Abstract
Background
Recent studies demonstrated low-grade inflammation in patients with Irritable Bowel Syndrome (IBS). However, these studies have been relatively small and do not enable examination of this factor in different subtypes of IBS and the possibility of confounding effects of co-morbidities that may be associated with inflammatory responses.
Goals
To investigate the association between high sensitive C - reactive protein (hs-CRP) and the diagnosis of IBS, IBS-subtypes, symptoms' severity and IBS-associated co-morbidities.
Study
This cross-sectional study uses data from a large matched case control study of IBS subjects and healthy controls (HC). hs-CRP levels were measured in all subjects. IBS diagnosis was determined by Rome III criteria, negative screening blood tests and normal colonoscopy. Subjects were evaluated for IBS severity and associated pain and psychological co-morbidities
Results
A total of 242 IBS patients and 244 HC were studied. Median hs-CRP levels in the IBS group were significantly higher than in HC (1.80, IQR 0.7-4.04 mg/l vs 1.20, IQR 0.5-2.97mg/l respectively, p<0.006,). Levels were highest in IBS-D patients with greater disease severity. Hs-CRP levels mildly correlated with symptoms severity (r=0.169, p=0.009); this correlation was stronger for the IBS-D patients (r=0.27, p=0.006). IBS was a significant independent predictor (p=0.025) for higher hs-CRP levels, whereas other pain and psychological co-morbidities were not.
Conclusions
Given these observations of cross-sectional differences in hs-CRP between IBS subtypes and severity, independent of pain and co-morbidities, more research is needed to explore a possible role of low-grade inflammation in the pathogenesis and/or clinical presentation of IBS.
Keywords: hs-CRP, IBS, low grade inflammation
INTRODUTCION
Irritable bowel syndrome (IBS) is a common disorder with a global prevalence of 8-23% of the adult population 1 and is slightly more prevalent in the industrialized world than in developing countries. The pathophysiology of IBS is not well understood. It is traditionally considered a multifactorial disorder associated with impaired brain-gut function, altered intestinal motility and visceral hypersensitivity 2. Although IBS is not considered an inflammatory disease, recent studies suggest a possible role for alterations in the intestinal immune function and low grade inflammation in its pathogenesis 3-5. The first evidence for an inflammatory component in IBS was reported in 1960 showing that IBS patients have a higher number of mast cells in their intestinal wall compared to healthy subjects 6. More recent studies have described additional histopathologic abnormalities in biopsies from the intestinal mucosa of patients with IBS, including increased numbers of activated immunocompetent cells, such as: intraepithelial lymphocytes, lamina propria CD3+ cells, CD25+ cells, neutrophils and mast cells compared with controls 7, 8.
Additional support for a potential role for low-grade inflammation in IBS came from epidemiological observational studies showing that in 6-17% of IBS patients the onset of symptoms may relate to an acute episode of gastrointestinal infection; usually referred to as “post-infectious IBS” (PI-IBS) 9, 10. Of particular interest are the findings of ongoing alterations in enteroendocrine cells, higher number of T-cell lymphocytes 11 and increased expression of interleukin 1β in mucosal biopsies of patients with PI-IBS 12. Other studies that investigated systemic immune function in patients with IBS have demonstrated that an underlying inflammatory response can also be identified in peripheral blood. Examples are genetic studies demonstrating reduced levels of IL-10 expression, findings of increased ratio of pro- to anti-inflammatory cytokines (i.e., IL-10 to IL-12) 13, increased release of pro-inflammatory cytokines (e.g., IL-1β_, IL-6, and TNF-α) from peripheral blood mononuclear cells (PBMCs) 14 and increased numbers of activated T cells in the peripheral blood 15 in patients with IBS compared to controls. In a recent case control study 16 we investigated the possibility of detectible systemic inflammatory response in IBS by comparing the levels of high-sensitive C-reactive protein (hs-CRP), a non-specific marker of inflammation, in patients with IBS and healthy controls (HC). We found a significantly higher levels of hs-CRP in patients with IBS (n=88, 1.17±1.26 mg/dl) compared to HC (n=352, 0.72mg/dl; p<0.001). However, that previous study did not have a sufficient statistical power to enable comparisons between the clinical subtypes of IBS and to investigate the possible confounding effect of co-morbidities commonly reported with IBS, which could be related to elevated hs-CRP.
