Abstract
Introduction
Inherited fXI deficiency has been an enigma since its discovery in 1953. The variable and relatively mild symptoms in patients with even the most severe form of the disorder seem out of step with the marked abnormalities in standard clotting assays. Indeed, the contribution of factor XI to hemostasis in an individual is not adequately assessed by techniques available in modern clinical laboratories.
Areas Covered
We discuss clinical studies, genetic/genomic analyses, and advances in laboratory medicine that are reshaping our views on the role of factor XI in pathologic coagulation. We review how the disorder associated with factor XI deficiency has contributed to changes in blood coagulation models, and discuss the complex genetics of the deficiency state and its relationship to bleeding. Finally, we cover new laboratory approaches that may distinguish deficient patients who are prone to bleeding from those without such predisposition.
Expert Commentary
Advances in understanding the biology of factor XI have led to modifications in treatment of factor XI-deficient patients. Factor replacement is used more judiciously, and alternative approaches are gaining favor. In the future, better laboratory tests may allow us to target therapy to those patients who would benefit most.
Keywords: Factor XI, Factor XI Deficiency, Factor XII, Prekallikrein, Contact Activation
1. INTRODUCTION
Between 1953 and 1955, Robert Rosenthal and his colleagues at the Beth Israel Hospital in New York described four generations of a family with a disorder characterized by mild to moderate bleeding after dental procedures or surgery [1,2]. Plasmas from affected individuals clotted slowly in glass tubes, similar to plasmas in the hemorrhagic disorders hemophilia A and hemophilia B. However, the clotting defect in patient plasma was corrected on mixing with hemophiliac plasma, indicating the patients lacked a factor different from those involved in hemophilia. Rosenthal named the missing entity plasma thromboplastin antecedent (PTA). In 1961, the International Committee for the Nomenclature of Blood Clotting Factors proposed factor XI (fXI), to distinguish it from the factors missing in hemophilia A (factor VIII [fVIII]), hemophilia B (factor IX [fIX]), and Hageman trait (factor XII [fXII]) [3].
The bleeding disorder in fXI-deficient patients (sometimes called hemophilia C or Rosenthal Syndrome) has presented practical and conceptual challenges for clinicians and basic scientists. While fXI is necessary for normal coagulation in in vitro systems such as the activated partial thromboplastin time (aPTT) assay used in hospital laboratories, bleeding in fXI deficiency is relatively mild [4]. Furthermore, the association between the amount of fXI in plasma as determined by the aPTT and bleeding propensity is weak [5–8]. A recent survey concluded that, of the known congenital coagulation factor deficiencies, the correlation between factor level and symptoms is poorest for fXI [6,8]. As numerous conditions can contribute to mild bleeding, these observations raise questions about the actual importance of fXI to blood coagulation in vivo. Here, we discuss fXI deficiency in light of advances in our understanding of the biochemistry and pathophysiology of blood coagulation over the sixty plus years since its discovery. We will start by considering the syndrome linked to fXI deficiency, as it has been instrumental in changing our thinking on how this protein contributes to blood coagulation.
1.1 Bleeding in Factor XI Deficiency
FXI-deficient patients consistently have prolonged aPTTs. Indeed, the aPTT is usually longer in plasma lacking fXI than in plasmas missing fVIII or fIX [9]. Despite this, the bleeding diathesis in fXI-deficient individuals is considerably milder than in hemophilia A or B, and involves different tissues [4–7,10]. The spontaneous soft tissue bleeds and hemarthroses characteristic of hemophilia A and B are not features of fXI deficiency. Rosenthal et al. noted that not all PTA-deficient patients experienced abnormal bleeding, and for those who did, hemorrhage was usually provoked [1,3]. Severe spontaneous bleeding is rare, although menorrhagia and epistaxis are relatively common [11]. FXI deficiency is most problematic when trauma involves the oral and nasal cavities or the urinary tract [4–10]. These tissues are rich in fibrinolytic activity, and the effectiveness of antifibrinolytic drugs such as tranexemic acid and ε-aminocaproic acid in controlling bleeding in fXI-deficient patients [12,13] has led to the hypothesis that the primary role of fXI is prevention of premature clot dissolution. Bleeding with injury at locations other than the urinary tract, mouth or nose is less frequent, and invasive procedures such as circumcision, appendectomy, and orthopedic surgery may be well tolerated without treatment [4,9,10,14]. There is a widely held impression that symptoms in fXI-deficient patients correlate poorly with fXI activity measured by aPTT-based assays [5,7,15,16]. Indeed, many patients with severe fXI deficiency (plasma fXI <15 to 20% of normal) do not experience abnormal bleeding, and several investigators report difficulty distinguishing severe and mild (fXI level 20–40% of normal) deficiency on clinical grounds [5,7,15,16]. However, others have reported hemorrhage in >60% of patients with severe deficiency undergoing tooth extraction, tonsillectomy, nasal surgery, or urologic surgery in the absence of factor replacement, and minimal symptoms with these procedures in mildly deficient individuals [4,10,14].
