Abstract
The current study examined the independent and combined effects of HIV and marijuana use (no use, light use, and moderate-to-heavy use) on neurocognitive functioning among a convenience sample of HIV-positive (HIV+) and HIV-negative (HIV−) individuals recruited from HIV community care clinics and advertisements in the Greater Los Angeles area. Marijuana (MJ) users consisted of individuals who reported regular use of marijuana for at least 12 months, with last reported use within the past month. Participants included 89 HIV+ (n = 55) and HIV− (n = 34) individuals who were grouped into non-users, light users, and moderate-to-heavy users based on self-reported marijuana use. Participants were administered a brief cognitive test battery and underwent laboratory testing for CD4 count and viral load. HIV+ individuals demonstrated lower performance on neurocognitive testing than controls, and moderate-to-heavy MJ users performed more poorly on neurocognitive testing than light users or non-users. Moderate-to-heavy HIV+ users performed significantly lower on learning/memory than HIV− moderate-to-heavy users (MD = −8.34; 95% CI: −16.11 to −0.56) as well as all other comparison groups. In the domain of verbal fluency, HIV+ light users outperformed HIV− light users (MD = 7.28; 95% CI: 1.62 to 12.39), but no HIV group differences were observed at other MJ use levels. HIV+ MJ users demonstrated lower viral load (MD = −0.58; 95% CI: −1.30 to 0.14) and higher CD4 count than non-users (MD = 137.67; 95% CI: 9.48 to 265.85). The current study findings extend the literature by demonstrating the complex relationship between HIV status and marijuana use on neurocognitive and clinical outcomes.
Keywords: Marijuana Use, Cannabis, HIV/AIDS, Neurocognition, Immune Status
1. Introduction
Approximately 23–56% of HIV+ individuals report using marijuana (MJ) to alleviate disease-related symptoms and medication side effects (Fogarty et al., 2007), indicating potential benefits of MJ. However, the cognitive consequences remain highly debated (Chang, Cloak, Yakupov, & Ernst, 2006; Lundqvist, 2005). Some studies of healthy populations have not found adverse cognitive effects following abstinence (Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003; Jager, Kahn, Van Den Brink, Van Ree, & Ramsey, 2006), whereas others have reported acute as well as long-term effects on cognition when compared to non-users (Abdullaev, Posner, Nunnally, & Dishion, 2010; Battisti et al., 2010; Gonzalez et al., 2012; Grant, Chamberlain, Schreiber, & Odlaug, 2012; Lisdahl & Price, 2012; Solowij et al., 2002; Thames, Arbid, & Sayegh, 2014; Tapert, Granholm, Leedy, & Brown, 2002). Furthermore, animal studies of Alzheimer’s disease and neuroinflammation-induced cognitive damage support the neuroprotective effects of cannabinoids (Fishbein-Kaminietsky, Gafni, & Sarne, 2014; Ramírez, Blázquez, Gómez del Pulgar, Guzmán, & de Ceballos, 2005).
While the adverse effects of MJ on cognitive functioning are still unclear, HIV-associated cognitive compromise is well-documented (Becker, Thames, Woo, Castellon, & Hinkin, 2011; Heaton et al., 2011). However, few investigations have examined the interactive effects of MJ and HIV status on cognitive functioning. One study found that self-reported frequent MJ use was associated with greater memory impairment, but only among symptomatic patients (Cristiani, Pukay-Martin, & Bornstein, 2014). Chang and colleagues (2006) found no additive effects on a measure of reaction time, a finding that was attributed to the relatively asymptomatic status of the HIV+ sample.
For the current study, we examined the combined effects of HIV status and marijuana use on neurocognitive and immune functioning among a sample with varying degrees of use.
