Abstract
Objective
The mechanisms by which chondrocytes convert biomechanical signals into intracellular biochemical events are not well understood. In this study, we sought to determine the intracellular mechanisms of the magnitude-dependent actions of mechanical signals.
Methods
Chondrocytes isolated from rabbit articular cartilage were grown on flexible membranes. Cells were subjected to cyclic tensile strain (CTS) of various magnitudes in the presence or absence of interleukin-1β (IL-1β), which was used as a proinflammatory signal for designated time intervals. The regulation of NF-κB was measured by reverse transcriptase–polymerase chain reaction, electrophoretic mobility shift assay, and immunofluorescence.
Results
CTS of low magnitudes (4–8% equibiaxial strain) was a potent inhibitor of IL-1β–dependent NF-κB nuclear translocation. Cytoplasmic retention of NF-κB and reduction of its synthesis led to sustained suppression of proinflammatory gene induction. In contrast, proinflammatory signals generated by CTS of high magnitudes (15–18% equibiaxial strain) mimicked the actions of IL-1β and induced rapid nuclear translocation of NF-κB subunits p65 and p50.
Conclusion
Magnitude-dependent signals of mechanical strain utilize the NF-κB transcription factors as common elements to abrogate or aggravate proinflammatory responses. Furthermore, the intracellular events induced by mechanical overload are similar to those that are initiated by proinflammatory cytokines in arthritis.
The pathology of osteoarthritis (OA) is associated with excessive mechanical load and trauma experienced by the joint tissue, and the inability of this tissue to tolerate that stress (1,2). In response to mechanical loading, articular chondrocytes are exposed to compressive, tensile, and shear forces (3,4). These cells have the necessary signaling and effector mechanisms to sense and react to applied mechanical forces by mounting a stream of cellular responses such as proliferation, matrix catabolism, and matrix synthesis (5–9). An accumulating body of evidence suggests that mechanical signaling plays a key role in regulating cartilage damage and repair. Exposure of cartilage to mechanical strain of high magnitudes leads to inflammation and synthesis of mediators of tissue destruction, such as interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), inducible nitric oxide synthase (iNOS), and matrix metalloproteinases (2,9–12). These mediators augment matrix degradation and inhibit the synthesis of matrix-associated proteins (10,11,13). In contrast, lower levels of tensile forces induce antiinflammatory and anabolic responses (12,14,15). The intriguing question is how cartilage cells adapt to mechanical loading, i.e., how intracellular signals generated by tensile strain of high magnitudes manifest themselves as proinflammatory responses.
The signals induced by proinflammatory cytokines such as IL-1β and TNFα are transmitted to the nucleus through activation of kinase cascades that lead to phosphorylation, ubiquitination, and ultimate degradation of the inhibitor of NF-κB (IκB), a protein that sequesters NF-κB in the cytoplasm (16–20). Upon release from IκB, NF-κB, a multifunctional transcription factor, translocates to the nucleus, where it binds to consensus sequences of several proinflammatory genes to initiate their expression (16–18). Mechanosensing in chondrocytes is closely related to inflammatory gene induction (11). Furthermore, NF-κB inhibition blocks bone erosion associated with inflammatory arthritis (21). Therefore, we speculated that pro- and antiinflammatory actions of cyclic tensile strain (CTS) are mediated by NF-κB transcription factors that are utilized by most inflammation mediators in arthritis. In this study, we found that chondrocytes perceive signals generated by tensile strain and respond to them in a magnitudedependent manner. Furthermore, the antiinflammatory effects of CTS of low magnitudes and the proinflammatory effects of CTS of high magnitudes are both regulated by the NF-κB signal transduction pathway.
MATERIALS AND METHODS
Cell culture and materials
Chondrocytes were isolated from the 150–200-µm-thick superficial layer of articular cartilage from 14–16-week-old female NZW rabbits, as previously described (14,15,22). Briefly, cartilage pieces were minced in Hank’s balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) and treated with 2.5% trypsin for 10 minutes. Subsequently, the tissue was transferred to 1% collagenase (Worthington, Lakewood, NJ) in HBSS and incubated at 37°C for 2 hours. The cells were then centrifuged at 800g for 10 minutes. The pellet containing chondrocytes was washed twice with HBSS, counted, and the cells were cultured in Ham’s F-12 medium (Mediatech, Herndon, VA) supplemented with 10% defined fetal calf serum (Hyclone, Logan, UT), 2 mM l-glutamine (Invitrogen), 100 units/ml penicillin (Mediatech), and 100 µg/ml streptomycin (Mediatech). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Chondrocytes that retained their phenotype as shown by the expression of aggrecan, type II collagen, and biglycan synthesis were used during the first 3 passages (22).