Several factors likely contribute to the variable presentation, and varying impressions, of fXI deficiency. First, there is a predilection for bleeding with injury to certain tissues, and many fXI-deficient patients do not experience the types of injury that lead to hemorrhage. Second, the primarily mucosal bleeding in fXI deficiency is mild when compared with symptoms with other coagulation factor deficiencies. This likely contributes to inconsistent criteria for what constitutes hemorrhage, and increases the likelihood that symptoms in fXI deficient individuals are unrelated to the deficiency. Third, other conditions such as low von Willebrand factor levels may have a greater effect on the range of symptoms in mild disorders such as fXI deficiency than in more severe disorders such as hemophilia [15,16]. Finally, different fXI mutations could contribute to phenotypic variation. Studies reporting associations between fXI levels and bleeding use data collected primarily from Jewish patients (discussed below) [4,10], while those reporting a lack of an association tend to involve ethnically diverse patient groups [15,16].
These issues aside, some conclusions can be drawn from available data and experience. FXI deficiency can exacerbate trauma-induced bleeding in some individuals, complicating injuries, surgical procedures and childbirth. FXI deficiency also contributes to menorrhagia, with 59% of fXI-deficient women reporting symptoms compared to 10% of the general population [11]. The disorder is significant for these reasons. However, the patterns of bleeding in fXI-deficient patients suggest the protein serves an ancillary role in hemostasis, and is not required under most circumstances. It is also apparent that some individuals do not require fXI. Currently, we lack methods that reliably distinguish fXI-deficient patients who may bleed from those without such predisposition. To appreciate recent work directed at developing such methods, we need to discuss how our understanding of coagulation has changed over the past 50 years.
1.2 Factor XI and the Cascade-Waterfall Model of Coagulation
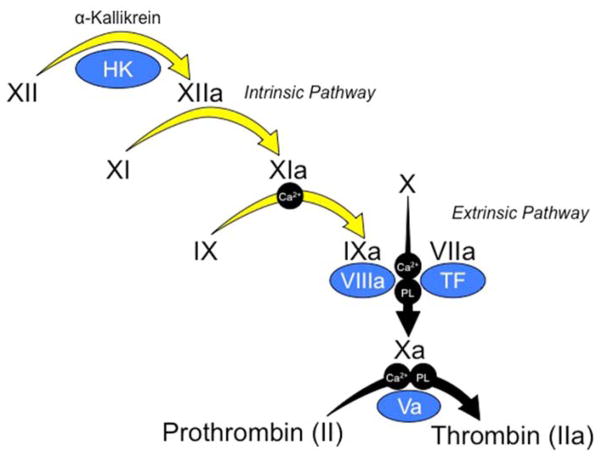
In 1964, Macfarlane, and Davie and Ratnoff, described the cascade-waterfall model of coagulation, which incorporated known coagulation factors into a series of enzymatic reactions leading to thrombin generation [18,19]. In schemes based on this hypothesis (Figure 1), coagulation starts with conversion of factor XII (fXII) to the enzyme fXIIa, and is amplified by sequential activation of fXI, fIX, factor X (fX), and prothrombin. Coagulation can also be initiated by factor VIIa (fVIIa), in a process bypassing fXII, XI, and IX. A feature of the model in Figure 1 is that there are two ways to initiate thrombin generation. Initiation through the intrinsic pathway, as in the aPTT assay, is triggered by contact activation (Figure 2, right panel). In the prothrombin time (PT) assay coagulation is initiated through the extrinsic pathway (Figure 2) by adding a cofactor for fVIIa called tissue factor (TF) to plasma.
Figure 1. Traditional Cascade-Waterfall Model of Thrombin Generation.
Black Roman numerals indicate inactive zymogens of plasma proteases, and the lowercase “a” indicates active protease. Co-factors are indicated in blue ovals. Requirements in some reactions for calcium ions (Ca2+) and phospholipid (PL) are indicated. In the activated partial thromboplastin time (aPTT assay), coagulation is initiated by activation of fXII on a charged surface in a process called contact activation, which involves the protease α-kallikrein and the cofactor high molecular weight-kininogen (HK). FXIIa triggers converting of fXI to fXIa, and fXIa in turn converts fIX to fIXa. The series of reactions indicated by yellow arrows are referred to as the intrinsic pathway. The intrinsic pathway converts fX to fXa. FXa then cleaves prothrombin to generation thrombin. The intrinsic pathway can be bypassed by the extrinsic pathway, which is comprised of fVIIa in complex with the cofactor tissue factor (TF). Activation of fX through the extrinsic pathway is the basis of the prothrombin time (PT) assay.
