Abstract
A novel non-toxic biodegradable lysine-di-isocyanate (LDI)-based urethane polymer was developed for use in tissue engineering applications. This matrix was synthesized with highly purified LDI made from the lysine diethylester. The ethyl ester of LDI was polymerized with glycerol to form a prepolymer. LDI–glycerol prepolymer when reacted with water foamed with the liberation of CO2 to provide a pliable spongy urethane polymer. The LDI–glycerol matrix degraded in aqueous solutions at 100, 37, 22, and 4°C at a rate of 27.7, 1.8, 0.8, and 0.1 mM per 10 days, respectively. Its thermal stability in water allowed its sterilization by autoclaving. The degradation of the LDI–glycerol polymer yielded lysine, ethanol, and glycerol as breakdown products. The degradation products of LDI–glycerol polymer did not significantly affect the pH of the solution. The glass transition temperature (Tg) of this polymer was found to be 103.4°C. The physical properties of the polymer network were found to be adequate to support the cell growth in vitro, as evidenced by the fact that rabbit bone marrow stromal cells (BMSC) attached to the polymer matrix and remained viable on its surface. Culture of BMSC on LDI–glycerol matrix for long durations resulted in the formation of multilayered confluent cultures, a characteristic typical of bone cells. Furthermore, cells grown on LDI–glycerol matrix did not differ phenotypically from the cells grown on the tissue culture polystyrene plates as assessed by the cell growth, and expression of mRNA for collagen type I, and transforming growth factor-β1 (TGF-β1). The observations suggest that biodegradable peptide-based urethane polymers can be synthesized which may pave their way for possible use in tissue engineering applications.
Keywords: Lysine-di-isocyanate, Glycerol, Urethane polymer, Osteoblasts, Cell culture
1. Introduction
Polyurethanes created by reacting polyesters or polyethers with polyisocyanates provide matrices with adequate mechanical and physical properties, and with the flexibility and blood compatibility needed for use in biomedical applications [1–3]. In fact, a number of non-biodegradable urethanes have been utilized in blood-contact applications such as, heart valves, dialysis membranes, breast implants, aortic grafts, and bone adhesives [1,2]. Additionally, bioabsorbable poly(ester)–urethanes have been synthesized and widely used in medical devices. However, these polymers invariably produce toxic by-products which have posed severe limitations on their use in vivo. For example, urethane formed by reacting poly(D,L-lactide) diol with methylene diisocyanate hydrolyzes in vivo into 4,4-methylenedianiline, a toxic by-product which reportedly produces hepatitis in humans [4]. Similarly, polyurethanes synthesized with toluene diisocyanate degrade into 2,4-diaminotoluene, which is a suspected carcinogen [2,5]. Aliphatic diisocyanates including 4,4-methylenedicyclohexyl diisocyanate and hexamethylene diisocyanate have also been considered, but their corresponding diamines are relatively toxic [1,2,6]. Nevertheless, except for their toxicity, urethanes possess unique attributes which make them ideal for tissue engineering applications. These attributes include a wide range of physical and mechanical properties, chemical functionality, and diversity in specific polymer characteristics.
In this paper, we have developed a new generation of poly(urea–urethane) which is peptide based, and have initiated testing of this polymer for its ability to support cell growth in vitro. Specifically, this polymer is composed of a lysine-di-isocyanate and glycerol that degrades into non-toxic components, lysine, glycerol, and ethanol. Thus this peptide-based urethane possesses the versatility of polyurethanes, but may lack the toxicity of other urethane degradation products. We expect that the LDI–glycerol polymer may provide better biocompatibility than presently used matrices due to its ability to: (i) degrade into non-toxic lysine and glycerol, (ii) allow incorporation of proteins of interest (cell attachment factors, and/or growth factors) to regionally promote a microenvironment optimal for cell organization and stimulation, and (iii) change topology by varying the functionality and mechanical properties from an elastomer, to a thermoplastic, to a thermoset. The degradation rates at various temperatures demonstrate that this polymer is biodegradable and its degradation products do not affect the pH of the environment significantly. While this polymer is suitable for supporting growth and phenotypic characteristics of osteoblast-like cells in vitro, its usefulness for in vivo applications is yet to be established.
2. Materials and methods
2.1. Materials
L-lysine ethyl ester dihydrochloride, hydroxy proline, chloramine T, glutamine (Glu), and penicillin–streptomycin solution (10 000 units penicillin and 10 μg streptomycin/ml saline; Pen/Strep), glycerokinase from E. coli, firefly luciferase and luciferin were obtained from Sigma Chemical Co. (St Louis, MO, USA). Glycerol was from EM Science (Gibbstown, NJ 08027). Methyl ester LDI was kindly provided by Dr. K. Tanaka, Chemical Division, Kyowa Hakko Kogyo Co. Ltd. (Tokyo, Japan). The tissue culture medium RPMI 1640 was obtained from Life Technologies (Grand Island, NY 14072, USA). Phosgene (20% in toluene) was purchased from Fluka Chemie AG (Buchs, Switzerland). Methylene chloride (CH2Cl2) and pyridine were obtained from Aldrich Chemical Co. (Milwaukee, WI), and were distilled over calcium hydride prior to use. All other reagents used were of analytical grade.
2.2. The synthesis of lysine-di-isocyanate (LDI) from lysine ethyl ester dihydrochloride
The synthesis of LDI was conducted according to the method described by Nowick et al [7]. In a typical experiment, 5.01 g lysine ethyl ester dihydrochloride (20 mmol), 100 ml of freshly distilled CH2Cl2, and 20 ml of pyridine (240 mmol) were added to a dry 250 ml, three-necked round-bottom flask, fitted with two rubber septa and a nitrogen inlet adapter. The mixture was stirred in a dry ice bath for 15 min. Subsequently, 60 ml of a 1.93 M phosgene solution in toluene (116 mmol) was added by syringe over a period of 20–30 s, and the resulting light yellow solution was stirred in a dry ice bath for 2 h. The reaction mixture was extracted two times with 300 ml of cold 0.5 M aqueous HCl over ice water. Each aqueous layer was re-extracted with 100 ml of CH2Cl2. The combined organic phases were dried over MgSO4, filtered, and concentrated by rotary evaporation to obtain the crude isocyanate as a light yellow oil. The product was purified by distillation under reduced pressure. The purity of product was examined by 1H NMR at 300 MHz (Brucker AC 300).