2. Method
HIV+ (n = 55) and HIV− (n = 34) participants recruited from HIV clinics and advertisements in the Greater Los Angeles area. All procedures received institutional approval and participants provided written informed consent. Screeners and questionnaires about neurological and medical history assessed for neurological, psychiatric, and medical confounds (see Thames et al., 2014). We grouped participants based upon their reported MJ use using a similar classification as outlined in Bolla, Brown, Eldreth, Tate, & Cadet (2002): light users [i.e., 2–14 times per week (n = 42)], moderate-to-heavy users [i.e., 18–90 times per week (n = 21)], non-users [reported never using marijuana (n = 26)]. Users had to report using MJ for at least 12 months for inclusion.
2.1 Measures
2.1.2 Drug Use
The Brief Drug Use History Form (DHQ; UCLA’s Center for Advanced Longitudinal Drug Abuse Research) was used to collect information about drug use. Participants underwent urine toxicology screening using Integrated E-Z Split Key (Innovacon, Inc., San Diego, CA). Participants were excluded if they reported MJ use within 24 hours of cognitive testing or regular use of other substances aside from marijuana and alcohol.
2.1.3 Neurocognitive Functioning and Immune Status
Participants were administered a brief cognitive test battery used in prior studies (Thames et al., 2014). Global neuropsychological performance was calculated by averaging t scores from individual cognitive tests (Heaton et al., 1991; Miller & Rohling, 2001). Participants provided a blood sample for CD4 and HIV viral load testing.
3. Statistical Analyses
3.1 Group Comparisons
HIV and Marijuana use groups
MJ groups did not significantly differ in age, years of education, or race/ethnicity (all p’s > .10). However, there were significant differences in gender [χ2 (4, N = 89) = 10.81, p = .03] and estimated premorbid IQ (WRAT-4 performance) [F (2,86) = 3.29, p = .04], with significantly greater proportion of males in the MJ use groups (light and moderate-to-heavy) than females, and significantly lower WRAT-4 scores among moderate-to-heavy MJ users. There were no significant differences between MJ use groups on alcohol use variables (all p’s > .10). We included WRAT-4 as a covariate given its association with overall neurocognitive performance, r (89) = .52, p < .001. HIV status groups did not significantly differ in age, education,, estimated premorbid IQ, or race/ethnicity (all p’s > .10). There was no statistically significant interaction between MJ use and HIV on age and education (p’s >.10). Please see table 1 for a summary of group differences.
Table 1.
Participant Demographics and Group Statistics
| HIV/MJ Groups | ||||||
|---|---|---|---|---|---|---|
| (a) | (b) | (c) | (d) | (e) | (f) | |