Application of cyclic equibiaxial strain
To study the effects of tensile forces in vitro, chondrocytes (5 × 105/well) were grown on pronectin-coated Bioflex II 6-well culture plates (Flexcell International, Hillsborough, NC) to 80% confluence (7–8 days). Various magnitudes of cyclic equibiaxial radial strain were applied to the cells at a rate of 0.05 Hz by a Flexercell strain unit (Flexcell International). After loading of the plates on a station (located in an incubator at 5% CO2 with 95% humidity), a vacuum deformed the membrane across the postface to create uniform biaxial strain. The strain was calculated as circumferential strain = 2π(change in radius)/2π(original radius) = (change in radius)/(original radius) = radial strain. The relationship between vacuum level and strain was linear. Cells grown on Bioflex II plates were assigned to 4 different treatment regimens: 1) untreated controls, 2) cells treated with recombinant human IL-1β (rHuIL-1β; EMD Biosciences, San Diego, CA), 3) cells treated with CTS, or 4) cells treated with rHuIL-1β and CTS.
Production of NO
NO production was measured based upon the Griess reaction, as previously described (14,23).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Extraction of RNA was performed with an RNA extraction kit according to the manufacturer’s recommended protocols (Qiagen, Santa Clara, CA). A total of 1.0 µg of RNA was reverse transcribed with 200 units of Moloney murine leukemia virus reverse transcriptase (Invitrogen) at 42°C for 25 minutes followed by 65°C for 5 minutes. Complementary DNA was amplified with 0.1 µg of specific primers in a reaction mixture (PCR supermix; Invitrogen) containing Taq DNA polymerase, Tris HCl, potassium chloride, magnesium chloride, and deoxynucleoside triphosphates. Amplification was carried out for 30 cycles of 45 seconds at 94°C, 45 seconds at 59°C, and 60 seconds at 72°C with an Eppendorf DNA thermal cycler (Brinkmann, Westbury, NY). The sequence of sense and antisense rabbit primers was as follows: for GAPDH (293 bp), sense 5′-TCACCATCTTCCAGGAGCGA-3′ and antisense 5′-CACAATGCCGAAGTGGTCGT-3′; for iNOS (243 bp), sense 5′-CGCCCTTCCGCAGTTTCT-3′ and antisense 5′-TCCAGGAGGACATGCAGCAC-3′; and for NF-κB p65 (186 bp), sense 5′-CACTGCCGAGCTCAAGATCTGCC-3′ and antisense 5′-GTCGGCGTACGGAGGAGTCCG-3′. The bands of ethidium bromide–stained DNA products on agarose gels were photographed and digitized with a Kodak Imager 1000 (Perkin Elmer, Emeryville, CA). The images were subjected to densitometric analysis and standardized with PCR products of GAPDH as an internal control. In some experiments, cells were incubated with various concentrations of caffeic acid phenethyl ester (CAPE; EMD Biosciences), a cell-permeable inhibitor of NF-κB, for 10 minutes to inhibit the nuclear translocation of NF-κB, and then subjected to CTS as described above.
Electrophoretic mobility shift assay (EMSA)
To determine the nuclear translocation of NF-κB, an EMSA was performed, as previously described (24). Briefly, nuclear extracts (4 µg) were incubated at 37°C for 15 minutes with 8 fmoles of 32P end-labeled, 45-mer, double-stranded NF-κB oligonucleotide containing the NF-κB binding site (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′) (Stratagene, La Jolla, CA) from the human immunodeficiency virus long terminal repeat. The DNA protein complex was separated from free oligonucleotide on 6% native polyacrylamide gels, and the specificity of binding was analyzed by competition with unlabeled oligonucleotide. Prior to analyzing the complexes by EMSA, the nuclear extracts were incubated with preimmune serum or antibodies against the NF-κB components p50, p52, p65, RelB, or c-Rel at room temperature for 30 minutes. The binding of NF-κB to its consensus sequences was visualized in dried gels, and the bands were quantitatively analyzed by scintillation counting.
Western blot analysis
Determination of cytoplasmic NF-κB proteins was performed by Western blot analysis. NF-κB was analyzed from cytoplasmic extracts of cells (2 × 106) subjected to the regimens described above and resolved on sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gels under reducing conditions. After electrophoresis, the proteins were electrotransferred to Immunolon membranes (New England Nuclear, Boston, MA), blocked with 5% nonfat dry milk, probed with rabbit anti–NF-κB p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and detected with horseradish peroxidase (HRP)–conjugated goat antirabbit IgG (Santa Cruz Biotechnology). To visualize the NF-κB bands, Western Lightening chemiluminescence reagent (Perkin Elmer, Boston, MA) was used as a substrate for HRP. The bands were semiquantitatively assessed by densitometric analysis using the Fluor-S Max imaging system (Bio-Rad, Hercules, CA).