2.3. Synthesis of the LDI–glycerol polymeric foam
Four ml of glycerol (55 mmol) was added drop wise to 16 ml of LDI (87 mmol) in a dry round-bottomed flask, flushed with nitrogen and then fitted with a rubber septa and sealed. The reaction mixture was stirred in the dark at room temperature for 7 days. Subsequently, the solution was cooled in a dry ice bath and the prepolymer precipitated by the addition of di-ethyl ether. The white solid was dissolved in CH2Cl2 again, and reprecipitated with di-ethyl ether. The formation of urethane linkages was monitored by FT-IR spectra of the prepolymer dissolved in dichloromethane (DCM). Finally, water was used to crosslink the prepolymer and generate a foam. Typically, 1 ml of water was added to 10 g of the pre-polymer at room temperature and stirred for 1 h. The polymer was then placed in a vacuum oven at 22°C over night to enhance foaming and to dry the material.
2.4. Analysis of glass transition temperature (Tg) of LDI–glycerol polymer
LDI–polymer (5 mg) was dried under vacuum at room temperature prior to sealing in aluminum pans. Subsequently, the thermal analysis was performed in a Thermal analyst 2000 (TA Instruments) with DSC 2910 differential scanning calorimeter. The heat was increase at a rate of 10°C/min under constant nitrogen purge.
2.5. Analysis of the degradation products of the LDI–glycerol polymer
Polymer (10 mg/ml PBS) was incubated at 4, 22, and 37°C for 1 to 60 days. Each day, 1 ml PBS was retrieved from each sample after thorough mixing. The concentration of lysine liberated from polymer was detected by the ninhydrin colorimetric reaction using UV spectrometry at 580 nm [8]. Glycerol was assessed according to the method described by Hellmèr et al. [9]. Briefly, 0.6 ml of 0.1 M Tris–HCl buffer (pH 8.0), 0.2 ml of ATP monitoring agent (10 μg of firefly luciferase, 1.4×10−5 M luciferin, 10 mM magnesium acetate in 1 ml 100 mM Tris–HCl, pH 8.0), 20 μg of glycerokinase and 0.01 mM ATP standard were mixed and measured as blank. Subsequently, 0.2 ml of sample or glycerol standard was added to the reaction mixture and the luminescence measured in a luminometer (EG & G Berthold LB 9501). The concentration of glycerol in the polymer degaradtion products was calculated against the glycerol standard curve. The changes in pH due to polymer degradation were assessed in parallel samples with the use of a pH meter.
2.6. Isolation and culture of bone marrow stromal cells (BMSC)
BMSC were obtained from 8 to 10 weeks old New Zealand white rabbits (Myrtle Labs, PA). Following euthanasia by intracardial injection of pentobarbital, a femur was excised aseptically, cleaned, and washed in TCM (RPMI 1640 containing 2 mM glutamine, 10% fetal calf serum, and 1% solution of penicillin (10 000 units/ml) and streptomycin (10 000 μg/ml). Subsequently, its metaphysis were removed and the marrow flushed from the bone with 5 ml TCM containing 10 units/ml heparin. The cells harvested were diluted in TCM, washed twice by centrifugation at 1100×g, and cultured in TCM containing 5 units/ml heparin at 37°C [10].
2.7. Culture of bone marrow stromal cells on the LDI–glycerol polymer
The polymer was washed 5 times each with sterile deionized water, 75% alcohol, and phosphate buffered saline (PBS). The polymer was left in PBS over night, followed by steam sterilization for 20 min at 121°C and 136 kPa pressure. Polymer was then washed in TCM and a total of 100 μl of TCM containing 3×105 BMSC were placed on the matrix. The polymer containing the cells was placed in a 6 well tissue culture plate and left undisturbed in an incubator for 4 h to allow the cells adherence. Subsequently, 1 ml TCM was gradually added in each well prior to replacing the cells in an incubator at 37°C, with 5% CO2 and 95% air. The cells were replenished with fresh TCM every 5 days.
Visualization of BMSC on the polymer was performed either by light or scanning electron microscopy. The polymer containing cells was fixed in 2.5% paraformaldehyde and 2% glutaraldehyde in PBS for 30 min. The polymer was rinsed and dehydrated in graded series of ethanol concentrations. For light microscopy the polymer was embedded in JB-4 embedding medium and sectioned at 2 μm thickness. For scanning electron microscopy, the polymer with or without cells was critically point dried and sputter coated with gold/palladium. The polymer was examined under a Joel scanning microscope with a accelerating voltage of 20 kV.
2.8. Determination of collagen type I production in BMSC grown on LDI–glycerol polymer and tissue culture polystyrene plates
To examine the collagen type I synthesis in BMSC grown on LDI–glycerol polymer, we assessed total hydroxyproline contents of the TCM supernatants of the cells. Cells were grown either on the tissue culture polystyrene (TCPS) plates or on the LDI–glycerol polymer for 5 days. Subsequently, 2 ml of TCM retrieved from each well of the cell culture plate was placed in an ampule, mixed with 2 ml of 6 N HCl, hydrolyzed at 100°C for 20 h. The reaction mixtures were then evaporated under reduced pressure to dryness and rehydrated with 2 ml water. The samples were then filtered through a syringe containing an HT tuffryn membrane (0.45 μM). A total of 0.2 ml of each sample was used to determine the concentration of hydroxyproline using the method described by Schwartz et al. [11]. The concentrations of hydroxyproline were calculated against a standard curve using medium as a blank, and P values calculated with Students’ t-test.