| HIV−/MJ none | HIV+/MJ none | HIV−/MJ light | HIV+MJ light | HIV−/MJ mod | HIV+/MJ mod | |
| Mean/% (Std) | Mean/% (Std) |
Mean/% (Std) | Mean/%(Std) | Mean/%(Std) | Mean/% (Std) | |
| (n = 12) | (n = 14) | (n = 12) | (n = 30) | (n = 10) | (n = 11) | |
| Age | 43.16 (3.43) | 52.35 (3.17) | 53.5 (2.17) | 48.6 (3.43) | 53.7 (3.71) | 48.7 (3.52) |
| Education | 15.08 (2.23) | 13.07 (0.82) | 13.33 (2.21) | 14.27 (2.13) | 12.96 (1.66) | 12.27 (1.72) |
| Gender (% Male) |
45% | 85% | 75% | 95% | 100% | 54% |
| Race/Ethnicity (%) | ||||||
| AA-Black | 50% | 57% | 58% | 66% | 30% | 72% |
| NH-White | 36% | 42% | 41% | 33% | 70% | 27% |
| Nadir CD4 | N/A | 233.09 (1.70) | N/A | 281.72 (172.92) | N/A | 176.75 (132.03) |
| CD4 count | N/A | 490.4 (275.71) | N/A | 609.64 (265.11) | N/A | 646.50 (258.80) |
| Viral Load (log) | N/A | 2.46 (1.36) | N/A | 1.79 (.85) | N/A | 1.97 (1.18) |
| Length of HIV | N/A | 10.1 (3.4) | N/A | 12.13 (1.9) | N/A | 12.98 (2.2) |
| Global NP | 46.79 (4.45) | 45.35 (4.74) | 49.52 (4.26) | 46.12 (4.05) | 42.72 (4.07) | 41.46 (5.89) |
| Attention | 44.42 (2.38) | 42.52 (2.04) | 46.22 (2.20) | 44.95 (1.42) | 43.67 (2.48) | 43.73 (2.36) |
| Processing Speed |
50.18 (2.15) | 50.31 (1.84) | 51.29 (1.98) | 47.53 (1.28) | 43.96 (2.24) | 42.53 (2.13) |
| Learning/Mem | 41.48 (2.58) | 39.90 (2.21) | 49.66 (2.38) | 38.13 (1.34) | 41.26 (2.69) | 32.92 (2.56) |
| Executive | 50.04 (1.99) | 46.95 (1.73) | 50.70 (1.84) | 47.32 (1.19) | 42.39 (2.08) | 42.06 (1.97) |
| Fluency | 49.85 (2.76) | 51.08 (2.37) | 48.78 (1.55) | 56.06 (1.65) | 46.68 (2.88) | 47.41 (2.73) |
| BDI-II | 6.56 (2.20) | 7.50 (5.99) | 8.0 (4.97) | 9.43 (9.08) | 10.43 (4.67) | 13.45 (8.5) |
| %Past Dependence |
||||||
| Alcohol | 0 | 0 | 50% | 14% | 75% | 18% |
| Stimulants | 0 | 0 | 0 | 0 | 0 | 0 |
| Opiates | 0 | 14% | 0 | 7% | 0 | 0 |
| Sedatives | 0 | 0 | 0 | 7% | 0 | 0 |
| Alcohol use | ||||||
| # days past 4- weeks |
3.5 | 1.2 | 3.2 | 8.3 | 10 | 4.8 |
| #drinks per day | 2.0 | 1.5 | 2.0 | 2.5 | 2.0 | 2.8 |
3.1.2 Statistical Procedures
We used Analysis of Covariance (ANCOVA) and Multivariate Analysis of Covariance (MANCOVA) to examine the independent and interactive effects of MJ use and HIV status on global neurocognitive functioning and individual cognitive domains.
4. Results
4.1. HIV status and marijuana use effects on neurocognitive performance
ANOVA demonstrated a significant main effect of MJ use [F (2, 82) = 9.08, p <.0001, partial η2 = .18] and a non-significant statistical trend towards a main effect of HIV status [F (1, 82) = 3.77, p = .05, partial η2 = .05] on global neurocognitive performance. There was no significant interaction between HIV status and MJ use on global neurocognitive performance [F (2, 82) = .519, p = .59]. Moderate-to-heavy MJ users demonstrated lower global neurocognitive performance than light users and non-users.