The aim of the current study was to investigate the association between hs-CRP and IBS, IBS subtypes, IBS symptoms severity, the presence of other pain syndromes (e.g., fibromyalgia, migraine headache) and psychological co-morbidities (e.g. depression, anxiety, somatization) in a large well defined cohort of patients with IBS. We hypothesized that hs-CRP levels in subjects with IBS: (1) are higher compared to HC, (2) vary between the IBS clinical subtypes, (3) are positively correlated with IBS symptoms severity, and (4) are not explained by other commonly reported co-morbidities.
MATERIAL AND METHODS
Study design
This cross-sectional study uses data collected as part of a large matched case control study of IBS subjects and HC conducted at the University of North Carolina at Chapel Hill (UNC-CH) during 2007-2009. Subjects were recruited by advertisement from the general population and the UNC-CH outpatient clinics. All subjects were evaluated at a single study visit at which a clinical exam was performed, questionnaires were administered, and blood samples were obtained. The study protocol was approved by the UNC-CH Institutional Review Board and subjects signed an informed consent form prior to enrollment in the study.
Study population
All study participants were 18 years old or older. IBS was diagnosed by an experienced physician based on Rome III criteria and negative screening blood tests. All IBS patients must have had IBS symptoms for 6 or more months and a normal colonoscopy or barium enema during the previous 12 months to rule out organic or obstructive disease. Subjects with any history of the following were excluded: evidence of a structural abnormality of the digestive tract, inflammatory bowel disease (IBD), intestinal resection, GI malignancy, microscopic colitis, celiac sprue, or within six months, any history of ileus, symptomatic cholelithiasis, pancreatitis, abdominal adhesions and/or stricture with evidence of small bowel obstruction, recurrent diverticulitis or unexplained rectal bleeding (including a positive fecal occult blood test (FOBT)).
HC were assessed by a study physician using ROME III criteria and were found negative for IBS at the time of enrollment. Persons were excluded if they had any history of IBS diagnosis in themselves or any first degree relatives, had been referred to a GI specialist for GI abdominal pain, had any history of functional constipation, infectious hepatitis B or C, HIV, celiac disease, and gastro-oesophageal reflux disease (GERD). If any had obtained screening colonoscopy or barium enema with prior 12 months, results must have been normal.
Measurements and evaluations
All participants underwent a detailed medical history and completed previously validated questionnaires. IBS diagnosis and its clinically relevant subtype were determined using the ROME III criteria for IBS 17. IBS severity was assessed using the Functional Bowel Disorders Symptom Severity Index (FBDSI)18. Somatic and psychological comorbidities were assessed using the Patient Health Questionnaire comprised of 15 somatic symptoms (PHQ-15) 19 and Hospital Anxiety and depression scale (HADS) 20. Other co-morbid pain syndromes (TMJ syndrome - Temporomandibular joint syndrome, migraine headache, fibromyalgia, chronic fatigue and pelvic pain) were assessed by self-report. All subjects provided blood samples for routine laboratory tests and serum hs- CRP.
Statistics
Continuous variables were summarized using the mean and standard deviation (SD) for normally distributed variables, or the median (IQR) for non-normally distributed variables; categorical variables were summarized using frequency distributions.
Using data from our prior study of 16, we determined that a minimum sample size of 77 in each group would be needed to detect a difference in hs-CRP levels of 0.5 mg/liter between patients and HD (β=0.2, α=0.05), assuming a SD of 1.26 and 0.91 in each group; for a difference of 0.4 mg/l, 120 per group would be needed.
Since hs-CRP has a non-normal distribution, all hs-CRP results in tables and figures are shown as median and IQR. For correlations and multivariable modeling, log-transformed hs-CRP values were used.
Independent t-test and chi square statistics were used for comparisons between cases and HC for socioeconomic and demographic variables, psychological characteristics and health status. Mann-whitney test was used to compare hs-CRP levels between IBS and HC, and Kruskal-Wallis tests to compare hs-CRP levels between clinical sub-types of IBS and between severity subgroups of IBS. Further investigation of the association between hs-CRP and IBS severity was done using Spearman correlation coefficients. We conducted two Mann-whitney tests for each stratum of co-morbidity, and multiple linear regression models to assess whether the higher hs-CRP levels in IBS patients were explained by co-morbid conditions.
For all analyses, we used a two-tailed significance test of p<0.05 to determine a statistical significance. The SPSS statistical package (Version 15, SSPS Inc., Chicago, IL), was used for all analyses.