2.9. Comparison of mRNA expression for collagen type I, TGF-β1 and osteocalcin in cells cultured on LDI–glycerol polymer and TCPS
Following culture of BMSC on LDI–glycerol polymer or TCPS, cells were briefly washed with PBS, and RNA extracted with the use of RNA extraction kit (Qiagen Inc., Santa Clara, CA). A total of 0.5 μg of RNA was mixed with 1 μg oligo dT (12–18 oligomer; Perkin Elmer, Norwalk CT) in reverse transcription buffer and incubated for 10 min at room temperature. Thereafter, the reaction mixture was cooled on ice and incubated with 200 U of M-MLV reverse transcriptase for 60 min at 37°C. The cDNA, thus obtained, was amplified with 0.1 μg of specific primers in a reaction mixture containing 200 μM dNTP, and 0.1 units of Taq polymerase in PCR buffer (Perkin Elmer, Norwalk CT). PCR was performed in a DNA thermal cycler (Perkin Elmer, Norwalk CT) for 30 cycles of 40 s at 94°C, 40 s at 62°C, and 60 s at 72°C [12]. The sequence of sense and antisense primers used were as follows: rabbit GAPDH (293 bp) sense 5′TCACCATCTTCCAGGAGCGA3′, antisense 5′CACAATGCCGAAGTGGTCGT3′; human osteocalcin [13], sense 5′ATGAGAGCCCTCACACTCCTC 3′, and anti-sense 5′CGGGCCGTAGAAGCGCCGATA3′; rabbit TGF-β1 [14] (271 bp) sense 5′CGGCAGCTGTACATTGACTT, antisense 5′AGCGCACGATCATGTTGGAC; and rabbit collagen type I [15] (514 bp), sense 5′TCAACGGTGCTCCTGGTGAAG3′,antisense5GGACCTTGGCTACC CTGAGAA3′.
3. Results and discussion
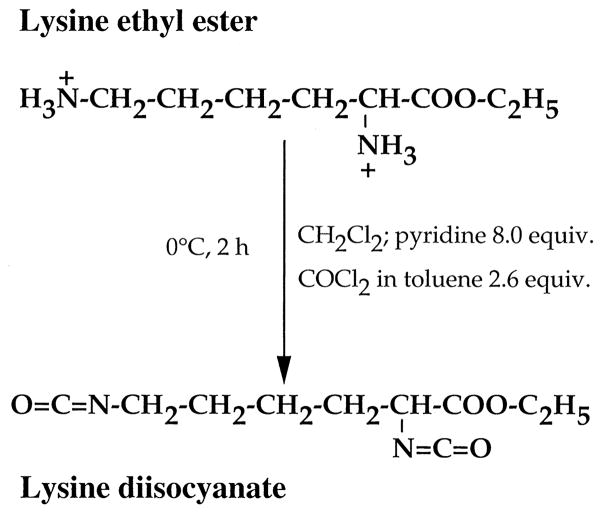
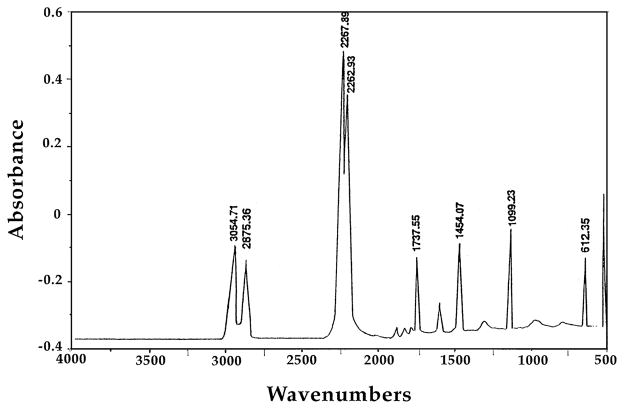
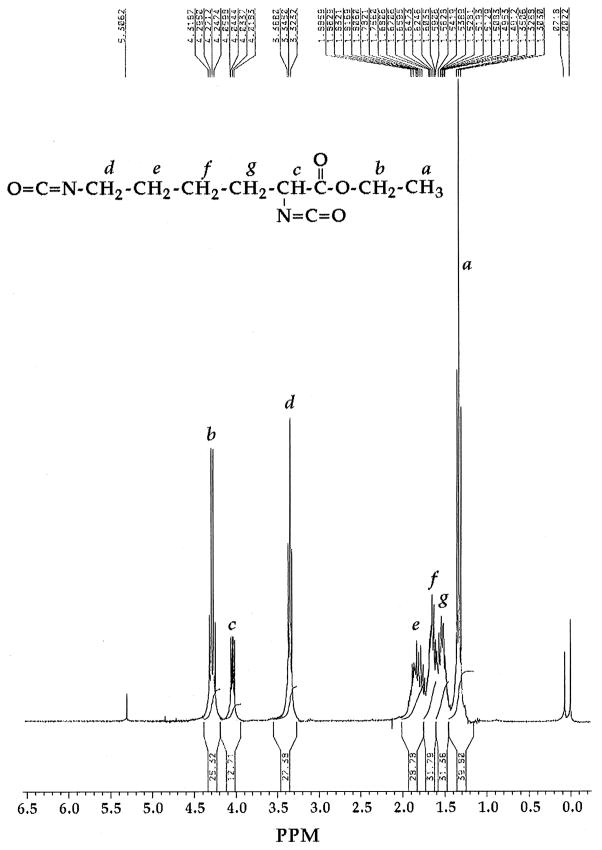
Lysine-di-isocyanate has been synthesized from lysine ethyl ester according to the scheme described in Fig. 1, providing a yield of 93% LDI. The IR spectrum exhibited characteristically strong peaks at 2260 to 2270 cm−1 associated with the isocyanate group (Fig. 2). Similarly, 1H NMR spectrum (Fig. 3) was also consistent with the structure of LDI (OCN–CH2–, δ = 3.34, 2H; OCN–CH–C(O), δ = 4.04, 1H; C(O)–O–CH2–, δ = 4.28, 2H; CH3, δ = 1.32, 3H; –CH2–, δ = 1.49–1.88, 6H). The yield of LDI obtained from this procedure is significantly higher than those previously described by Storey et al. [16] (10%), and Knolker et al. [17] (12%) using other methods. Furthermore, there was no evidence of contaminating dimers or trimers often observed during diisocyanate production. Thus LDI synthesized with this procedure was of adequate yield and purity for the synthesis of peptide-based prepolymers needed for the production of matrices with potential use in tissue engineering.
Fig. 1.