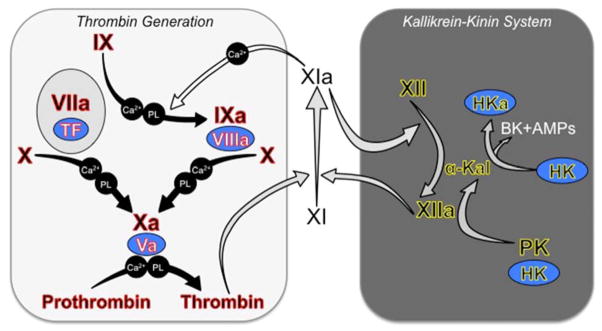
Figure 2. Current Models of Thrombin Generation and Contact Activation.
Black Roman numerals indicate inactive zymogens of plasma proteases, and a lowercase “a” indicates active protease. Co-factors are indicated in blue ovals. Requirements for calcium ions (Ca2+) and phospholipid (PL) in some reactions are indicated. (Left Panel) Tissue Factor-Initiated Thrombin Generation. Thrombin generation is initiated by activation of fX by the fVIIa/TF complex. FXa then converts prothrombin to thrombin in the presence of fVa. FVIIa/TF also activates fIX, and fIXa is responsible for sustaining fX and prothrombin activation [22]. The reactions indicated by the black arrows form the core of the thrombin generation mechanism in vertebrate animals [30]. Complete absence of one of the proteins highlighted in red causes either a severe bleeding disorder or is not compatible with life. FXI is a coagulation protein found only in mammals [31]. FXI provides another mechanism for fIX activation (white arrow) that supplements fIX activation by fVIIa/TF [34]. It is thought that fXI is activated during hemostasis by thrombin in a reaction requiring an anionic cofactor [24,25]. Polyphosphate (polymerized inorganic phosphate), which is released from platelets upon activation, is a leading candidate for such a cofactor [28]. Note that fXI during hemostasis is not thought to require fXIIa. (Right Panel) Contact Activation (Kallikrein-Kinin System). Exposure of blood to a variety of artificial and biologic surfaces triggers contact activation [21,33]. FXII and prekallikrein (PK) bind to the surface and convert each other to fXIIa and α-kallikrein. High molecular weight-kininogen (HK) is a cofactor for the reaction, facilitating PK binding to the surface. FXIIa can activate fXI leading to thrombin generation. There is also evidence that fXIa, like α-kallikrein, can activate fXII [51]. Contact activation triggers thrombin generation in the aPTT assay, but it is doubtful that it contributes to hemostasis, as congenital fXII, PK or HK deficiency does not cause a bleeding disorder. Because of this, fXII, PK and HK are often considered to form a system separate from fXI referred to as the kallikrein-kinin system (KKS components highlighted in yellow) [21,33]. Activation of the KKS results in cleavage of HK by α-kallikrein generating the potent vasoactive peptide bradykinin (BK) and antimicrobial peptides (AMPs) that likely play a role in host-defense. In this figure, gray arrows indicate reactions that are enhanced by polyanions such as polyphosphate, DNA and RNA.
The PT and aPTT are rate-based assays. That is, the assay end points are the speed with which a clot forms. However, during coagulation most thrombin forms after clot formation, and is involved in clot consolidation and maintenance [20]. Such activities are not assessed by the PT or aPTT. While the clinical syndrome in fXI-deficient patients suggests fXI serves a supporting role in clot consolidating, it is commandeered for initiating coagulation in the aPTT. The trigger for the aPTT is contact activation [10,21], a process involving fXII and prekallikrein (PK) and high molecular weight kininogen (HK) (Figure 2, right panel). FXIIa generated during contact activation converts fXI to fXIa leading to thrombin generation. Individuals lacking fXII or PK, like fXI-deficient patients, have long aPTTs. However, they do not bleed abnormally [9,21]. In the aPTT assay, then, fXI is activated by a process not required for hemostasis, and then drives initial clot formation, an activity unlikely to reflect its physiologic role. The different bleeding propensities in patients lacking fIX, fXI and fXII demonstrate that this series of reactions does not accurately reflect processes in vivo.