RESULTS
1) Study population characteristics
A total of 486 participants (242 IBS patients and 244 HC) were included in the analysis after excluding 18 patients (8 IBS and 10 HC) with abnormally elevated (≥ 35 mg/l) hs-CRP (n=2), missing hs-CRP levels (n=21), withdrawn consent, not meeting ROME III criteria for IBS, or diagnoses of other GI disease.
There were no differences between IBS patients and HC for sex, age or BMI. All participants were of Caucasian ethnicity. IBS patients were significantly less educated, and had lower income compared to HC (Table 1).
Table 1.
Comparison of demographic and socioeconomic characteristics, psychological profile, pain syndromes and co-morbid conditions between IBS patients and controls
| IBS (242) | HC (244) | p value1 | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex - % Women (n) | 79.8% (193) | 79.5% (194) | N.S. |
| Age (mean±S.D.) (years) | 38.78±14.16 | 39.87±15.04 | N.S. |
| BMI (mean±S.D.) (kg/m2) | 26.93±6.55 | 25.97±5.95 | N.S. |
| Socioeconomic status | |||
| Education - % more than 15 years (n) | 64.0% (155) | 74.6% (182) | 0.042 |
| Family income – % >100,000$per year (n) | 16.7% (40) | 28.3% (68) | 0.006 |
| Psychological health profile | |||
| Depression - % (n) | 9.6% (23) | 0.4% (1) | 0.000 |
| Anxiety – % (n) | 25.0% (60) | 6.1% (15) | 0.000 |
| Somatization - % (n) | 82.2% (199) | 58.6% (143) | 0.000 |
| Prevalence of pain syndromes | |||
| Migraine - % (n) | 35.1% (85) | 14.3% (35) | 0.000 |
| Fibromyalgia – % (n) | 14.9% (36) | 0.8% (2) | 0.000 |
| Chronic fatigue - % (n) | 7.0% (17) | 0.4% (1) | 0.000 |
| Pelvic pain - % (n) | 12.4% (30) | 5.3% (13) | 0.007 |
| TMJ syndrome - % (n) | 25.2% (60) | 10.7% (26) | 0.000 |
| Prevalence of co-morbid conditions | |||
| Hay fever - % (n) | 36.4% (88) | 25.8% (63) | 0.014 |
| Asthma - % (n) | 19.4% (47) | 12.7% (31) | 0.048 |
| Drug allergy - % (n) | 50.0% (121) | 29.5% (72) | 0.000 |
| Lactose intolerance - % (n) | 13.6% (33) | 4.1% (10) | 0.000 |
| Prevalence of co-morbid conditions | |||
| Functional dyspepsia - % (n) | 2.1% (5) | 0.0% (0) | 0.030 |
| GERD - % (n) | 28.1% (68) | 5.7% (14) | 0.000 |
| Cystitis - % (n) | 5.4% (13) | 2.0% (5) | 0.058 |
Chi square for categorical variables and t-test for continuous variables
N.S. – Non significant
HC – Healthy controls
IBS - Irritable bowel syndrome
TMJ syndrome - Temporomandibular joint syndrome
GERD –Gastro-esophageal reflux disease
Most (41.7%; n=101) of the IBS group were classified as either diarrhea-predominant (IBS D) or unspecified subtype (36.8%; n=89), with smaller numbers of constipation-predominant (IBS-C) (16.1%; n=39), and mixed (IBS-M) subtypes (5.4%; n=13).
Most IBS patients scored mild on symptom severity (52.5%; n=126), followed by moderate (36.3%, n=87) and severe (11.3%; n=27) by FBDSI.
A significant higher percentage of IBS patients suffered from depression and anxiety and had more somatic symptoms (Table 1).
The prevalence of pain syndromes was significantly higher in the IBS group. The most prevalent syndromes were migraine headache (35.1%) followed by TMJ syndrome (25.2%), fibromyalgia (14.9%), pelvic pain (12.4%) and chronic fatigue (7.0%). Moreover, the prevalence of all other non-pain co-morbid conditions was significantly higher in the IBS group with a borderline significance for cystitis (Table 1).