Schematic representation of the synthesis of LDI from lysine ethyl ester.
Fig. 2.
IR spectrum of the ethyl ester of LDI exhibiting absorbance of synthesized isocyanate at 2226 cm−1.
Fig. 3.
1H NMR spectrum demonstrating the purity and structure of LDI-ethyl ester.
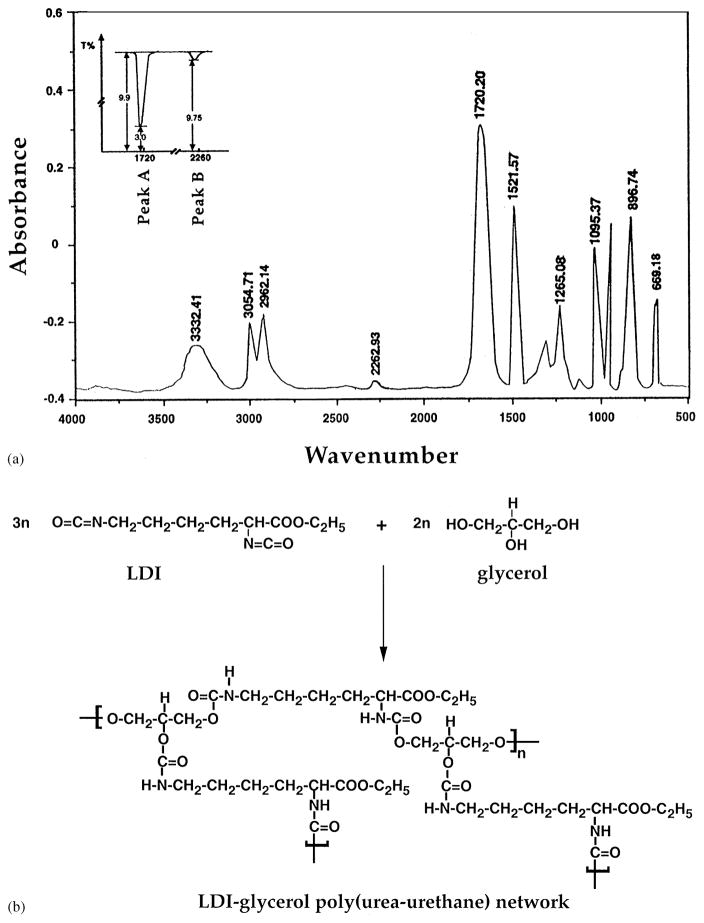
We next synthesized urethane prepolymer with the use of LDI and glycerol. The reaction of LDI and glycerol in a ratio of 1.60 : 1 for 7 days resulted in the formation of LDI–glycerol poly(urea–urethane), as demonstrated by the strong absorption band at approximately 1720 cm−1 in the IR spectrum (Fig. 4). This absorption was attributed to the formation of –NHCOO– group with concomitant quantitative disappearance of the isocyanate group (–NCO) at 2262 cm−1 during the reaction. Under these experimental conditions, FT-IR spectrum suggests that more than 98% LDI polymerized with glycerol (Fig. 4). The intensities of the peaks at 1720 cm−1 showed that only 1.26% free isocyanate remained in the reaction mixture (Fig. 4, inset). This is based on the following calculations: the absorption of the polymer peak A (–NHCOO–) = log 9.9/3.0 = 0.5185; while that of the isocyanate peak B (–NCO) = log 9.9/9.75 = 0.0063. If the concentration of the polymer is C1, and that of free isocyanate is C2, then C1/C2 = 0.5185/0.0063 = 78.2; in the reaction mixture, C1 + C2 = 1, if C1 = 98.74% then C2 = 1.26%.
Fig. 4.
(a) IR spectrum of LDI–glycerol prepolymer showing formation of urea linkages at 1720 cm−1 and concomitant disappearance of isocyanate from 2226 cm−1. Inset shows the % transmittance of urethane linkages formed at 1720 nm (Peak A = log 9.9/3.0 = 0.5185) and free isocyanate at 2260 nm (Peak B = log 9.9/9.75 = 0.0063). Accordingly, the reaction mixture contains 98.74% urethane and 1.26% free isocyanates; (b) schematic representation of LDI–glycerol (urea–urethane) synthesis and structure showing possible linkages of glycerol to LDI.
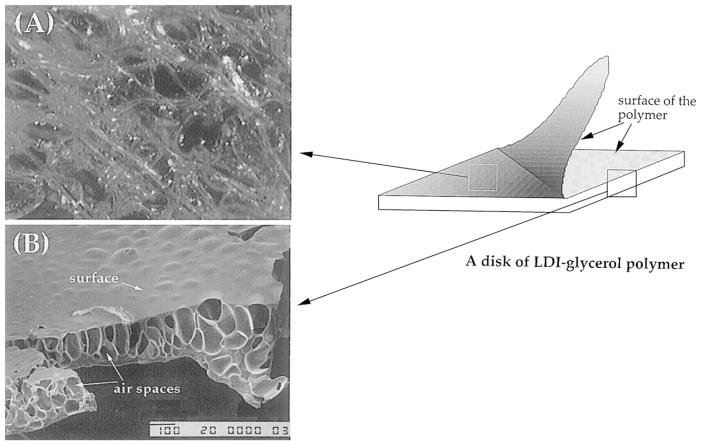
The addition of water to the LDI–glycerol prepolymer resulted in the formation of a foamed polymer. Beneath the surface, the polymerization of LDI and glycerol resulted in cross-link points forming a network of matrix (Fig. 5A). The scanning micrograph of the polymer showed that the surface topology of the foam was smooth with a cobblestone appearance. The cross-sectional view exhibited sponge-like cavities apparently formed due to the liberation of CO2 during foaming or polymerization process (Fig. 5B). The porosity of the polymer varied in various areas, with pore sizes ranging between 10 μm and 2 mm in diameter. The cross-sectional view of the polymer showed that not only the pores in the polymer provided a large surface area to support cell growth, these pores were interconnected to allow free fluid flow for circulation of nutrients and other metabolites (Fig. 5A and B). Thus structurally the LDI–glycerol polymer resembled the microporous polyurethanes presently being used in biomedical applications [1,18].