1.3 Factor XI in Tissue Factor-Initiated Coagulation
In current coagulation models, thrombin generation is initiated by the enzyme fVIIa bound to TF, a membrane protein exposed to blood at sites of injury [22,23]. In addition to activating fX (as in the PT assay), fVIIa/TF converts fIX to fIXa, sustaining fXa and thrombin production (Figure 2, left panel). Here, as in the cascade-waterfall scheme, fXIa is assigned a role in fIX activation. But instead of acting as the sole fIX activator, fXIa supplements fIXa produced by the fVIIa/TF complex. This supporting role is more consistent with the variable symptoms observed in fXI-deficient patients. As fXII is not required for hemostasis, the model predicts a fXII-independent mechanism for fXI activation. For example, thrombin generated early during coagulation activates fXI [24–26]. When compared with fXIIa, thrombin is a slow activator of fXI in vitro. However, it is well recognized that anionic polymers such as dextran sulfate and heparin can accelerate fXI activation by thrombin [24–26]. Anionic polymers of phosphate groups (polyphosphate) are present in many tissues, and are released from dense granules upon platelet activation [27]. In vitro, platelet-sized polyphosphate (60–80 phosphate units) enhances fXI activation by thrombin several thousand-fold [28,29], suggesting it is a pathophysiologically relevant cofactor for fXI activation in vivo.
The features of the scheme in the left hand panel of Figure 2 that distinguish it from the cascade-waterfall model are that (1) there is only one mechanism for initiating thrombin generation; (2) there are two mechanisms for fIX activation, explaining the different phenotypes associated with fIX and fXI deficiencies; and (3) there is a fXII-independent mechanism for fXI activation. With this model in hand, we can propose simple explanations for variable bleeding in fXI deficiency. There is undoubtedly variation in the strength of the procoagulant stimulus initiated by fVIIa/TF within a population, and in different tissues within an individual. Similarly, activities countering clot formation and integrity such as fibrinolysis also vary. The importance of fXI to hemostasis likely depends on the balance between these processes. In situations where thrombin generation is weak or fibrinolysis brisk, fXI may play a significant role in stemming injury-induced bleeding. A more robust fVIIa/TF response or weak fibrinolytic response may obviate the need for fXI.
1.4 The Natural History of Factor XI
Analyses of vertebrate genomes have provided insight into the evolution of fXI that lends support to the coagulation model shown in Figure 2 [30,31]. The coagulation factors highlighted in red form the core of the thrombin generation mechanism in nearly all vertebrates [30]. Genes for fXII, PK and a kininogen similar to HK are found in most land vertebrates, but not fish, indicating they arose later than the thrombin generation mechanism [31,32]. FXI is a recent addition to the overall scheme, being found only in mammals [31], in keeping with the impression that fXI is not as important to hemostasis as the core proteins highlighted in red. Furthermore, it is apparent that during much of vertebrate evolution fXII did not communicate directly with the thrombin generation mechanism in the manner described by the cascade-waterfall model because of the absence of fXI (Figure 1). This is consistent with the observation that fXII, PK and HK are not required for hemostasis in modern vertebrates. This does not necessarily mean that these proteins do not support thrombin generation in mammals for purposes other than hemostasis. FXII, PK and HK comprise the kallikrein-kinin system (KKS, highlighted in yellow. Figure 2, right panel), which contributes to several host-defense and homeostatic functions including the initial response to infection [21,33]. Thrombin plays an important role in the response to infection, and it would make sense that the KKS can trigger thrombin generation through fXI activation for this purpose.
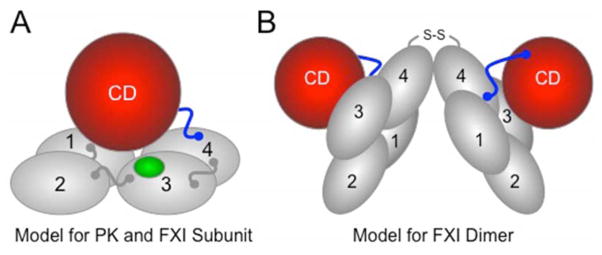
The FXI gene is the result of a duplication of the PK gene [30,31]. PK and fXI, therefore, are homologs, consistent with the observation that they are both fXIIa substrates. However, fXI has acquired features that facilitate interaction with the thrombin generation mechanism that distinguish it from PK. Each PK and fXI polypeptide contains four apple domains and a trypsin-like catalytic domain (Figure 3A) [34,35]. FXI differs from PK in that it has a fIX binding site in the apple 3 domain [36], and an activation site that permits cleavage by thrombin as well as fXII [26]. Also, unlike PK, fXI is a dimer containing two identical polypeptides (Figure 3B) [34,35]. The functional significance of this feature is poorly understood, but it has important implications for inheritance of fXI deficiency (discussed in the following section). The combination of novel features and PK-like properties allow fXI to function as a fXIIa-independent coagulation factor, and as a fXIIa-dependent contact factor, as shown in Figure 2.
