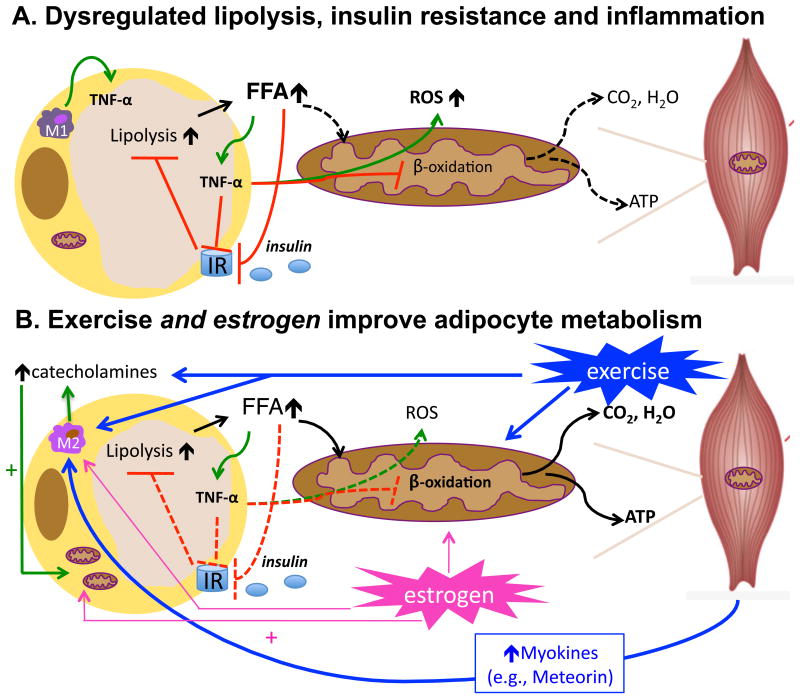
Figure 1. Proposed mechanism by which exercise reduces adipose tissue inflammation.
Depicted in A., adipocyte hypertrophy, as occurs in obesity, leads to dysregulated adipocyte lipolysis and insulin resistance. Insulin's ability to suppress lipolysis becomes impaired leading to increased free fatty acid (FFA) mobilization, even in the presence of insulin. In the absence of FFA utilization, FFAs trigger adipocyte inflammation and an increase in inflammatory (M1) Mϕs which also produce TNF-α, a cytokine that inhibits insulin signaling and impairs mitochondrial function (12) leading to increased reactive oxygen species (ROS) production; ROS further contribute to inflammation, leading to a vicious cycle. Depicted in B., exercise stimulates adipocyte lipolysis via catecholamine activation while also stimulating FFA oxidation in skeletal muscle. Exercise training also reduces adipocyte size, enhances adipocyte insulin sensitivity, and may also stimulate mitochondrial function in adipocytes thereby further limiting the inflammatory effects of FFA within the adipocyte. In addition, exercise triggers the release of myokines, which are cytokines and other peptides released by skeletal muscle. One such recently discovered myokine, meteorin-like, enhances adipocyte insulin sensitivity and reduces adipoctye inflammation (24). Meteorin-like also promotes alternative (M2) Mϕ activation, which is characteristic of healthy insulin-sensitive adipocytes. Similar to exercise, estrogen improves adipocyte metabolism by facilitating FFA oxidation and mitochondrial biogenesis and also via activation of M2 Mϕ, which increases local catecholamine production. Dashed lines = inactive processes; solid lines = active processes.