Fig. 5.
Morphology of the LDI–glycerol poly(urea–urethane): (A) a view of the network of matrix under the surface of polymer showing interconnected spaces in the matrix; and (B) a scanning electron micrograph exhibiting smooth surface topology and spongy internal structure with pores interconnected to support fluid flow.
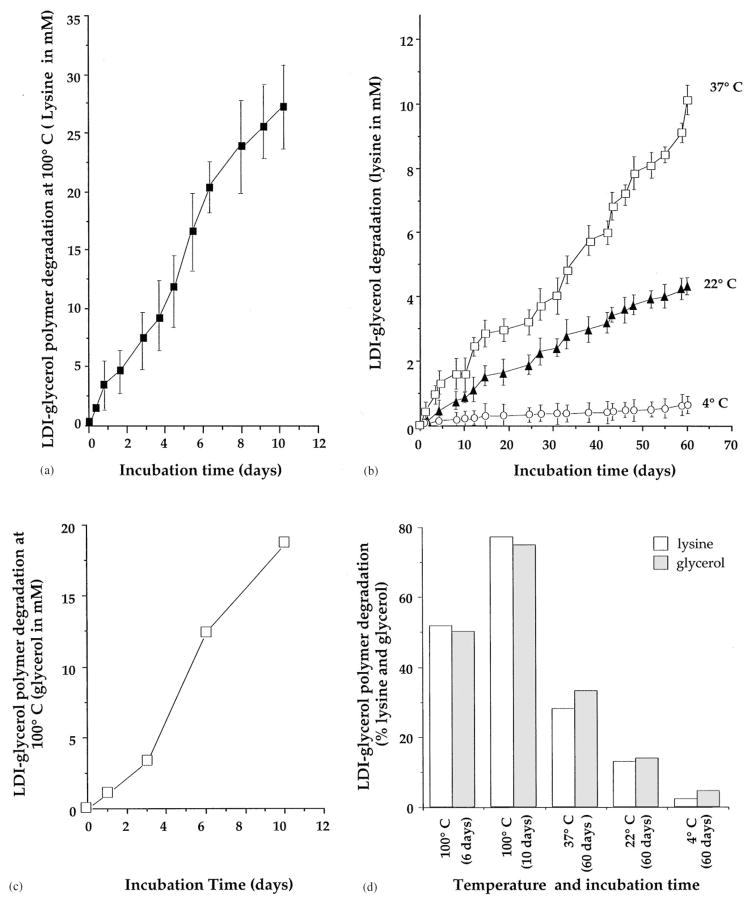
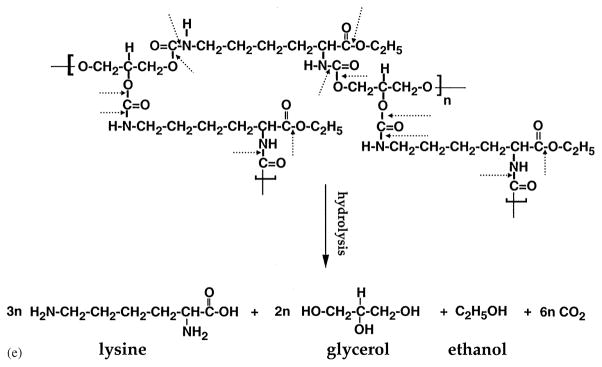
We have examined the rate of polymer degradation as a function of total lysine released in the PBS over a period of 60 days. The LDI–glycerol polymer matrix degraded in a temperature dependent manner. As shown in Fig. 6a, at 100°C approximately 77.2% of the lysine (10 mg/ml H2O) was recovered from the medium in 10 days, while about 2% degradation was observed during the first 6 h of treatment in boiling water. Thus, the LDI–glycerol polymer was thermally stable and exhibited minimal change when heated to 100°C for brief periods of time or sterilized by autoclaving for 20 min. The degradation rate of LDI–glycerol polymer was linear at 37°C and showed a consistent breakdown of polymer to yield 1.8 mM/10 days. The degradation rate at 22°C was more than 50% of that observed at 37°C, while at 4°C the total degradation of polymer was almost 95% less than that observed at 37°C (Fig. 6b). Further examination of the degradation products of LDI–glycerol polymer showed the presence of glycerol at concentrations similar to lysine at all temperatures tested (Fig. 6c and d). These results agree with the expected hydrolysis of the urethane bonds of LDI–glycerol polymer, which would result in the liberation of lysine, glycerol, ethnol, and CO2 (Fig. 6e). In these experiments the degradation rates were measured under slow agitation (rotation of 2 c/min). It has been shown that biodegradable polyurethanes synthesized with lactide or glycolide and ε-caprolactone joined by lysine-diisocyanates degrade twice as rapidly in vivo than in vitro [19]. Therefore, we suspect the degradation rate of LDI–glycerol polymer to be higher in vivo, due to the presence of circulating body fluids and physiologically active cells.
Fig. 6.
Degradation of LDI–glycerol polymer in aqueous solution at various temperatures and time intervals; (a) lysine (mM) release from LDI–glycerol polymer in PBS at 100°C over a period of 10 days; (b) lysine release from LDI–glycerol polymer in PBS at 37, 22 or 4°C over a period of 60 days; (c) release of glycerol from the LDI–glycerol polymer in PBS at 100°C over a period of 10 days; (d) comparitive assessment of lysine and glycerol release from LDI–glycerol polymer at different temperatures and time intervals; (e) schematic representation of LDI-glycerol hydrolytic degradation.
The degradation products of polymers made of poly-lactide and poly-glycolide create an acidic environment in vivo [20–22]. To examine whether the breakdown products of the LDI–glycerol polymer also change the pH of the surrounding solution, we measured the pH of the PBS containing 1 mg/ml polymer over a period of 60 days. We selected PBS as a medium because it is isotonic, and its buffering capacity and pH resemble those of biological fluids. As evident in Fig. 7, the degradation products of LDI–glycerol polymer did not affect the pH of PBS significantly at any temperature tested i.e., 37, 25 or 4°C (Fig. 7). The lack of change in the pH of PBS suggests that biodegradation of LDI–glycerol polymer may not create a low/high pH environment in the medium. Nevertheless, the possibility of pH changes in the immediate micro-environment of the polymer cannot yet be excluded.