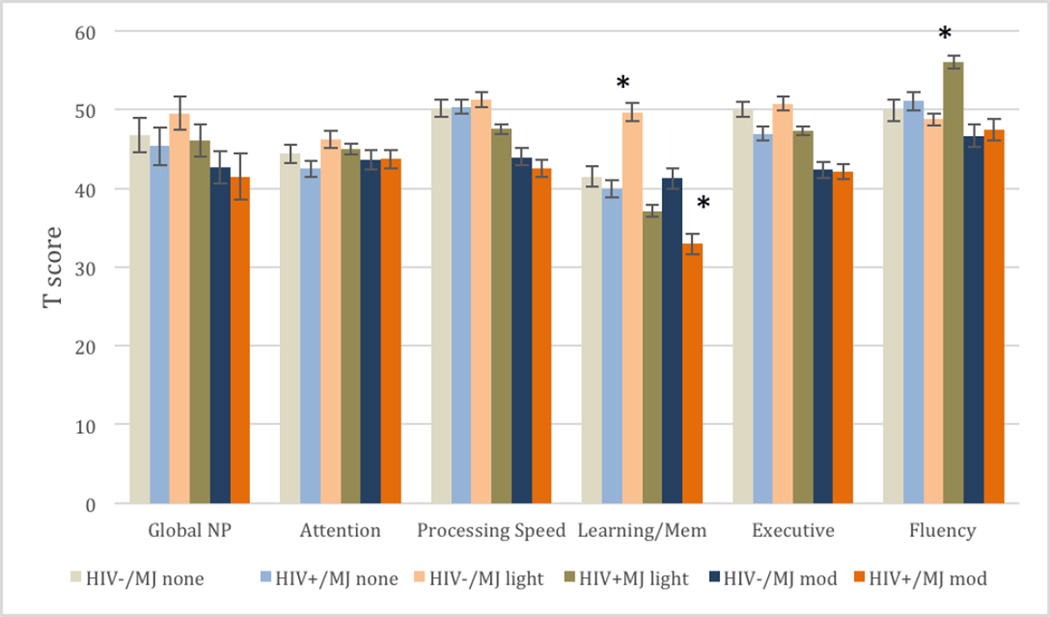
MANCOVA demonstrated a main effect for HIV status [F (5, 78) = 3.708, p = .005, Λ = .81, η2 = .19], MJ use [F (5, 78) = 2.84, p = .003, Λ = .71, partial η2 = .16] and an HIV*MJ interaction effect [F (5, 78) = 2.53, p = .04, Λ = .92, partial η2 = .08] on individual cognitive domain scores. Main MJ effects were in the domains of processing speed [F (2, 82) = 6.12, p = .003, η2 = .05], learning/memory [F (2, 82) = 3.46, p = .03, partial η2 = .07], and executive functioning [F (2, 82) = 7.22, p = .01, partial η2 = .15], such that moderate-to-heavy users performed significantly lower in these domains than light users and non-users. There were no significant differences between non-users and light users across these domains. HIV+ individuals performed lower in cognitive domains of learning/memory [F (1, 82) = 15.65, p < .001, partial η2 = .16], and executive functioning [F (1, 82) = 3.23, p = .03, partial η2 = .07] than HIV− individuals. There was a significant HIV*MJ interactive effect in learning and memory [F (2, 82) = 8.82, p = .004, partial η2 = .07], such that HIV+ moderate-to-heavy users demonstrated significantly lower learning and memory performance than all other comparison groups. There was also a significant HIV*MJ interactive effect such that HIV+ light users outperformed HIV− light users in verbal fluency, but no HIV group differences were found at other MJ use levels in the domain of verbal fluency [F (2, 82) = 10.24, p = .001, partial η2 = .09]. See Figure 1.
Figure 1.
HIV status and marijuana use effects on neurocognitive performance
*Significant at FDR corrected p < .05
4.1.2. MJ use group differences on HIV-disease markers
There were no statistically significant differences between marijuana use groups on Nadir CD4 [F (2, 52) = 1.13, p = .32]. Non-users demonstrated significantly lower current CD4 than light or moderate-to-heavy users, [F (2, 52) = 3.14, p = .04]. Higher viral load was found among non-users compared to light and moderate-to-heavy users, F (2, 52) = 3.76, p = .03.
5. Discussion
The current study found main effects for both HIV status and MJ use on neurocognitive functioning. HIV+ moderate-to-heavy users performed significantly worse on learning/memory than other comparison groups, whereas HIV+ light users performed significantly better on verbal fluency than HIV− light users. HIV+ MJ users (light and moderate-to-heavy) evidenced higher plasma CD4 and lower viral load than HIV+ non-users, suggesting healthier immune functioning. This is consistent with a recent investigation by Constantino and colleagues (2012) that found a 40% reduction in HIV-1 infected CD4+ cells that were pre-treated with a cannabinoid receptor 2 agonist..