Relationship between adipose tissue inflammation and mitochondrial function
Enhancement of adipocyte mitochondrial function may play a major role in exercise training-mediated improvements in adipose tissue immunometabolism. The effects of exercise training on the capacity of skeletal muscle cells to efficiently utilize FFAs is well-known and supported by evidence of muscle fiber type switching and enhanced mitochondrial function and density in skeletal muscle. The role of FFA oxidation and mitochondrial function in adipocytes with exercise training is much less studied; however, recent evidence implicates adipocyte mitochondria as having a role in training adaptations (29), albeit not to the degree of skeletal muscle mitochondria. This exercise-mediated effect on adipocyte mitochondria was first shown by Stallknecht and colleagues in 1991 (28). We recently highlighted literature demonstrating the interrelationships between adipocytes, their mitochondria, and adipocyte inflammation (37). Briefly, “healthy” adipocytes are characterized by highly functional mitochondria (e.g., sufficient capacity to oxidize FFA), a resident Mϕ phenotype characterized as “M2” with highly efficient lipid handling capabilities and less pro-inflammatory cytokine production, and high insulin sensitivity. In contrast, “unhealthy” adipocytes are characterized as having dysfunctional mitochondria which produce reactive oxygen species (ROS), undergo dysregulated lipolysis due to insulin insensitivity, and contain “M1” Mϕs which are recruited to the tissue and perpetuate the inflammatory situation by secreting pro-inflammatory cytokines.
Greater mitochondrial density is characteristic of BAT. Exercise increases mitochondrial biogenesis and brown adipocyte-specific gene expression and may even induce a phenotypic switch from WAT to BAT (22). Slocum et al. showed that, even with low-intensity exercise training, there was increased mitochondrial content mainly due to increased uncoupling protein-1 (UCP-1) and peroxisome proliferator-activated receptor gamma (PPARγ) expression in BAT suggestive of enhanced BAT activation (27). Another study found that exercise increased UCP-1 and PPARγ expression in PGAT, suggesting exercise enhances brown adipocyte progenitor cells in WAT (39). Importantly, BAT activation and/or regeneration in animal models has been associated with enhanced metabolic function including obesity reduction and increased insulin sensitivity (1). While the mechanisms by which exercise training may increase mitochondrial function in WAT and/or BAT are not completely understood, a new hormone recently recognized to be induced in muscle with exercise training, meteorin-like (24), increases energy expenditure and improves insulin sensitivity. Interestingly, it also up-regulates anti-inflammatory cytokines and alternative (M2) Mϕ activation in WAT suggesting an inverse relationship between inflammation and mitochondrial function perhaps due to alternative Mϕ activation (Figure 1).
Interestingly, recent data suggest that the type of Mϕs present in WAT not only determine the local inflammatory state, but also lipid dynamics within the adipocyte; and, this relationship appears to involve adipocyte mitochondria (37). In a recent cell culture study using the adipocyte cell line 3T3L1, Hahn et al. (11) revealed a direct relationship between the inflammatory cytokine, TNF-α (but not other cytokines investigated such as IL-6 and IL-1β), produced by adipose tissue Mϕs, and adipocyte mitochondrial function. Exposure of the mitochondria to TNF-α decreased their function as indicated by reductions in regulators of mitochondrial biogenesis, peroxisome proliferator-activated receptor co-activator (PGC1α) and endothelial nitric oxide synthase (eNOS). Moreover, the mitochondria showed other signs of dysfunction including fragmentation and dysregulated fusion causing them to produce more ROS and perpetuate the inflammatory situation. Meanwhile, activation of the important cellular fuel gauge associated with greater FFA oxidation and mitochondrial function, AMP kinase (AMPK), is potently activated by exercise in both skeletal muscle as well as adipose tissue and has been shown to associate with reduced cellular inflammation. On the other hand, M2 Mϕs, which release anti-inflammatory cytokines, associate with increased FFA oxidation and decreased availability of potentially toxic lipid species. Importantly, exercise increases M2 Mϕ activation, which has been proposed as a potential mechanism by which exercise reduces inflammation in adipose tissue. In a study by Kawanishi et al. (2010), C57BL6 mice fed HFD and exercise trained did not experience a reduction in fat mass yet marked reductions in pro-inflammatory cytokines in WAT were observed. Those changes were associated with both a suppression of Mϕ infiltration as well as a Mϕ phenotype switch from M1 to M2 (16). Unfortunately, that study did not assess WAT mitochondrial function. The link between M2 Mϕ activation and mitochondrial function recently was illustrated by the finding that M2 Mϕ activation and associated local catecholamine production is mechanistically related to the “browning” of WAT (17). What occurs metabolically to adipocytes when they undergo the browning process is an increase in mitochondrial density, function, and uncoupling of oxidative phosporylation due to the increase in expression of the uncoupling protein, UCP-1(1). Interestingly, another factor that has recently been shown to increase M2 Mϕ activation in WAT is the hormone, estrogen (E2) (30). Further, studies have implicated E2 in increasing mitochondrial biogenesis, activating AMPK, and BAT activation (20).