Fig. 7.
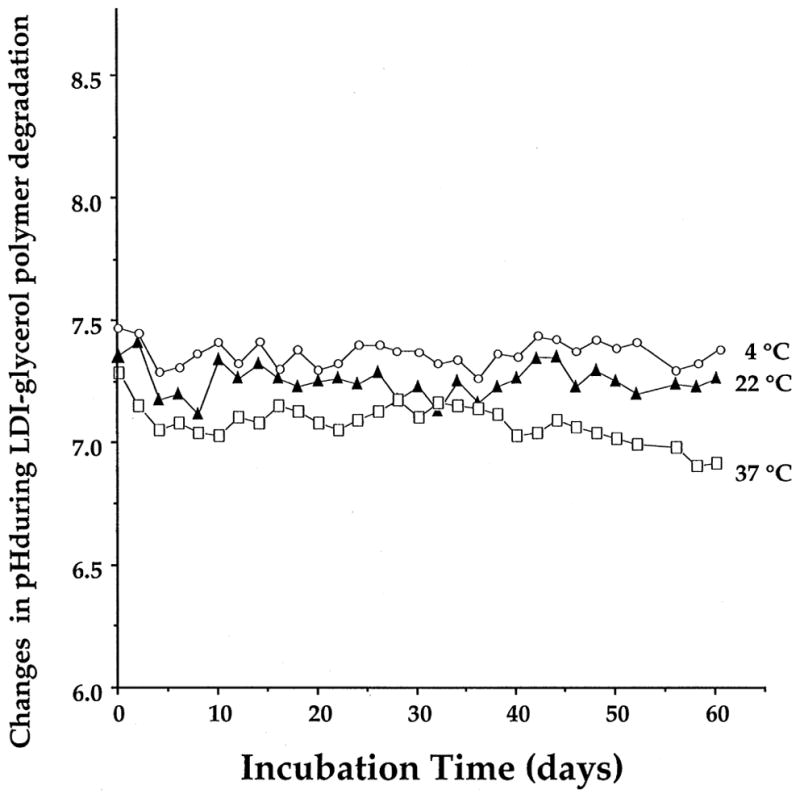
Effect of degradation products of LDI–glycerol polymer on the pH of PBS. The LDI–glycerol polymer was incubated at 37, 22 or 4°C with gradual mixing, over a period of 60 days, and the pH of the solution measured every 24 h.
The glass transition temperature (Tg) of a biologically suitable polymer must be high enough to provide stable structural rigidity during its use [1,2]. The assessment of the Tg of the LDI–glycerol polymer showed that its heat capacity does not change below 103.4°C (Fig. 8), suggesting its suitability for use in biological systems.
Fig. 8.
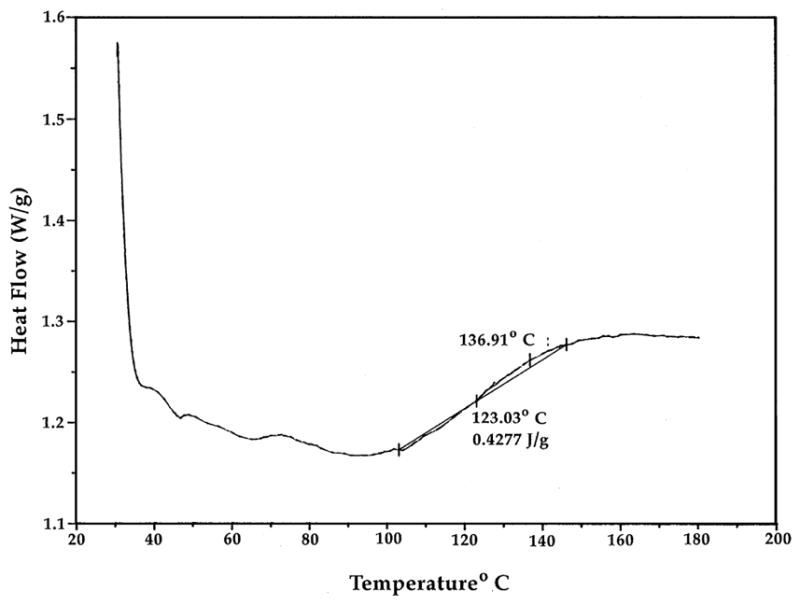
Determination of Tg of LDI–glycerol polymer. The LDI–glycerol polymer (5 mg) was dehydrated, and subjected to temperatures increasing at a rate of 10°C/min under constant N2 to assess the Tg.