2) Associations of hs-CRP with IBS diagnosis and IBS-subtypes
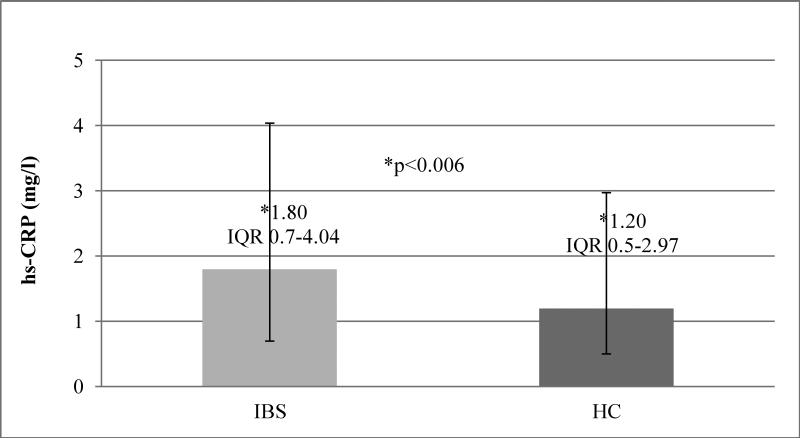
The median hs-CRP level was significantly higher in the IBS group compared with the HC (1.80, IQR 0.7-4.04 mg/l vs 1.20, IQR 0.5-2.97 mg/l, p<0.006) (Figure 1).
Figure 1. hs-CRP levels in IBS vs. HC.
Median hs-CRP (mg L−1) level higher in the IBS group compared with the HC (1.80, IQR 0.7-4.04 mg/l vs 1.20, IQR 0.5-2.97 mg/l, p<0.006)
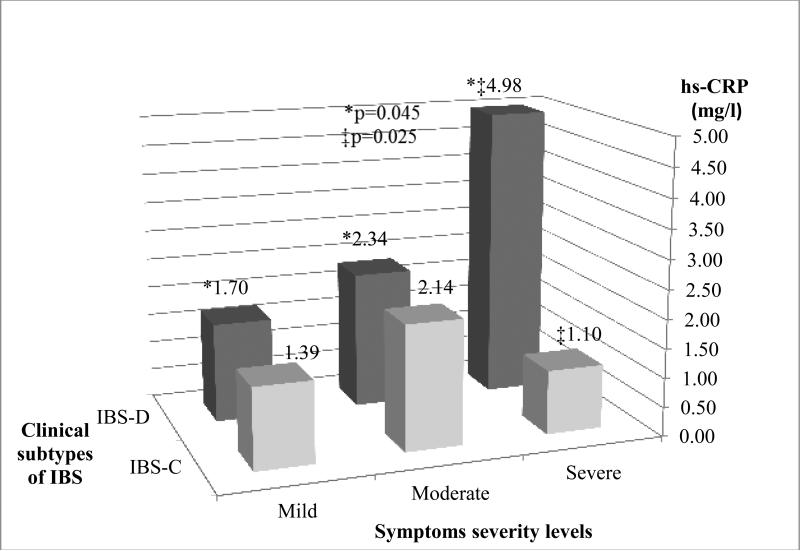
Among IBS patients in the highest severity subgroup, median hs-CRP was significantly higher in IBS-D patients than IBS-C (4.98, IQR 2.22-8.96 vs. 1.10, IQR 0.55-2.6 mg/l respectively, p=0.0025) (Figure 2). However, this relationship was not observed among the mild and moderate severity subgroups, where median levels of hs-CRP were similar between IBS-D and IBS-C subtypes (Figure 3). Median hs-CRP levels increased with mild, moderate, and severe symptom severity (Figure 3) but only among persons with IBS-D subtype (1.7, 2.34, 4.98 respectively, p=.045).
Figure 2. hs-CRP levels in subtypes of IBS with severe symptoms.
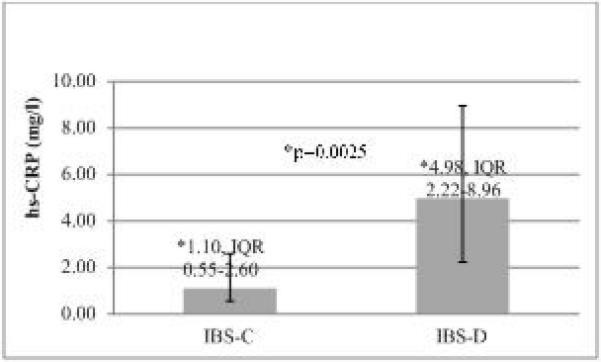
Median hs-CRP (mg L−1) levels in two subtypes of IBS having severe symptoms. hs-CRP was higher in IBS-D than IBS-C (4.98, IQR 2.22-8.96, IQR 0.74-5.18 mg/l, vs. 1.10, IQR 0.55-2.6 mg/l respectively, p=0.0025).