Nevertheless, there was a trend for moderate-to-heavy MJ use to be associated with worse performance on cognitive functioning for HIV+ and HIV− individuals, which is consistent with previous reports (Bolla et al., 2002; Cristiani et al., 2014; Solowij et al., 2002). Light users on average demonstrated better performance than heavy users, but it is unclear why HIV+ light users outperformed HIV− light users in the domain of verbal fluency. We should note that although the performance differences were statistically significant, from a clinical standpoint, the scores obtained from the HIV+ light users (T = 56.06; 73rd %ile) and HIV− light users (T = 48.78; 47th %ile) fall well within the average range.
These results highlight the complex relationship between MJ use and neurocognitive functioning as a function of chronic disease. If light or occasional marijuana use protects against disease progression or helps with maintaining adequate immune functioning (perhaps through reducing inflammation) without associated cognitive compromise, such use may have a neuroprotective role in several neuroinflammation related diseases (Klein, 2005).
However, the mechanisms by which marijuana act upon immune and neurocognitive functioning cannot be determined from the current study. Further, our sample was from a region that has legalized the use of medical marijuana. Perhaps there is more variability in the sources and preparations of marijuana used among our sample in comparison to prohibited areas. Finally, we were unable to gather information about age of onset of MJ use and our abstinence period was very short (24 hours). This limits our interpretation as we cannot determine if moderate-to-heavy smokers performed worse on cognitive testing as a function of starting at an earlier age, or if the observed effects would remain after a prolonged period of abstinence. In a previous study we found that individuals who abstained from smoking cannabis for four weeks continued to demonstrate deficits in executive functioning, although most other performances were similar to non-users (Thames et al., 2014).
In sum, based on the needs of this population and the rapidly advancing legislation of medicinal cannabis use, there is a pressing need for future investigations to isolate the benefits for medicinal purposes. There is a mix of low-quality and moderate-quality evidence supporting the therapeutic effects of cannabinoids across clinical trials (Whiting et al., 2015). As more studies adhere to CONSORT guidelines, appropriate dosage levels (based upon CB receptor effects), formulations, and delivery mechanisms may be established.
Acknowledgments
Funding for this study was provided by NIMH Grant K23-MH095661 and the Society for Clinical Neuropsychology (PI: A. Thames). NIMH or SCN had no role in the study design, collection analysis or interpretation of data, writing the manuscript, or the decision to submit the paper for publication.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behavioural Brain Research. 2010;215(1):45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Battisti Ra, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212(4):613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon Sa, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2006;1(1):65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino CM, Gupta A, Yewdall AW, Dale BM, Devi LA, Chen BK. Cannabinoid receptor 2-mediated attenuation of CXCR4-tropic HIV infection in primary CD4+ T cells. PloS one. 2012;7(3):e33961. doi: 10.1371/journal.pone.0033961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana Use and Cognitive Function in HIV-infected People. The Journal of Neuropsychiatry and Clinical Neurosciences. 2014;16(3):330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- Fishbein-Kaminietsky M, Gafni M, Sarne Y. Ultralow doses of cannabinoid drugs protect the mouse brain from inflammation-induced cognitive damage. Journal of neuroscience research. 2014;92(12):1669–1677. doi: 10.1002/jnr.23452. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Rawstorne P, Prestage G, Crawford J, Grierson J, Kippax S. Marijuana as therapy for people living with HIV/AIDS: social and health aspects. AIDS care. 2007;19(2):295–301. doi: 10.1080/09540120600841930. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychological Society. 2003;9(05):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and Alcohol Dependence. 2012;121(1–2):159–162. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead–Reitan Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology. 2006;185(3):358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nature Reviews Immunology. 2005;5(5):400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacology Biochemistry and Behavior. 2005;81(2):319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Miller LS, Rohling ML. A statistical interpretive method for neuropsychological test data. Neuropsychology Review. 2001;11:143–169. doi: 10.1023/a:1016602708066. [DOI] [PubMed] [Google Scholar]
- Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. The Journal of Neuroscience. 2005;25(8):1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Thames AD, Arbid N, Sayegh P. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addictive Behaviors. 2014;39(5):994–999. doi: 10.1016/j.addbeh.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]