Figure 3. Models for Prekallikrein and Factor XI.
(A) PK and fXI subunit structure. PK and fXI are homologs [31]. Each subunit for these proteins is comprised of four apple domains (1 through 4) and a trypsin-like catalytic domain (CD) [34,35]. The apple domains form a planar disk on which the catalytic domain rests. Activation of PK and fXI by fXIIa occurs by a single proteolytic cleavage within the portion of the proteins indicated in blue. The sequence around the fXIIa cleavage site on fXI differs from that in PK, allowing fXI to also be activated by thrombin [26]. There is a fIX binding site on the fXI apple 3 domain (indicated in green) that is not present on PK [36]. The fIX binding site is not exposed in fXI, but becomes available for fIX binding when fXI is converted to fXIa. (B) The factor XI dimer. FXI is a homodimeric protein in all species in which it has been evaluated [34,35]. The apple 4 domain serves as the interface for two fXI subunits which are oriented at a 70° angle to each other [34] In most species, a disulfide bond (S-S) connects the subunits. While dimeric and monomeric forms of fXI behave similarly in aPTT assays, the monomeric forms perform poorly in mouse thrombosis models, indicating that the dimeric conformation serves a critical function in vivo [29].
1.5 The Genetics of Congenital Factor XI Deficiency
While the incidence of severe fXI deficiency in the general population is estimated at only one per million persons [8], the disorder is common in Ashkenazi Jews, in whom the carrier frequency for an abnormal fXI allele is ~5%, and severe deficiency is present in one in every 450 persons [37,38]. Rosenthal’s first impression that PTA-deficiency was a dominant trait [1,2] was probably influenced by an under-appreciation of the frequency of abnormal fXI alleles in the Jewish population. Two mutations account for >90% of abnormal alleles in Jews [37,38]. The Glu117Stop (type II) mutation introduces a premature termination codon in the apple 2 domain, while the Phe283Leu (type III) mutation in the apple 4 domain interferes with dimer formation and reduces protein secretion [39,40]. In Ashkenazi Jews 2.2% and 2.5% of fXI alleles carry the types II and III mutations, respectively [38]. The type II mutation is also found in Iraqi, Sephardic/North African, and other Middle Eastern Jews, and in Arabs, consistent with an origin > 2500 years ago [37]. The type III mutation appears to be of more recent European origin [38]. Over 200 other fXI gene mutations have been identified in fXI deficient patients (http://www.hgmd.org) [4,9]. A few, such as Cys38Arg (allele frequency ~0.5% in French Basques) [41] and Cys128Stop (~10% of mutant fXI alleles in Great Britain) [42] may be relatively common.
With few exceptions, fXI mutations interfere with protein secretion and/or stability. Reduced plasma activity is, therefore, a consequence of low protein level. Patients homozygous for the type II mutation do not have fXI antigen in their plasmas, while type III homozgyotes have ~10% of the normal antigen and activity [10]. Compound heterozygotes have intermediate levels. Carriers for either mutation, have levels of 50 to 65% of normal [10]. In a study of 45 mostly Jewish families, the odds ratios for excessive bleeding were 13.0 and 2.6 for homozygotes and heterozygotes, respectively [43]. Thus, mild deficiency appears to confer only a slightly increased risk for bleeding, consistent with other autosomal recessive coagulation factors deficiencies.
While the preponderance of evidence indicates fXI deficiency is an autosomal recessive disorder in Jewish patients, the dimeric fXI structure could allow the product of a single mutant allele to exert a negative effect on the product of a normal allele [44,45]. This can occur if a mutant subunit forms a dimer with a normal subunit, and the resulting heterodimer cannot be secreted. Such a “dominant negative” effect has been demonstrated for the fXI mutations Ser225Phe, Cys398Tyr, Gly400Val, and Trp569Ser [44,45], and may account for families in which symptomatic severe to moderate factor XI deficiency (levels of 15–40% of normal) is convincingly transmitted as a dominant trait. Mutant alleles that do not produce a protein product (i.e. Glu117Stop) or that interfere with dimer formation (i.e. Phe283Leu) would not produce a dominant negative effect [44], consistent with the recessive inheritance pattern observed Jews. The dominant negative mechanism may in fact be relatively common in non-Jewish patients, with an estimated incidence of 1 in 30,000 [8]. Given that the fXI levels in affected individuals tend to run at intermediate levels between those for homozygotes and heterozygotes for the type II and III mutations, this may partly explain the poorer correlation between fXI levels and symptoms in non-Jewish patients.