Immunofluorescence
Nuclear translocation of NF-κB was analyzed by immunofluorescence using rabbit anti–NF-κB p65 IgG (Santa Cruz Biotechnology) and Cy3-conjugated goat anti-rabbit IgG (The Jackson Laboratory, Bar Harbor, ME). Phalloidin–fluorescein isothiocyanate was used as a counterstain to visualize F-actin (Santa Cruz Biotechnology). The cells adhered to Bioflex membranes were mounted in phosphate buffered saline with 20% glycerol. The cells were observed under 20× or 40× objectives with a BX50 epifluorescence microscope (Olympus, Lake Success, NY). The images were captured with an air-cooled camera and Magnafire image-capturing software (Olympus).
RESULTS
Magnitude of CTS determines its antiinflammatory or proinflammatory actions on chondrocytes
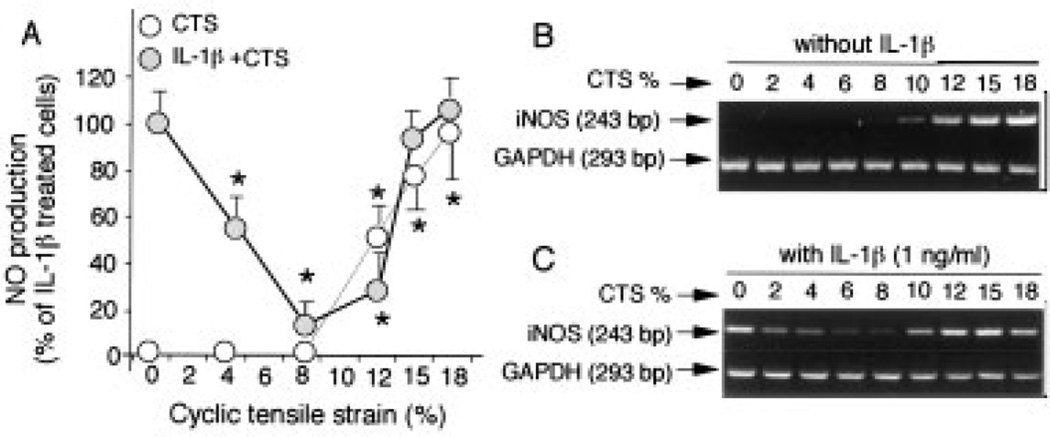
IL-1β is intricately involved in the pathogenesis of OA as well as rheumatoid arthritis. Since IL-1β up-regulates multiple proinflammatory genes such as iNOS, we used IL-1β–dependent NO production to probe the intracellular target sites of CTS in the IL-1β signal transduction pathway. As shown in Figure 1A, under normal cell conditions, accumulation of NO was not observed in the culture supernatants of control cells at 24 hours. The effects of CTS on chondrocytes were magnitude dependent. Over a period of 24 hours, NO production was not induced by lower magnitudes (4% and 8%) of CTS. In contrast, exposure of chondrocytes to CTS of higher magnitudes (12%, 15%, and 18%) resulted in an increased production of NO. As expected, IL-1β treatment also induced a significant up-regulation of NO synthesis (Figure 1A). Coexposure of chondrocytes to CTS of lower magnitudes and IL-1β resulted in a magnitude-dependent inhibition (P ≤ 0.05) of the IL-1β–induced NO production. The inhibitory effect of CTS on IL-1β–induced NO production was decreased at 12% CTS but was still significant.
Figure 1.
Magnitude-dependent response of chondrocytes to mechanical signals. A, Regulation of nitric oxide (NO) production by various magnitudes of cyclic tensile strain (CTS) in the presence and absence of interleukin-1β (IL-1β) (1.0 ng/ml). Accumulation of NO in the culture supernatants was assessed after 24 hours. Values are the mean and SEM of triplicate determinations. * = P ≤ 0.05 between unstretched cells and cells subjected to CTS in the absence and presence of IL-1β, by Student’s t-test. B and C, The regulation of inducible NO synthase (iNOS) mRNA expression by various magnitudes of CTS was determined in B, the absence and C, the presence of IL-1β (1.0 ng/ml). Expression of iNOS mRNA was measured by reverse transcriptase–polymerase chain reaction after 4 hours. Representative results from 1 of 3 separate experiments are shown.