Figure 3. hs-CRP levels in clinical subtypes of IBS patients by symptom severity.
Median hs-CRP (mg L−1) levels by IBS subtypes and severity levels. Among IBS-D group, hs-CRP levels increased with mild-moderate-severe symptom severity, ( 1.70, IQR 0.56-4.00 mg/l, vs. 2.34, IQR 0.88-4.26 mg/l , vs. 4.98, IQR 2.22-8.96 mg/l, respectively, p=0.0045).
3) Association of hs-CRP with symptoms severity
Hs-CRP level correlated with symptom severity of IBS (r=0.169 p=0.009), and this correlation was stronger among IBS-D patients (r=0.27, p=0.006). Symptoms severity also correlated with depression, anxiety and somatization (r=0.203, p=0.002; r=0.235, p=0.000; r=0.259, p=0.000, respectively)
4) Association of hs-CRP with IBS and co-morbid conditions
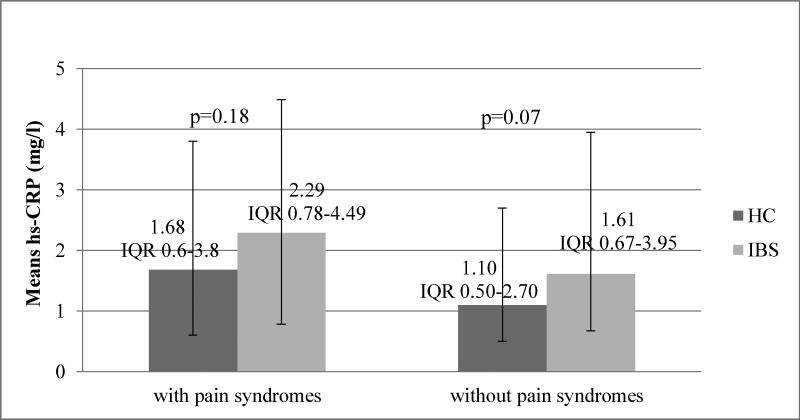
We used stratified analyses to adjust for possible inflammation associated with co-morbid pain and psychological syndromes, comparing hs-CRP levels between the IBS group and HC within strata defined by presence of pain and/or psychological (depression, anxiety and somatization) syndromes. In all strata, hs-CRP levels were consistently higher in the IBS group compared with the HC. For pain syndrome-defined strata, hs-CRP was 36-46% higher (Figure 4) in IBS patients (2.29, vs. 1.68 mg/l, p=0.18 and 1.61 vs. 1.10 mg/l, p=0.07, with and without pain syndrome, respectively).
Figure 4. hs-CRP levels in IBS vs. HC by morbidity.
Median hs-CRP (mg L−1) levels in IBS vs. HC in both strata: presence or absence of any pain syndrome. Meian hs-CRP levels was higher in IBS group compared with the HC in both strata (2.29, IQR 0.78-4.49 mg/l vs. 1.68, IQR 0.6-3.8mg/l, p=0.18 in patients with pain syndrome, and 1.61 IQR 0.67-3.95 mg/l vs. 1.10, IQR 0.5-2.7 mg/l, p=0.07 in patients without a pain syndrome, respectively).
Similar differences were observed within psychological syndrome strata: IBS versus HC hs-CRP levels were 1.90 vs. 0.58 mg/l (p=0.067), and 1.77 vs. 1.20 mg/l (p=0.043) in participants with and without psychological co-morbidities, respectively. This relationship of higher hs-CRP in IBS patients remained within strata defined by both pain and psychological syndromes (any pain or psychological co-morbidity, versus neither) 1.81 vs 1.20 mg/l in the co-morbid stratum (p=0.042) , and 1.32 vs 1.06 mg/l in the stratum without co-morbidities (p=0.460).
In order to test whether the higher hs-CRP levels in subjects with IBS are explained by other commonly reported co-morbidities, we created a multivariate linear model, designed to identify which, if any, co-morbid variables significantly predicted hs-CRP levels. In this model IBS was found to be a significant (p=0.025) predictor for hs-CRP whereas pain syndromes and psychological co-morbidities were not. Moreover, when adjusted to age, gender, pain syndromes (fibromyalgia, pelvic pain, migraine and chronic fatigue ) and psychological co-morbidities (somatization) factors that affect hs-CRP - hs-CRP levels were higher by 33% (95% CI 1.05-1.68) among IBS patient compare to HC.