What can we learn about the immunoregulatory role of exercise on adipose tissue through sex comparison studies?
There is growing appreciation for the fact that premenopausal females are protected from metabolic dysfunction compared to age-matched males. Senthil et al. (2014) compared male and female mice for their metabolic responses to HFD and found that, despite similar body composition changes, females were protected against the development of inflammation and insulin resistance observed in the males (26). This sex difference in metabolic response has also been observed in other species such as sheep and rats. There is good evidence that E2 is a key player in mediating this sex difference. Shen and colleagues (2014) demonstrated that, unlike SHM-operated mice, female OVX mice developed WAT inflammation and insulin resistance on HFD. In addition, these detrimental metabolic effects were rescued with E2 replacement, even while the OVX-E2-HFD and OVX-HFD (vehicle only) groups gained the same amount of weight. Those authors concluded that E2 contributed to the maintenance of insulin sensitivity during the early phase of metabolic dysfunction development and that the mechanism may have involved a decrease in WAT inflammation (26).
Although it has long been known that important sex differences exist in terms of cardio-metabolic risk factors, the vast majority of animal studies assessing mechanistic links between obesity and metabolic dysfunction have been done using male rodents. However, it is becoming increasingly evident that the relationships observed in male rodents may not translate to female rodents, and more importantly, female humans. Some sex-specific metabolic differences are well established, such that females have a greater percentage of body fat, a different fat distribution profile, and different presentation of cardiovascular risk factors. Recent evidence illustrates that premenopausal women also have better insulin sensitivity compared to age-matched men. Work that we conducted several years ago suggested that the immune profile changes that occur in young women with obesity may differ from those of young males; in fact, the increase in elevated levels of circulating immunoglobulin E (IgE) specifically among young obese women, is suggestive of a Th2 immune profile which associates with higher levels of IL-4 and IL-13 and a predisposition for allergy (34). Incidentally, those same cytokines (IL-4, IL-13) are essential in alternative Mϕ activation. Similarly, animal studies have shown that female rodents are “protected” against classical (M1) WAT inflammation as well as insulin resistance. However, this “protection” disappears when ovarian hormone production is removed. In a study we conducted using female OVX mice, we found that OVX significantly increased WAT inflammation, which was associated with the development of insulin resistance (32). Importantly, this effect was independent of adiposity suggesting that ovarian hormone loss, and not the associated increase in adiposity per se, caused the changes. That study suggests that the protection against WAT inflammation and systemic insulin resistance in young females is largely due to the presence of ovarian hormones, the leading contender among them being E2.