To determine if the effects of CTS of lower magnitudes were perceived at the transcriptional level, we measured the IL-1β–induced iNOS messenger RNA (mRNA) expression in the presence of CTS of various magnitudes at 4 hours. Unstretched chondrocytes and cells exposed to CTS of lower magnitudes did not show iNOS mRNA expression (Figure 1B). Interestingly, iNOS mRNA expression was markedly up-regulated with CTS at 10–18%. IL-1β (1.0 ng/ml) induced significant levels of iNOS mRNA expression (Figure 1C). More important, CTS of low magnitudes (CTS-L; 2–8%) strongly suppressed the IL-1β–dependent iNOS mRNA expression. CTS of 10% and 12% was less effective in inhibiting iNOS mRNA induction, but the effect was still significant. CTS of high magnitudes (CTS-H; 15% and 18%) did not suppress iNOS mRNA expression. Collectively, these observations suggested that mechanical signals act on chondrocytes in a magnitude-dependent manner. Additionally, the intracellular target sites of both CTS-L and CTS-H lie upstream of iNOS mRNA expression and may involve proinflammatory pathways similar to those regulated by IL-1β.
Inhibition of IL-1β–induced NF-κB gene expression by CTS-L
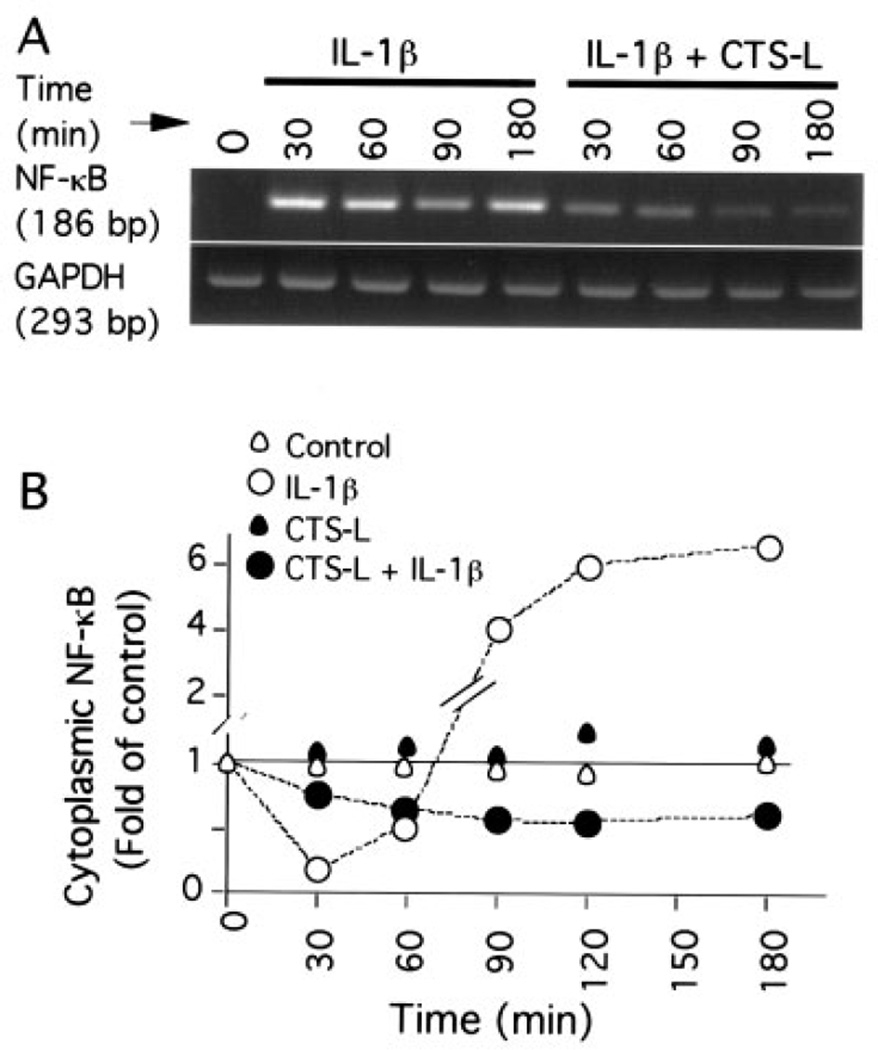
We next investigated the nuclear translocation of NF-κB, the key transcription factor involved in signal transduction of proinflammatory cytokines, to explore the intracellular mechanisms by which CTS-L attenuates IL-1β–induced proinflammatory responses. Analysis by EMSA did not reveal nuclear translocation of NF-κB in controls and cells treated with CTS-L (6%) over a period of 15–90 minutes (Figure 2A). Rapid nuclear translocation of NF-κB was observed within 15 minutes of IL-1β treatment, and a further 6.2-fold increase in the nuclear NF-κB was apparent after 90 minutes (Figure 2B). In contrast, cells treated simultaneously with CTS-L and IL-1β exhibited a time-dependent suppression of the IL-1β–induced NF-κB nuclear translocation (Figures 2A and B). Quantitative assessment of EMSA gels revealed that >98% of the IL-1β–induced nuclear translocation of NF-κB was inhibited by CTS-L at 60 minutes, and the inhibitory effect was sustained over the next 30 minutes.