1.6 Laboratory Testing to Assess Factor XI Activity
The lack of standardized assays to assess fXI’s physiologically relevant functions places limits on our ability to predict bleeding tendencies in fXI deficient patients. This leads, out of necessity, to a largely empiric treatment approach to the disorder. Clinical laboratories use the aPTT to screen for fXI deficiency, with the diagnosis established by comparing the capacities of patient and normal plasma to correct the aPTT of fXI-deficient plasma [9]. While these assays establish how much fXI is present in plasma as a percentage of the mean normal level, as we have discussed the values do not reflect the importance of fXI to hemostasis in an individual very well. Our current models predict that fXI sustains thrombin generation after clot formation, and that this activity is particularly important for clot stability in the face of fibrinolysis. Two types of assays have been developed that focus on these functions.
Thrombin generation assays use a fluorescent substrate to follow thrombin formation in plasma over time, irrespective of clot formation [20,46]. Coagulation in plasma collected into sodium citrate may be initiated by recalcification with or without low concentrations of TF. An inhibitor of fXIIa such as corn trypsin inhibitor (CTI) may be added to block contact activation-induced fXI activation, isolating the pathway involving thrombin-mediated fXI activation. Livnat et al. reported that thrombin generation in recalcified platelet poor-plasma can be used to distinguish fXI deficient patients with bleeding propensities from those without symptoms, particularly when fXI levels were 2 to 20% of normal [47]. Curiously, this discriminatory ability was lost if TF or CTI was added, indicating fXIa generated by fXIIa was being measured. In contrast, Pike et al. noted significant differences in thrombin generation between fXI deficient bleeders and non-bleeders in platelet-rich plasma with 0.5 pM TF and CTI, [48]. Here, the inclusion of platelets may have provided a co-factor for fXI activation by thrombin in the form of polyphosphate [27–29]. These promising studies suggest that it may be possible to develop tests that identify fXI-deficient patients with bleeding propensities, but it is clear that the optimal assay conditions have not been established. Furthermore, sample collection methods that avoid induction of contact activation or platelet activation are required to obtain useful results. In this regard, thrombin generation assays are far less forgiving than PT and aPTT assays. Proper phlebotomy techniques may be difficult to introduce into clinical settings.
Zucker et al. studied the importance of fXI to clot resistance to fibrinolysis, using platelet poor plasma supplemented with TF in the presence or absence of tissue plasminogen activator [tPA] to activate fibrinolysis [49]. Plasmas from patients with bleeding histories exhibited lower fibrin network density (determined by turbidity and laser scanning confocal microscopy) and lower clot stability in the presence of tPA than plasmas from asymptomatic patients. While this type of analysis is technically demanding, it addresses the role of fibrinolysis and clot instability in bleeding in fXI deficiency. More recently, Colucci et al. reported that variation in the sensitivity of plasma clots to fibrinolysis in fXI-deficient patients may be due to a thrombin-independent defect in TAFI activity [50]. While the mechanisms involved remain to be worked out, the finding could be relevant to the varying propensity to bleed in these patients.
2. EXPERT COMMENTARY
As part of the traditional intrinsic pathway of coagulation (Figure 1), fXI provides a link between contact activation and factor IX, and is critical for driving thrombin and fibrin formation in the aPTT assay, [9]. As we have seen, the syndrome associated with fXI deficiency is not compatible with this hypothesis. In our opinion, the enigmatic nature of fXI deficiency derives largely from the use of the aPTT to establish the “severity” of the disorder. FXI is clearly not a key part of the core thrombin generating mechanism comprised of vitamin K-dependent proteases and their cofactors (Figure 2, left panel) [22]. Its role in hemostasis is supplemental and, indeed, its primary functions may lie outside of hemostasis, perhaps in inflammatory responses to injury. We feel that fXI should be classified both as a component of the KKS and as a coagulation protein [21,51]. In contrast to its role in the cascade-waterfall model, fXI may actually operate as a bidirectional interface between thrombin generation and the pro-inflammatory KKS (Figure 2), facilitating communication between the systems in the host response to injury or infection [21,51].