While the sex differences in metabolic response to HFD are not completely understood, convincing data implicate E2 and signaling through estrogen receptor alpha (ERα) as playing an important role. Estrogen receptors (ERs) are present in adipocytes, within the cell membrane, in the nucleus, and within the mitochondrial membrane. There is evidence of both ERα and ERβ mediated increases in mitochondrial biogenesis, which likely plays a role in the increased fat oxidation capacity among females. More recently, it has been discovered that E2 also stimulates intracellular signaling pathways via binding to membrane associated ERs and this rapid signaling pathway results in, among other endpoints, nitric oxide production via activation of eNOS which is associated with improved mitochondrial function. Estrogen is also a known regulator of the transcription of Nuclear Respiratory Factor 1 (NRF-1) which promotes transcription of mitochondrial transcription factor (Tfam) which transcribes many mitochondrial genes. Studies have also shown that estrogens activate mitochondrial respiration (10). And, E2 increases transcription of genes associated with protection against mitochondrial damage, such as glutathione peroxidase (Gpx3) and superoxide dismutase (MnSOD). Taken together, those lines of evidence suggest that female WAT, which presents with less inflammation compared to that from equally-obese males, likely also contains more or better functioning mitochondria. In fact, adipocytes from female mice have been shown to have more mitochondria, and the mitochondria take on a different phenotype consisting of more densely packed cristae (25). Moreover, the higher fat oxidation rates among females compared to males diminishes following menopause. Interestingly, ER knock out studies have shown that ablation of ERα not only leads to increased total adiposity, but also reduced energy expenditure while E2 replacement increases energy expenditure and reduces adiposity in humans and animals. Finally, E2 has been shown to up-regulate electron transport chain subunits including COXIV and OVX leads to decreased mitochondrial energetics, an effect which can be completely restored with E2.
Considering the evidence cited above which demonstrates a remarkable sex difference in inflammation and insulin resistance that may be related to an increase in mitochondrial function in premenopausal/estrogen-sufficient females, a question that arises is: Might exercise training or aerobic fitness, both of which associate with improved mitochondrial function/oxidative metabolism, reduce inflammation via this mechanism? Based on this idea, ongoing work by our group attempts to identify the specific role played by intrinsic aerobic fitness on adipose tissue inflammation, energy expenditure, and insulin resistance. In fact, we have demonstrated that, just as OVX females present with reduced metabolic protection compared to ovary-intact controls, low aerobically fit female rats (i.e., low capacity runners, “LCR”) have reduced metabolic protection compared to high aerobically fit rats (i.e., high capacity runners, “HCR”). Moreover, compared to HCR rats, LCR rats respond more adversely to OVX in terms of reduction in energy expenditure, reduced mitochondrial function, and increased insulin resistance (38). Furthermore, in comparing WAT immunometabolic profiles between HCR and LCR rats, we observe increased gene expression of inflammatory markers including F4/80, CD3, CD8, and TNF-α (Vieira-Potter et al., unpublished) suggesting that the “protection” among HCR females is somewhat reduced in a setting of impaired mitochondrial function; meanwhile, the HCR/LCR model has been described as lines divergent in oxidative metabolism (21).
Summary
Exercise is one of the most powerful behavioral strategies to improve metabolic health. Even when body weight or adiposity is not reduced, exercise training has significant cardiometabolic benefits for reasons that remain incompletely understood. A body of work beginning about two decades ago has revealed a powerful anti-inflammatory effect of exercise, specifically in the WAT, while WAT inflammation plays a major role in the development of metabolic diseases. Although it is difficult to dissociate exercise training and WAT reduction, convincing new evidence suggests that the effects of exercise are not fully explained by its fat-reducing effects. For example, recent important data demonstrated that training imparts protection against an acute inflammatory stimulus thereby supporting a fat-loss independent anti-inflammatory effect. In that study, the protection observed in trained animals was associated with a reduction in a cation channel protein (TRPV4) suggesting a molecular mechanism by which training reduces WAT inflammation (7).