DISCUSSION
Several lines of data implicate a possible pathomechanistic role for alterations in the intestinal immune function and low grade inflammation in some patients with IBS 3-5, 21. Genetic studies have demonstrated genetic polymorphisms in TNF-α 22, 23, IL-2 24, IL-4 24, IL-6 22, and IL-10 24, 25 in patients with IBS 4. Several studies have shown ongoing inflammatory changes (e.g., increased IL-1β expression 12 and lymphocytes infiltration 26) in the mucosa of patients with PIIBS and other studies have demonstrated increased infiltration of inflammatory cells (e.g., mast cell 27, and T lymphocytes 28) in the intestinal mucosa of patient with IBS, not related to PI-IBS.
However, it is recognized that inflammatory processes at the intestinal mucosal level are not always reflected in peripheral blood. Indeed, the data documenting systemic inflammatory responses in peripheral blood in patients with IBS is much more limited. Pullis et al 29 have reported a significantly higher hs-CRP levels in patients with IBS-D (n=117, 1.435 mg/l) compare to patients with IBS-C (n=42, 0.383 mg/l; p<0.0001) . However this study did not compare these levels to HC. In a recent case control study 16 we found that the levels of hs-CRP were significantly higher in patients with IBS (n=88, 1.17±1.26 mg/dl) compared to HC (n=352, 0.72mg/dl; p<0.001), although these values were within the normal range (0-5 mg/l). We also found a correlation between hs-CRP levels and IBS severity, however, this correlation was observed only among men and we could not demonstrate a similar correlation among women. Although these findings suggest a detectible systemic inflammatory response in IBS our previous study had several limitations. First, due to the small sample size we were not able to investigate and compare the levels of hs-CRP between the clinically relevant subtypes of IBS. Thus, although it is reasonable to hypothesize that inflammatory responses are associated with specific subtypes of IBS (e.g., IBS-D; as suggested by Pullis et al 29) we were not able to provide information on this issue. Second, due to limitations of the dataset used in our previous study, we could not investigate possible confounding effects of commonly reported co-morbidities that can be associated with elevated hs-CRP such as fibromyalgia, migraine headache, pelvic pain, and TMJ syndrome. Third, our previous results may not be generalizable to the general population of clinic patients with various chronic diseases given that patients with chronic diseases and CRP higher than 10 mg/dl were excluded.
Using data from a large study which was carried out at UNC we were able to confirm our previous findings of higher hs-CRP serum levels in IBS patients compared to HC 16, 29. In the current study hs-CRP levels were higher in both IBS and HC groups compared to our previous study. However, as in our previous study, the hs-CRP levels in both groups were within the normal range of 0-5 mg/dl. It should be noted that 36 out of 244 HC and 53 out of 242 IBS patients had hs-CRP levels above 5 mg/dl suggesting a possible slightly higher prevalence of low grade inflammation in a subgroup of patients with IBS compared to HC. The differences in hs-CRP levels between the two studies could be due to disparity in the exclusion criteria: in the previous study we excluded subjects with CRP levels>10 mg/dl 30 and those having inflammatory conditions which may affect CRP levels, whereas in the current study we used a cut-off hs-CRP level of 35 mg/dl in order to extend the generalization of our results.
The larger dataset in this study also enabled us to investigate and compare hs-CRP levels in different subgroups of patients based on symptoms severity and bowel characteristics. As hypothesized, and consistent with Pullis et al study, the highest hs-CRP levels were found in the subgroup of patients with IBS-D (Figure 3).
In addition, we were able to demonstrate, for the first time, that hs-CRP levels correlate with symptoms severity of IBS suggesting that IBS, and specifically IBS-D, may be associated with systemic inflammatory responses. However, as evident by the correlation analysis the association between hs-CRP and symptoms severity are significant although relatively weak, both in the general IBS population (r=0.169 p=0.009) and in IBS-D patients (r=0.27, p=0.006). These weak correlations can be partially explained by differences in the distribution of symptom severity in our study population, as most IBS patients reported mild symptom severity (n=126, 52.5%), followed by moderate (n=87, 36.3%) and only a minority (n=27, 11.3%) had severe symptom severity. Alternatively, these findings can suggest that systemic inflammation is not a prominent phenomenon in IBS or that hs-CRP in peripheral blood is not a sensitive marker to detect possible mucosal inflammatory responses in these patients.