Figure 2.
Abrogation of interleukin-1β (IL-1β)–induced nuclear translocation of NF-κB by cyclic tensile strain of low magnitudes (CTS-L). A, Nuclear proteins of untreated cells (control) and cells treated for 15, 30, 60, or 90 minutes with IL-1β (1.0 ng/ml) and/or CTS-L (6%) were extracted. Subsequently, the presence of NF-κB in the nuclear extract was determined by electrophoretic mobility shift assay (EMSA). B, Quantitative analysis of net 32P associated with each band from the EMSA gel shown in A was performed by scintillation counting to assess NF-κB nuclear translocation. C, NF-κB subunits involved in the actions of CTS-L were analyzed by supershift EMSA (SS-EMSA) using antibodies against p65 (RelA) and p50 subunits of NF-κB. D, Chondrocytes were treated for 30, 60, 120, or 180 minutes with IL-1β (1.0 ng/ml) and/or CTS-L (6%). Untreated cells were used as controls. Nuclear translocation of NF-κB was assessed by immunofluorescence staining. NF-κB was stained with rabbit anti–NF-κB p65 IgG, with Cy3-conjugated goat anti-rabbit IgG as secondary antibody (red). Cellular β-actin was stained with fluorescein isothiocyanate–conjugated phalloidin (green). Representative results from 1 of 3 separate experiments are shown.
NF-κB consists of heterodimers or homodimers of various types of Rel proteins. The composition of these dimers is important for the regulation of proinflammatory gene expression. Therefore, we next examined the subunit structure of NF-κB involved in the actions of CTS-L. IL-1β induced nuclear translocation of NF-κB heterodimers composed of p65 and p50 subunits. CTS-L directly inhibited the IL-1β–induced nuclear translocation of NF-κB, as evidenced by a 66% reduction in nuclear p65 and p50 subunits by supershift EMSA (Figure 2C). CTS-L did not induce nuclear translocation of RelB, p52, or c-Rel (results not shown).
Using immunofluorescence, we further confirmed the CTS-L–induced inhibition of NF-κB nuclear translocation. These experiments revealed that CTS-L inhibited NF-κB activation by its sequestration in the cytoplasm. IL-1β–activated chondrocytes exhibited translocation of NF-κB to the nucleus within 30 minutes. During the ensuing 150 minutes, a further increase of NF-κB was observed, mainly in the nuclear compartment, which was also accompanied by an increase in the cytoplasmic chamber (Figure 2D). However, cells exposed to CTS-L (6%) and IL-1β simultaneously exhibited cytoplasmic retention of NF-κB during the first 30 minutes. Thereafter, the effect of CTS-L on the IL-1β–dependent nuclear translocation of NF-κB resulted in almost total inhibition during the next 150 minutes. Treatment of cells with 6% CTS alone did not induce nuclear translocation of NF-κB, with results found to be similar to those in untreated control cells.
Examination by RT-PCR of NF-κB mRNA expression revealed that the IL-1β–induced nuclear translocation of NF-κB was followed by a time-dependent increase in NF-κB mRNA synthesis (Figure 3A). Semiquantitative assessment of PCR products revealed that CTS-L markedly inhibited 92% of the IL-1β–induced NF-κB p65 mRNA expression within 30 minutes, and this inhibition increased to 98% over the next 150 minutes. Densitometric analysis of Western blots of cytoplasmic NF-κB revealed that IL-1β treatment resulted in a near total depletion of NF-κB p65 from cytoplasm within the first 30 minutes (Figure 3B). Nevertheless, cytoplasmic NF-κB was rapidly replenished during the next 150 minutes, with a >6-fold increase over that present in untreated control cells. The densitometric analysis also showed that IL-1β failed to induce up-regulation of NF-κB p65 in the presence of CTS-L (6%); instead, a nearly 30% reduction in NF-κB was observed over the period of 180 minutes when compared with untreated control cells. Control cells and cells exposed to CTS-L alone did not exhibit significant changes in NF-κB p65 levels during the experiment. These observations are consistent with the results of the immunofluoresence analysis shown in Figure 2D, in which IL-1β induced a dramatic time-dependent increase in cytoplasmic NF-κB p65.