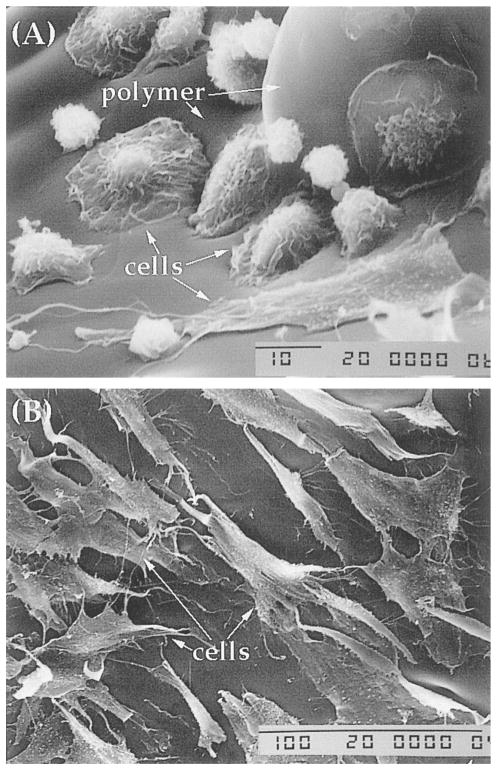
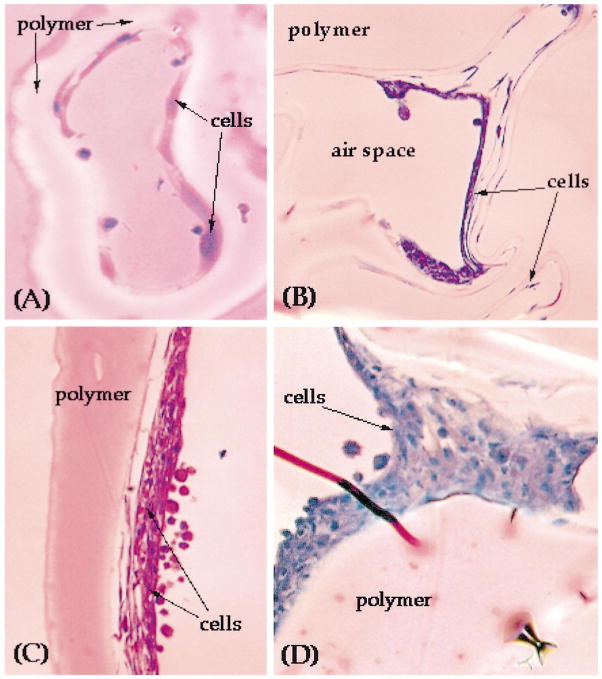
We next examined the ability of LDI–glycerol polymer to support cell growth. Scanning micrographs of cells cultured on LDI–glycerol polymer for various time intervals showed that following seeding, the cells spread on the polymer surface, and gradually adhere to polymer within few hrs (4–6 h; Fig. 9A). Whether the cells attach to the polymer through binding to lysine groups, or through fibronectin synthesized by cells for attachment is as yet not clear [23]. Continuous culture of BMSC on polymers for 7 days showed that BMSC retained their morphology similar to the cells grown on tissue culture polystyrene (TCPS; data not shown). The cells seeded on the surface of the polymer migrated in the pores of polymer suggesting that the porosity of the polymer is adequate for free fluid flow to support cell growth, and pores are interconnected to allow cell migration in vitro (Fig. 10A and B). More importantly, BMSC cells formed multiple layers of confluent cells in the pores of the foamed polymer over a period of 30 days, a typical characteristic of BMSC grown on TCPS (Fig. 10C and D). Nevertheless, only a fraction of cells (approximately 40%) seeded on the polymer remained on the polymer, while the remaining of the cells migrated to the polystyrene surface in the plate [11,24]. Since the cells that migrated to the TCPS from the polymer remained healthy and indicates that the polymer and its degradation products were not significantly toxic in vitro. In addition to the morphology, the growth rates of cells grown on LDI–glycerol polymer and TCPS were similar (Fig. 11a).
Fig. 9.
Assessment of BMSC attachment and growth characteristics following their culture on LDI–glycerol polymer or TCPS: (A) BMSC showing various stages of cell attachment to the polymer after a period of 6 h; (B) BMSC forming a monolayer on the polymer after 7 days of culture.
Fig. 10.
BMSC growth characteristics in LDI-polymer network: (A) a cross section of LDI–glycerol polymer showing BMSC cells growing on the surface of an air pocket following 1 week of culture; (B) BMSC forming multiple layers in the air pockets after 4 weeks of culture; (C) higher magnification of a multilayered nodule of BMSC shown in (B); (D) monolayer of cells growing on the surface of the air space following 4 weeks of culture.
Fig. 11.
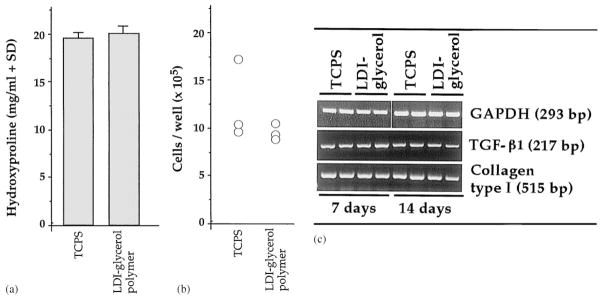
Comparison of phenotypic characteristics of BMSC grown on LDI–glycerol polymer and TCPS: (a) total hydroxyproline contents as a measure of synthesis and secretion of collagen type I in BMSC grown on LDI–glycerol polymeric foam and TCPS; (b) comparison of BMSC proliferation on LDI–glycerol and TCPS. BMSCs were grown on TCPS or LDI–glycerol poly(urea–urethane) for 7 or 14 days, were trypsinized and viable cells counted by trypan blue exclusion. Non-viable cells were less than 1% in each case; (c) comparison of mRNA expression for collagen type I (COL I) and TGF-β1 in BMSC cultured on LDI–glycerol polymer or TCPS for 7 or 14 days. Expression of mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a house keeping gene, was used as a control to show equivalent input of RNA in all lanes.
Collagen type I is hydroxyproline-rich protein secreted in significant concentrations by BMSC cells [23,24]. Since collagen type I contains 10–15% hydroxyproline and it is the major protein synthesized by BMSC, we measured hydroxyproline contents in the culture supernatents to compare collagen synthesis in BMSC cultured on LDI–glycerol polymer or on the TCPS. As shown in Fig. 11b, following 5 days of culture, synthesis of collagen type I was not significantly different in BMSC grown on LDI–glycerol polymer or on the TCPS, suggesting that the degradation of LDI–glycerol polymer does not alter the ability of BMSC to synthesize and secrete procollagen significantly. Additionally, we have examined the ability of BMSC to express mRNA for collagen type I and TGF-β1 following culture on LDI–glycerol polymer or TCPS. The BMSC grown LDI–glycerol polymer or TCPS expressed equivalent concentrations of mRNA for collagen type I and TGF-β1/μg total RNA, as assessed by RT/PCR (Fig. 11c).
In summary, our results demonstrate that we have synthesized a novel polymer with properties that may be useful in biomedical applications. This is based on the facts that: (1) LDI synthesized from lysine ethyl ester dihydrochloride obtained is of high purity and can be obtained in high yield for the preparation of large quantities of polymer. (2) LDI polymerized with glycerol yields a matrix which can be foamed in aqueous solutions. (3) LDI–glycerol polymer foam degrades in aqueous solutions, and its breakdown products are non-toxic and do not alter the pH of PBS. (4) LDI–glycerol foam exhibits Tg that is suitable for use in biological systems. (5) In vitro, LDI–glycerol polymer supports growth and phenotype of BMSC over a period of 30 days. The in vitro properties of the LDI–glycerol polymer suggest that this polymer may prove useful for biomedical applications.