As mentioned above, the majority of IBS patients in our study suffered from a mild disease severity, which represent the general IBS population 18. Moreover, the higher percentage of women 1, 31, and the age range (18-81 years) with most subjects younger than 45 years, are typical of IBS populations as previously reported 32. In addition, the clinical subtype distribution, IBS-D being the most common (41.7%), followed by IBS-C (16.1%) and IBS-M (5.4%), is also typical of IBS 33.
With regard to psychological and pain co-morbidities, as expected we found that a significantly higher percentage of IBS patients suffered from psychological disorders (e.g., depression, anxiety and somatization) and had more pain syndromes (e.g., pelvic pain, fibromyalgia, migraine, chronic fatigue and TMJ syndrome) and other co-morbid conditions compared to HC (Table 1). These findings are in agreement with previous studies34-36. Using a multivariate model, we found that IBS significantly predicted higher hs-CRP levels, whereas co-morbidities including psychological disorders and pain syndromes were not. This, together with the finding of similar difference in hs-CRP levels (higher by 0.5-0.6 mg/l, 36-46%) in IBS cases compared to controls and within strata of presence of co-morbidities, suggest that the differences in hs-CRP levels between cases and controls are independent of the commonly reported co-morbidities in IBS.
Moreover, we found that hs-CRP levels among IBS patients are higher by 33% (95% CI 1.05-1.68) in a multivariate linear model after adjustments for multiple factors that might affect CRP. This finding confirms the crude association shown in Figure 1.
Our study has several strengths. The IBS status was rigorously defined by validated questionnaires, physician diagnosis, laboratory screening blood tests and recant normal colonoscopy/barium enema. The large population size allowed for adequately powered comparisons between the different clinical subtypes of IBS and according to severity levels. The instruments used to measure possible confounders enabled adjustment for other factors known to affect CRP levels such as psychological disorders 37 and additional pain syndromes 38. Another strength is the similarity of age, BMI, ethnicity and gender (variables known to affect CRP level) between the IBS and HC by groups.
In addition, over one-third of IBS patients (89 patients, 36.8%) did not meet any of the subtype's definition, due to the meticulous definition of ROME III, and therefore they were classified as “unspecified subtype”, and were not included in the analysis. This should have reduced possible misclassification bias due to differing bowel syndromes
A limitation of our study is the use of inflammatory markers measured in peripheral blood which may not fully reflect inflammatory processes and immune responses at the level of the intestinal mucosa, leading to possible mischaracterization of outcomes. An additional potential limitation is the substantial variability between the patients within groups. Thus, while medians are significantly different, the individual values would not provide much discriminant ability to diagnose IBS or rule it out.
In addition, while we had 242 total IBS patients, we only had 153 subtyped IBS patients due to the strict definition of ROME III for IBS subtypes. Once these 153 patients are further divided into 3 subtypes of IBS-D (n=101), IBS-C (n=39), and IBS-M (n=13), power is limited, especially for IBS-M subtype. Thus, comparisons of hs-CRP stratified by severity were performed between IBS-D vs. IBS-C.
In conclusion, the results of our study support the hypothesis that intestinal inflammation may play a role in the pathogenesis of symptoms in a subgroup of patients with IBS. Our findings suggest that inflammatory responses in IBS may be identified in peripheral blood, specifically in IBS-D patients with severe symptoms. Our study underline the need for further investigation to identify additional sensitive inflammatory biomarkers for IBS and to determine whether inflammatory biomarkers can contribute to the diagnosis, direct specific treatment or serve as potential markers of response to therapy.
Acknowledgments
Financial support
None for the design, funding, conduction, analysis, interpretation and writing of this study. The collection of the data set used in this study was supported by GSK.