For the moment, fXI deficiency remains important to clinicians because it can cause excessive trauma-induced bleeding and complicate childbirth [4–9]. Pending availability of laboratory assays that can distinguish fXI-deficient bleeders from non-bleeders, we must rely on clinical experience and fXI levels measured through aPTT-based assays. When preparing patients with severe deficiency (fXI level <15 to 20% of normal) for invasive procedures it should be remembered that a negative bleeding history does not establish a low bleeding risk in the future [4,19], that certain procedures are associated with more bleeding than others [4–9,14–16], and that patients with very low fXI levels (<1%) are at substantial risk for develop neutralizing antibodies after factor replacement [52]. These considerations have led to refinements in treatment strategies that limit exposure to fXI. For Jewish patients, fXI deficiency is essentially a recessive disorder [4,9,53], and those with severe (homozygous or compound heterozygous) deficiency will require the majority of treatment. Patients with fXI levels >40% of normal generally do not experience abnormal bleeding and do not require special preparation for surgery. This approach may not hold for some fXI deficient individuals who are not of Jewish ancestry [6–8,15,16], in whom autosomal dominant forms of the disorder are more common. Intermediate deficiency (20 to 40% of normal) is more prevalent in this group, and patients may benefit from factor replacement for high-risk procedures involving the prostate, mouth or nasal cavity.
Factor replacement started prior to surgery is recommended for most major procedures in fXI deficient patients. Those undergoing surgery on the oropharynx, nasopharynx or urinary tract should be treated with plasma or fXI concentrate to keep the plasma trough level >40% of normal for at least seven days [4,7–9]. Supplementation with antifibrinolytic therapy can be considered, although caution should be exercised in patients receiving fXI concentrate because of the potential for thrombotic events. Factor replacement is appropriate for neurosurgery, head and neck surgery, cardiothoracic procedures, and major abdominal or pelvic surgery [4,7–9]. A strategy in which replacement is withheld unless bleeding occurs is gaining favor for procedures with low bleeding rates such as circumcision, appendectomy and some orthopedic surgery [4,16,43,53]. In a recent phase 2 trial in which antisense olignonucleotide (ASO) therapy was used to reduce plasma fXI as prophylaxis to prevent venous thrombosis, patients underwent knee replacement with fXI levels as low as a few percent of normal without experiencing excessive bleeding [54]. Avoiding factor replacement unless bleeding occurs has also been advocated in pregnancy for normal vaginal deliveries [4,55], although this approach is not universally favored [8,11,56]. There is little data to evaluate the safety of epidural anesthesia in the absence of factor coverage, and replacement is generally advised [4,11,56]. However, some patients in the fXI ASO knee replacement study with low fXI levels did undergo epidural anesthesia without adverse effect [54]. Obviously, the “wait and see” approach requires an appreciation of the consequences of bleeding for a specific procedure, which must be incorporated into decisions to use or not use prophylactic replacement.
Other agents have been used in place of factor replacement in some situations. In patients with fXI antibody inhibitors, a strategy based on a single dose of recombinant factor VIIa (15–30 μg/kg) followed by antifibrinolytic therapy has maintained hemostasis in patients undergoing major surgery, including one case of aortic dissection repair [13]. Factor VIIa (15–30 μg/kg) every two to four hours in conjunction with antifibrinolytic therapy has been used successfully during surgery [57,58] or epidural anesthesia [59] in lieu of factor replacement in patients without inhibitors who wish to avoid plasma exposure. This strategy may be preferable in patients with very low plasma fXI levels (<1% of normal), such as homozygotes for the type II mutation, ~30% of who develop neutralizing antibodies after exposure to fXI [52]. Certain procedures such as tooth extraction or skin biopsies can be managed with ε-amino caproic acid or tranexamic acid alone starting 12 hours before the procedure and continuing for seven days, and dental procedures such as scaling and root canal can be covered with ε-amino caproic acid or tranexamic acid mouthwash prepared from the intravenous formulation three to four times daily with or without systemic antifibrinolytic therapy [4,9,53].
3. 5-YEAR VIEW
Looking forward, improvements in assays that better reflect the roles of fXI in clot stability will hopefully assist in identifying fXI deficient patients who are prone to bleeding prior to an invasive procedure. This will facilitate targeting therapy to the subsets of patients who would benefit most, while reducing exposure to potentially prothrombotic agents in those who would derive little benefit.