Acknowledgments
The authors are indebted to Mr. Eric Krauland, Scott Beaver, and Ms. Feng Liu for their technical assistance. This work was supported by grants provided by Pittsburgh Tissue Engineering Initiative and Central Medical Research Funds, University of Pittsburgh.
References
- 1.Lamba MK, Woodhouse KA, Cooper SL. Polyurethanes in biomedical applications. Washington, DC: CRC Press; 1998. [Google Scholar]
- 2.Gogolewski S. Biomedical polyurethanes. In: Arshady R, editor. Desk reference of functional polymers. Washington, DC: Am Chem Soc; 1997. [Google Scholar]
- 3.Sinclair TM, Kerrigan CL, Buntic R. Biodegradation of the polyurethane foam covering of breast implants. Plast Reconstr Surg. 1993;92:1003–13. [PubMed] [Google Scholar]
- 4.McGill DB, Motto JD. An industrial outbreak of toxic hepatitis due to methylene-dianiline. New Engl J Med. 1974;291:278–82. doi: 10.1056/NEJM197408082910604. [DOI] [PubMed] [Google Scholar]
- 5.Benoit FM. Degradation of polyurethane foams used in the meme breast implant. J Biomed Mater Res. 1993;27:1341–8. doi: 10.1002/jbm.820271014. [DOI] [PubMed] [Google Scholar]
- 6.Planck H, Syre I, Danner M, Egbers G. Polyurethanes in biomedical engineering II. New York: Elsevier; 1987. [Google Scholar]
- 7.Nowick JS, Powell NA, Nguyen TM, Noronha G. An improved method for the synthesis of enantimerically pure amino acid ester isocyanates. J Org Chem. 1992;57:7364–6. [Google Scholar]
- 8.Beckwith AC, Paulis JW, Wall JS. Direct estimation of lysine in corn meals by the ninhydrin color reaction. J Agric Food Chem. 1975;23:194–6. doi: 10.1021/jf60198a015. [DOI] [PubMed] [Google Scholar]
- 9.Hellmèr J, Arner P, Arner L. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989;177:132–7. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 10.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–24. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz CE, Hellerqvist CG, Cunningham LW. Attaching human fibroblasts secrete a type I procollagen rich in 3-hydroxyproline. Biochem Biophys Res Commun. 1979;90:240–6. doi: 10.1016/0006-291x(79)91616-4. [DOI] [PubMed] [Google Scholar]
- 12.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanvich-Racic M, Piesco NP, Evans CH, Agarwal S. Cyclic tensile stress exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Fleet JC, Hock JM. Identification of Osteocalcin mRNA in non-osteoid tissue of rats and humans by reverse transcription—polymerase chain reaction. J Bone Miner Res. 1994;9:1565–73. doi: 10.1002/jbmr.5650091009. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang H, Wang W, Tahernia AD, Levitz CL, Luchetti WT, Brighton CT. Mechanical strain-induced proliferation of osteoblastic cells parallels increased TGF-β1 mRNA. Biochem Biophys Res Commun. 1996;229:449–53. doi: 10.1006/bbrc.1996.1824. [DOI] [PubMed] [Google Scholar]
- 15.Hart DA, Sciore P, Boykiw R, Reno C. Pregnancy induces complex changes in the pattern of mRNA expression in knee ligaments of the adolescent rabbit. Matrix Biol. 1998;17:21–34. doi: 10.1016/s0945-053x(98)90122-6. [DOI] [PubMed] [Google Scholar]
- 16.Storey RF, Wiggins JS, Mauritz KA. Bioabsorbable composites. II: nontoxic, L-lysine-based poly(ester-urethane) matrix composites. Polymer Comp. 1993;14:17–25. [Google Scholar]
- 17.Knolker HJ, Braxmeier T, Schlechtingen G. A novel method for the synthesis of isocyanates under milder conditions. Angew Chem Int Ed Engl. 1995;34:2497–500. [Google Scholar]
- 18.Zhang Z, King MW, Guidoin R, Therrien M, Pezelot M, Adnot A, Ukpabi P, Vantal MH. Morphological, physical and chemical evaluation of the Vascugraft arterial prosthesis: comparison of a novel polyurethane device and other microporous structures. Biomaterials. 1994;15:483–92. doi: 10.1016/0142-9612(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 19.Bruin P, Veenstra GJ, Nijenhuis AJ, Pennings AJ. Design and synthesis of biodegradable poly(ester–urethane) elastomer networks composed of non-toxic building blocks. Makromol Chem. 1988;9:589–97. [Google Scholar]
- 20.Anderson JM. Biomedical applications of synthetic biodegradable polymers. Boca Raton: CRC Press; 1995. Perspectives on the in vivo responses of biodegradable polymers; pp. 223–33. [Google Scholar]
- 21.Athanasiou KA, Niederauer EG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical application of polylactic acid/polyglycolic acid co-polymer. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 22.Penco M, Marcioni S, Ferruti P, D’Antone S, Deghenghi R. Degradation behaviour of block copolymers containing poly(lactic-glycolic acid) and poly(ethylene glycol) segments. Biomaterials. 1996;17:1583–90. doi: 10.1016/0142-9612(95)00323-1. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto-Iwamoto M, Iwamoto M, Nakashima K, Mukudai Y, Boettiger D, Pacifici M, Kurisu K, Suzuki F. Involvement of α5β1 integrin in matrix interactions and proliferation of chondrocytes. J Bone Miner Res. 1997;12:1124–32. doi: 10.1359/jbmr.1997.12.7.1124. [DOI] [PubMed] [Google Scholar]
- 24.Martinez ME, Medina S, del Campo MT, Sanchez-Cabezudo MJ, Sanchez M, Munuera L. Effect of polyethylene on osteocalcin, alkaline phosphatase and procollagen secretion by human osteoblastic cells. Calc Tiss Int. 1998;62:453–6. doi: 10.1007/s002239900459. [DOI] [PubMed] [Google Scholar]