ABBREVIATIONS
- ESR
Erythrocyte sedimentation rate
- FOBT
Fecal occult blood test
- GERD
Gastrooesophageal reflux disease
- GI
Gastrointestinal
- HC
Healthy controls
- Hs-CRP
High-sensivite C-Reactive protein
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- IBS-C
Constipation-predominant irritable bowel syndrome
- IBS-D
Diarrhea-predominant irritable bowel syndrome
- IBS-M
Mix irritable bowel syndrome
- IBS-U
Unspecified irritable bowel syndrome
- TMJ syndrome
Temporomandibular joint syndrome
Footnotes
Conflict of interest disclosure
None
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Mach T. The brain-gut axis in irritable bowel syndrome--clinical aspects. Med Sci Monit. 2004;10:RA125–31. [PubMed] [Google Scholar]
- 3.Chan J, Gonsalkorale WM, Perrey C, et al. IL-10 and TGF-B genotypes in irritable bowel syndrome: Evidence to support an inflammatory component gastroenterology. 2000;118:A184. [Google Scholar]
- 4.Ringel Y, Maharshak N. The Intestinal Microbiota and Immune Function in the Pathogenesis of Irritable Bowel Syndrome. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–35. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt RB, Katz L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541–5. [PubMed] [Google Scholar]
- 7.Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–9. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary NA, Truelove SC. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962;31:307–22. [PubMed] [Google Scholar]
- 10.Spiller RC. Irritable bowel syndrome: bacteria and inflammation--clinical relevance now. Curr Treat Options Gastroenterol. 2007;10:312–21. doi: 10.1007/s11938-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 11.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–6. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 14.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Ohman L, Isaksson S, Lindmark AC, et al. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–12. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 16.Hod K, Dickman R, Sperber A, et al. Assessment of high-sensitivity CRP as a marker of micro-inflammation in irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:1105–10. doi: 10.1111/j.1365-2982.2011.01788.x. [DOI] [PubMed] [Google Scholar]
- 17.Dorn SD, Morris CB, Hu Y, et al. Irritable bowel syndrome subtypes defined by Rome II and Rome III criteria are similar. J Clin Gastroenterol. 2009;43:214–20. doi: 10.1097/MCG.0b013e31815bd749. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Li Z, Toner BB, et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986–95. doi: 10.1007/BF02064187. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hartono JL, Mahadeva S, Goh KL. Anxiety and depression in various functional gastrointestinal disorders: do differences exist? J Dig Dis. 2012;13:252–7. doi: 10.1111/j.1751-2980.2012.00581.x. [DOI] [PubMed] [Google Scholar]
- 21.Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(Suppl 2):1–9. doi: 10.1111/j.1365-2036.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- 22.Barkhordari E, Rezaei N, Ansaripour B, et al. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J Clin Immunol. 2010;30:74–9. doi: 10.1007/s10875-009-9342-4. [DOI] [PubMed] [Google Scholar]
- 23.van der Veek PP, van den Berg M, de Kroon YE, et al. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–6. doi: 10.1111/j.1572-0241.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 24.Barkhordari E, Rezaei N, Mahmoudi M, et al. T-helper 1, T-helper 2, and T- regulatory cytokines gene polymorphisms in irritable bowel syndrome. Inflammation. 2010;33:281–6. doi: 10.1007/s10753-010-9183-6. [DOI] [PubMed] [Google Scholar]
- 25.Gonsalkorale WM, Perrey C, Pravica V, et al. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–3. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HS, Lim JH, Park H, et al. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–57. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 28.Barbara G, De Giorgio R, Stanghellini V, et al. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):i41–4. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poullis AP, Zar S, Sundaram KK, et al. A new, highly sensitive assay for C- reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409–12. doi: 10.1097/00042737-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, O'Keefe G, Jialal I. Defining the proinflammatory phenotype using high sensitive C-reactive protein levels as the biomarker. J Clin Endocrinol Metab. 2005;90:4549–54. doi: 10.1210/jc.2005-0069. [DOI] [PubMed] [Google Scholar]
- 31.Ringel Y, Sperber AD, Drossman DA. Irritable bowel syndrome. Annu Rev Med. 2001;52:319–38. doi: 10.1146/annurev.med.52.1.319. [DOI] [PubMed] [Google Scholar]
- 32.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–9. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 33.Yao X, Yang YS, Cui LH, et al. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2011;27:760–5. doi: 10.1111/j.1440-1746.2011.06930.x. [DOI] [PubMed] [Google Scholar]
- 34.Vandvik PO, Wilhelmsen I, Ihlebaek C, et al. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther. 2004;20:1195–203. doi: 10.1111/j.1365-2036.2004.02250.x. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Agnihotri A, Pathak MK, et al. Psychiatric, somatic and other functional gastrointestinal disorders in patients with irritable bowel syndrome at a tertiary care center. J Neurogastroenterol Motil. 2012;18:324–31. doi: 10.5056/jnm.2012.18.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogelzangs N, Duivis HE, Beekman AT, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tak LM, Bakker SJ, Slaets JP, et al. Is high-sensitive C-reactive protein a biomarker for functional somatic symptoms? A population-based study. Brain Behav Immun. 2009;23:1014–9. doi: 10.1016/j.bbi.2009.05.059. [DOI] [PubMed] [Google Scholar]