The body of work illustrating the joint mitochondrial-enhancing and anti-inflammatory effects of E2 lends support to the idea that exercise may serve as an E2 “mimetic”. That is, exercise and E2 appear to improve adipocyte health similarly: by enhancing mitochondrial function and reducing inflammation. The “protection” among females against insulin resistance and inflammation and the loss of this protection following OVX or menopause, along with the data highlighting the metabolically important role of ERα, supports the contention that E2 is a “protective factor.” Thus, emerging evidence suggests that exercise makes fat “fit” and that adipocyte “fitness” (i.e., greater mitochondrial function) may be mechanistically related to its anti-inflammatory effect. It should be noted that the idea that exercise makes fat “fit” is not new; this exercise-mediated effect on adipocyte mitochondria was first shown by Stallknecht and colleagues in 1991 and in 2009, David Wright's group elegantly showed that one mechanism involves exercise-mediated catecholamine production (29). The emergence of research studies on the metabolic importance of BAT underscores the potential utility of exercise on improving adipose tissue immunometabolism, as exercise increases BAT activity and may increase “browning” of WAT. Although mechanisms are not fully elucidated, mitochondrial function in adipose tissue appears to be intimately related to inflammation, lipid metabolism and overall metabolic function; thus, exercise improves adipocyte immunometabolism. The similarities between the metabolic protection imparted by exercise training and estrogen are striking; thus, sex comparison studies may offer important insight about the anti-inflammatory role of exercise.
Nonetheless, it should not be understated that both exercise and E2 represent master regulatory factors. That is, the effects of both exercise and E2 are multifactorial; suggesting that they both work in virtually the same manner to improve adipocyte immunometabolism would be a grossly simplistic model. It is intriguing nonetheless, that major mechanisms by which exercise has been postulated to have anti-inflammatory effects in adipose tissue (e.g., increased catecholamine secretion, alternative Mϕ activation, AMPK activation) are also stimulated by E2 either via direct signaling pathways or via E2-mediated genomic effects. Additionally, there are numerous indirect potential mechanisms by which exercise improves adipose tissue inflammation including increased angiogenesis and enhanced anti-oxidant defenses; similarly, E2 activates angiogenesis in models of tumor progression and increases anti-oxidant defenses via genomic mechanisms. It is also possible that other newly recognized strategies to improve immunometabolism such as cold exposure may be working via similar mechanisms (e.g., increased catecholamine release, increased mitochondrial function). In conclusion, exercise training, similar to E2 signaling, improves the metabolic health of the adipose tissue, and this effect likely contributes to the global metabolic health benefits of exercise.
Acknowledgments
J.P. is supported by NHLBI K01HL125503.
Footnotes
Conflicts: None.
Exercise and Sport Sciences Reviews articles in the Published Ahead-of-Print section have been peer-reviewed and accepted for publication. However, during copyediting, page composition, or proof review changes may be made that could affect the content.
References
- 1.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nature reviews Endocrinology. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 2.Baturcam E, Abubaker J, Tiss A, et al. Physical exercise reduces the expression of RANTES and its CCR5 receptor in the adipose tissue of obese humans. Mediators of inflammation. 2014;2014:627150. doi: 10.1155/2014/627150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baynard T, Vieira-Potter VJ, Valentine RJ, Woods JA. Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators of inflammation. 2012;2012:767953. doi: 10.1155/2012/767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. American journal of physiology Endocrinology and metabolism. 2008;295(3):E586–94. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt C, Jakobsen AH, Adser H, et al. IL-6 regulates exercise and training-induced adaptations in subcutaneous adipose tissue in mice. Acta physiologica. 2012;205(2):224–35. doi: 10.1111/j.1748-1716.2011.02373.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. American journal of physiology Endocrinology and metabolism. 2006;290(5):E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 7.Castellani L, Root-Mccaig J, Frendo-Cumbo S, Beaudoin MS, Wright DC. Exercise training protects against an acute inflammatory insult in mouse epididymal adipose tissue. Journal of applied physiology. 2014;116(10):1272–80. doi: 10.1152/japplphysiol.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. American journal of physiology Endocrinology and metabolism. 2010;298(4):E824–31. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 9.Crissey JM, Jenkins NT, Lansford KA, et al. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(8):R596–606. doi: 10.1152/ajpregu.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigli I, Bussmann LE. Exercise and ovarian steroid hormones: their effects on mitochondrial respiration. Life sciences. 2001;68(13):1505–14. doi: 10.1016/s0024-3205(01)00954-7. [DOI] [PubMed] [Google Scholar]