Figure 3.
Inhibition of IL-1β–induced NF-κB mRNA expression and NF-κB synthesis by CTS-L. A, IL-1β–induced NF-κB p65 mRNA expression over a period of 30–180 minutes in the absence and presence of CTS-L (6%) was analyzed by reverse transcriptase–polymerase chain reaction. B, Cytoplasmic NF-κB p65 in untreated cells and cells treated with IL-1β (1.0 ng/ml) and/or CTS-L (6%) over a period of 30–180 minutes was determined by densitometric analysis of Western blots. Representative results from 1 of at least 3 separate experiments are shown. See Figure 2 for definitions.
Involvement of NF-κB nuclear translocation and synthesis in proinflammatory gene induction by CTS-H
We next examined whether CTS-H also utilizes the NF-κB signaling pathway for its proinflammatory actions. The time course of NF-κB nuclear translocation, as determined by EMSA, revealed that CTS-H (15%) or treatment with IL-1β resulted in a progressive increase in nuclear NF-κB accumulation over a period of 30–180 minutes (Figure 4A). RT-PCR analysis of cells exposed to CTS-H (15%) showed that nuclear translocation of NF-κB was paralleled by a time-dependent up-regulation of NF-κB mRNA expression (Figure 4B). CTS-H induced NF-κB mRNA expression within 30 minutes, and a further 2.8-, 5.8-, and 6.4-fold increase in the NF-κB mRNA expression was found at 60, 120, and 180 minutes, respectively, by densitometric analysis.
Figure 4.
Induction of NF-κB nuclear translocation and synthesis by CTS of high magnitudes (CTS-H). A, Nuclear proteins of untreated cells (control) and cells treated for 30, 60, 120, or 180 minutes with IL-1β (1.0 ng/ml) and/or CTS-H (15%) were extracted. Subsequently, the presence of NF-κB in the nuclear extract was determined by EMSA. Quantitative analysis of net 32P associated with each band determined by EMSA was performed to assess NF-κB nuclear translocation. Each point is the mean of triplicate values. B, NF-κB p65 mRNA expression induced by CTS-H (15%) and/or IL-1β (1.0 ng/ml) over a period of 180 minutes was analyzed by reverse transcriptase–polymerase chain reaction. C, NF-κB subunits involved in CTS-H actions (15%, 30 minutes) were analyzed by supershift EMSA using antibodies against p65, p50, p52, RelB, and c-Rel subunits of NF-κB. D, Immunofluorescence staining of chondrocytes subjected to CTS-H (15%) for 30, 60, or 120 minutes was performed in the presence or absence of IL-1β (1.0 ng/ml). Untreated cells were used as controls. NF-κB was stained with rabbit anti–NF-κB p65 IgG, with Cy3-conjugated goat anti-rabbit IgG as secondary antibody (red). Cellular β-actin was stained by fluorescein isothiocyanate–conjugated phalloidin (green). Representative results from 1 of 3 separate experiments are shown. See Figure 2 for other definitions.
Similar to IL-1β, the heterodimers of NF-κB that translocated to the nucleus in response to CTS-H were composed of p65 and p50 subunits. CTS-H did not induce a nuclear translocation of RelB, p52, or c-Rel, as revealed by supershift EMSA (Figure 4C). Immunofluorescence analysis confirmed that CTS-H induced a rapid and sustained nuclear translocation of NF-κB between 30 and 120 minutes, which was also paralleled by an increase of NF-κB in the cytoplasm (Figure 4D).
To further confirm that CTS-H actions were mediated by NF-κB, chondrocytes were either untreated or treated with CAPE for 10 minutes prior to being subjected to CTS-H (15%). Quantitative assessment of EMSA gels demonstrated that CAPE (100 µM) inhibited 53% of CTS-H–induced nuclear translocation of NF-κB within 30 minutes and led to near-complete inhibition by 60 minutes (Figure 5A). Additionally, CAPE inhibited the ability of NF-κB to drive CTS-H–induced transcription of iNOS mRNA at 4 hours, in a dose dependent manner (5, 25, 50, and 100 µM) (Figures 5B and C). In parallel experiments, iNOS expression induced by IL-1β and/or CTS-H was also inhibited by CAPE.
Figure 5.
Abrogation of CTS of high magnitudes (CTS-H)–induced nuclear translocation of NF-κB by caffeic acid phenethyl ester (CAPE). A, Induction of CTS-H–dependent nuclear translocation of NF-κB was inhibited by CAPE over a period of 30–180 minutes. Chondrocytes were either untreated or were treated with CAPE (100 µM) for 10 minutes prior to being subjected to CTS-H (15%) in the presence or absence of IL-1β (1.0 ng/ml). Subsequently, the presence of NF-κB in the nuclear extract was determined by EMSA. The radioactivity associated with bands determined by EMSA was measured with a scintillation counter. Values are the mean and SEM of triplicate determinations. B, Effect of various concentrations of CAPE on inducible nitric oxide synthase (iNOS) mRNA expression induced by IL-1β and/or CTS-H. Chondrocytes were exposed to various concentrations of CAPE (0, 5, 25, 50, or 100 µM) for 10 minutes prior to being treated with IL-1β (1.0 ng/ml) and/or CTS-H (15%) for 4 hours. The iNOS mRNA expression was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR). C, Densitometric analysis of the RT-PCR gels shown in B. Representative results from 1 of 3 separate experiments are shown. See Figure 2 for other definitions.
DISCUSSION
The mechanisms by which chondrocytes convert biomechanical signals into intracellular events have become an area of intense interest in orthopedic research. The results presented here describe intracellular mechanisms by which biomechanical signals are converted into biochemical events. Our foremost findings are that chondrocytes perceive mechanical signals and respond to them in a magnitude-dependent manner. Mechanical signals of low magnitude (2–8% CTS) were not perceived as inflammatory signals and did not affect iNOS or NO synthesis. However, the actions of CTS-L were evident in the presence of IL-1β, i.e., CTS-L directly abolished the actions of inflammatory insults by inhibiting IL-1β–induced iNOS mRNA expression and NO production. CTS-L also inhibits chondrocytic transcription of a number of other proinflammatory genes, such as cyclooxygenase 2 and matrix metalloproteinases (15,22). Exposure of chondrocytes to 15% dynamic compression also inhibits IL-1β–induced prostaglandin E2 production in chondrocytes in vitro (25). In vivo, during normal movement, articular chondrocytes experience compression loads of 15%, which leads to 5% elongation of chondrocytes. Collectively, these observations suggest that signals generated by CTS-L may be equivalent to those experienced by compressive loading of chondrocytes in vivo.
In contrast, CTS-H (15–18%) is associated with proinflammatory gene expression, as evidenced by enhanced iNOS mRNA expression and NO production. Mechanical signals of high magnitudes also markedly up-regulate matrix degradation and decrease matrix synthesis (1,2,6,7). Since these actions of mechanical strain can also be induced by IL-1β or TNFα (10,11,13,19,20), CTS-H and proinflammatory mediators appear to exert similar effects to up-regulate inflammatory responses. These findings further suggest that the magnitude of mechanical strain is a critical determinant of chondrocytic responses.
The next most striking finding is that the NF-κB signal transduction pathway is central to the proinflammatory and antiinflammatory actions of mechanical strain. NF-κB transcription factors have an established role in cytosolic signaling of proinflammatory cytokines through their nuclear translocation and subsequent transactivation of a plethora of genes (16,18). The antiinflammatory signals generated by CTS-L act directly on the NF-κB pathway and abrogate the IL-1β–induced nuclear translocation of NF-κB in a sustained manner. The time course of the CTS-L–mediated inhibition of IL-1β–induced NF-κB nuclear translocation was rapid and could be observed within 15 minutes. Because NF-κB regulates its own gene expression (26), we investigated whether CTS-L also inhibits IL-1β–induced NF-κB induction. Our findings demonstrate that CTS-L–mediated suppression of NF-κB nuclear translocation also resulted in the inhibition of its mRNA expression and synthesis. Hence, the antiinflammatory actions of CTS-L were mediated both by suppression of IL-1β–induced NF-κB nuclear translocation and by inhibition of IL-1β–induced NF-κB synthesis.
NF-κB/Rel proteins exist as homo- or heterodimers of 5 different subunits of NF-κB, including p65 (RelA), c-Rel, RelB, p50, and p52, which constitute different NF-κB DNA binding complexes (16). Targeted disruption studies provide evidence that combinatorial interactions between different NF-κB subunits exhibit distinguishable DNA binding specificity and transcriptional activity. For example, p50 homodimers lack the transactivation domain and act as repressors of gene expression (16,18,27). In our experiments, we investigated the subunit composition of the inducible NF-κB complexes using specific antibodies against different NF-κB proteins. Exposure of chondrocytes to CTS-L resulted in the suppression of the IL-1β–induced nuclear translocation of NF-κB consisting of p65 and p50. Since a clear inhibition of IL-1β–induced p65 and p50 subunits of NF-κB was observed, CTS-L appears to directly intercept IL-1β–induced nuclear translocation of these NF-κB subunits to attenuate IL-1β–induced proinflammatory gene induction. This is also supported by immunofluorescence analysis, which demonstrates cytoplasmic retention of NF-κB p65 in cells exposed simultaneously to IL-1β and CTS-L. Nevertheless, a role for p50 homodimers in inhibiting proinflammatory responses cannot yet be completely excluded.