- 11.Hahn WS, Kuzmicic J, Burrill JS, et al. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. American journal of physiology Endocrinology and metabolism. 2014;306(9):E1033–45. doi: 10.1152/ajpendo.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 13.Higa TS, Spinola AV, Fonseca-Alaniz MH, Evangelista FS. Remodeling of white adipose tissue metabolism by physical training prevents insulin resistance. Life sciences. 2014;103(1):41–8. doi: 10.1016/j.lfs.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Medicine and science in sports and exercise. 2013;45(9):1684–93. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 16.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise immunology review. 2010;16:105–18. [PubMed] [Google Scholar]
- 17.Lee MW, Odegaard JI, Mukundan L, et al. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell. 2014 doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard H. Adipose tissue interleukin-18 mRNA and plasma interleukin-18: effect of obesity and exercise. Obesity. 2007;15(2):356–63. doi: 10.1038/oby.2007.528. [DOI] [PubMed] [Google Scholar]
- 19.Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149(7):3559–68. doi: 10.1210/en.2008-0053. [DOI] [PubMed] [Google Scholar]
- 20.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell metabolism. 2014;20(1):41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naples SP, Borengasser SJ, Rector RS, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35(2):151–62. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. The FEBS journal. 2014;281(3):739–49. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 23.Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism: clinical and experimental. 2006;55(10):1375–81. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Rao RR, Long JZ, White JP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. The Journal of biological chemistry. 2002;277(45):42958–63. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- 26.Senthil Kumar SP, Shen M, Spicer EG, et al. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocrine journal. 2014;61(5):457–70. doi: 10.1507/endocrj.ej13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slocum N, Durrant JR, Bailey D, et al. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2013;65(5):549–57. doi: 10.1016/j.etp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. The American journal of physiology. 1991;261(3 Pt 1):E410–4. doi: 10.1152/ajpendo.1991.261.3.E410. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. The Journal of physiology. 2009;587(Pt 7):1607–17. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toniolo A, Fadini GP, Tedesco S, et al. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. The Journal of clinical endocrinology and metabolism. 2015;100(1):E50–8. doi: 10.1210/jc.2014-2751. [DOI] [PubMed] [Google Scholar]
- 31.Trachta P, Drapalova J, Kavalkova P, et al. Three months of regular aerobic exercise in patients with obesity improve systemic subclinical inflammation without major influence on blood pressure and endocrine production of subcutaneous fat. Physiological research / Academia Scientiarum Bohemoslovaca. 2014;63(Suppl 2):S299–308. doi: 10.33549/physiolres.932792. [DOI] [PubMed] [Google Scholar]
- 32.Vieira Potter VJ, Strissel KJ, Xie C, et al. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology. 2012;153(9):4266–77. doi: 10.1210/en.2011-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira VJ, Hu L, Valentine RJ, et al. Reduction in trunk fat predicts cardiovascular exercise training-related reductions in C-reactive protein. Brain, behavior, and immunity. 2009;23(4):485–91. doi: 10.1016/j.bbi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Vieira VJ, Ronan AM, Windt MR, Tagliaferro AR. Elevated atopy in healthy obese women. The American journal of clinical nutrition. 2005;82(3):504–9. doi: 10.1093/ajcn.82.3.504. [DOI] [PubMed] [Google Scholar]
- 35.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. American journal of physiology Endocrinology and metabolism. 2009;296(5):E1164–71. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009;46(3):339–45. doi: 10.1016/j.cyto.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cellular microbiology. 2014;16(10):1484–92. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 38.Vieira-Potter VJ, Padilla J, Park YM, et al. Female Rats Selectively Bred for High Intrinsic Aerobic Fitness Are Protected from Ovariectomy-Associated Metabolic Dysfunction. American journal of physiology Regulatory, integrative and comparative physiology. 2015 doi: 10.1152/ajpregu.00401.2014. ajpregu 00401 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Ying Z, Cai M, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(5):R1115–25. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]