CTS-H is a potent proinflammatory signal, and thus it is not surprising that its actions are mediated by NF-κB nuclear translocation and synthesis. In this respect, the actions of CTS-H are similar to classic proinflammatory cytokines that involve NF-κB transcription factors to initiate inflammation. Consistent with our observation that CTS-H actions were mediated by NF-κB nuclear translocation was the finding that CAPE completely abrogated the CTS-H–induced NF-κB nuclear translocation and ultimately iNOS mRNA expression. Furthermore, the finding that CTS-H induced the nuclear translocation of p65 and p50 heterodimers of NF-κB also suggests that CTS-H utilizes proinflammatory pathways for its signal transduction. Thus, despite being a physical signal, CTS-H acts in a manner similar to molecular activators that stimulate the transcriptional activity of NF-κB.
Taken together, these findings show that chondrocytes can perceive biomechanical signals and convert them into biochemical events that regulate proinflammatory gene induction. In this process, the NF-κB pathway is critical in regulating the antiinflammatory and proinflammatory actions of mechanical signals. Although the effects of low and high magnitudes of mechanical strain are diametrically opposed, their signals interact with the same Rel proteins to elicit very different physiologic responses. Low levels of CTS generate signals that inhibit NF-κB nuclear translocation to limit IL-1β–inducible expression of proinflammatory genes. In contrast, CTS of high magnitudes generates signals that are similar to IL-1β in that it employs NF-κB transcription factors to initiate expression of proinflammatory genes involved in soft and hard tissue destruction. How different magnitudes of cyclic strain on chondrocytes could lead to opposite effects is perplexing. It is conceivable that low and high magnitudes of mechanical strain act on different upstream kinases of the NF-κB signal transduction cascade. Alternatively, these signals may act on pathways that are known to regulate NF-κB nuclear translocation.
While these studies clearly show a role of the NF-κB signal transduction pathway in mechanotransduction, a role for other signaling pathways involved in proinflammatory and antiinflammatory signaling cannot be ruled out. Continued studies will allow us to address whether signals generated by CTS of low and high magnitudes have similar target sites upstream of the NF-κB translocation that regulate the magnitude-dependent responses to mechanical signals.
Acknowledgments
Supported by NIH grants AR-48781, AT-00646, and HD-40939.
REFERENCES
- 1.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 2.Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 3.Soltz MA, Ateshian GA. A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng. 2000;122:576–586. doi: 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84–93. doi: 10.1115/1.1531656. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 6.Ragan PM, Badger AM, Cook M, Chin VI, Gowen M, Grodzinsky AJ, et al. Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836–842. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- 7.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces [review] Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 8.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 9.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729–737. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 10.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models [review] Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 11.Lotz M. Cytokines in cartilage injury and repair [review] Clin Orthop. 2001;391(Suppl):S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 12.Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes [review] Curr Opin Clin Nutr Metab Care. 2003;6:289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology [review] Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 14.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanovich-Racic M, Piesco NP, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 15.Long P, Gassner R, Agarwal S. Tumor necrosis factor α–dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthritis Rheum. 2001;44:2311–2319. doi: 10.1002/1529-0131(200110)44:10<2311::aid-art393>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle [review] Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 18.Karin M, Lin A. NF-κB at the crossroads of life and death [review] Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 19.Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208–217. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 20.Seguin CA, Bernier SM. TNFα suppresses link protein and type II collagen expression in chondrocytes: role of MEK1/2 and NF-κB signaling pathways. J Cell Physiol. 2003;197:356–369. doi: 10.1002/jcp.10371. [DOI] [PubMed] [Google Scholar]
- 21.Clohisy JC, Roy BC, Biondo C, Frazier E, Willis D, Teitelbaum SL, et al. Direct inhibition of NF-κB blocks bone erosion associated with inflammatory arthritis. J Immunol. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1β actions in chondrocytes. J Immunol. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide and cartilage metabolism [review] Methods Enzymol. 1996;269:75–88. doi: 10.1016/s0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi MM, Kumar A, Darnay BG, Chainy GB, Agarwal S, Aggarwal BB. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-κB activation, IκBα phosphorylation, and degradation. J Biol Chem. 1997;272:30129–30134. doi: 10.1074/jbc.272.48.30129. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE2 by IL-1β-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285:1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- 26.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system [review] Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 27.Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-κB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]