The nature of fXI replacement therapy is likely to evolve over the next several years, although the direction it will take is not certain. In many parts of the world, including the United States, plasma is used for replacement because fXI concentrates are not readily available. Two plasma-derived concentrates, Hemoleven (a high purity product from LFB Biomedicaments, Les Ulis, France) and FXI Concentrate (a partially purified product from Bioproducts Laboratory, Elstree, UK) have shown efficacy, but have yet to be widely used [8,60,61]. A three-year post-marketing analysis of Hemoleven indicated it was effective as prophylaxis for surgery, invasive procedures and pregnancy, and for treating active bleeding [62]. However, safety concerns have led to recommendations to use it sparingly [8, 60,61]. The first fXI concentrates appeared to be responsible for cases of thrombosis and DIC, mostly in older patients with cardiovascular disease receiving doses >30 units/kg [4,60,63]. Traces of fXIa in the concentrates were thought to be responsible, and current preparations contain serine protease inhibitors (antithrombin, C1-inhibitor) and heparin to blunt this effect [60,61]. Recent analyses indicate that patients receiving lower doses (20 to 30 units/kg) are still at risk for thrombosis, and it has been suggested that initial doses should not exceed 10 to 15 U/kg, and that they should be avoided in patients with a history of thrombosis, or with thrombotic risk factors [60,62]. Because of these concerns, some practitioners have opted to use other products, such as recombinant factor VIIa with or without thrombolytics. At this point it is not clear if such approaches will supplant factor replacement in some or most clinical situations.
Finally, fXI appears to contribute to venous thrombosis and ischemic stroke, and perhaps myocardial infarction [23,64]. The recent ASO trial mentioned above demonstrated that lowering plasma fXI levels to ≤20% of normal was effective in preventing venous thrombosis during knee replacement [54]. During this study abnormal intraoperative bleeding did not occur, and post-operative bleeding was rare, despite low (in some cases undetectable) fXI levels at the time of surgery. This exciting result suggests that it may be possible to dissociate (at least partly) a therapeutic antithrombotic effect from an anti-hemostatic (anticoagulant) effect by targeting fXI. Antibody and small molecule inhibitors to fXI and fXIa are entering phase I studies, with the goal of developing a new class of antithrombotics with low bleeding risk [65]. If such drugs prove efficacious, they may be introduced into clinical practice over the next 5 to 10 years, and hematologists will face a new set of questions regarding what procedures can be safely performed on patients receiving these drugs.
KEY ISSUES.
As a component of the intrinsic pathway of coagulation, fXI is required for driving clot formation in the aPTT assay. Patients lacking fXI will have very long aPTTs.
FXI levels measured by aPTT-type assays do not correlate well with bleeding patterns in fXI deficient patients.
Abnormal bleeding in fXI-deficient patients is seldom severe, and usually accompanies trauma or surgery to tissues rich in fibrinolytic activity such as the mouth, nose and urinary tract.
Bleeding in fXI-deficient patients is rarely spontaneous, and some deficient patients do not bleed abnormally, even with trauma to susceptible tissues.
The poor correlation between fXI levels determined by the aPTT assay and bleeding symptoms in fXI-deficient patients reflects the inability of the assays to assess physiologically relevant fXI activities.
FXI is not a component of the core thrombin generation mechanism responsible for hemostasis. Instead, it supports thrombin generation, and is only required in certain situations, and in certain individuals.
FXI may function as an interface between the thrombin generation mechanism and pro-inflammatory mechanisms such as the kallikrein-kinin system. Given this, its major roles may not be in hemostasis.
Novel tests that assess more relevant fXI physiologic functions, such as thrombin generation assays and clot stability assays, may eventually allow us to distinguish fXI deficient patients with a bleeding propensity from those without such a propensity, prior to an invasive procedure.
Footnotes
Declaration of Interest
AP Wheeler has received consultants fees from the following companies (Bayer and Novo Nordisk). D Gailani has received consultants fees and research support from the following companies (Aronora, Bayer, Bristol-Myers Squibb, Dyax Corp., Instrument Laboratory, Ionis, Novartis, and Ono). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
*= Papers of interest, **=Papers of particular interest
- 1.Rosenthal RL, Dreskin OH, Rosenthal N. New hemophilia-like disease caused by deficiency of a third plasma thromboplastin factor. Proc Soc Exp Biol Med. 1953;82:171–4. doi: 10.3181/00379727-82-20057. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal RL, Dreskin OH, Rosenthal N. Plasma thromboplastin antecedent (PTA) deficiency; clinical, coagulation, therapeutic and hereditary aspects of a new hemophilia-like disease. Blood. 1955;10:120–31. [PubMed] [Google Scholar]
- 3.Wright IS. The nomenclature of blood clotting factors. Canad Med Ass J. 1962;86:373–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Duga S, Salomon O. Congenital factor XI deficiency: an update. Sem Thromb Hemost. 2013;39:621–31. doi: 10.1055/s-0033-1353420. [DOI] [PubMed] [Google Scholar]
- 5.Santoro C, Di Mauro R, Baldacci E, et al. Bleeding phenotype and correlation with factor XI (FXI) activity in congenital FXI deficiency: results of a retrospective study from a single centre. Haemophilia. 2015;21:496–501. doi: 10.1111/hae.12628. [DOI] [PubMed] [Google Scholar]
- 6**.James P, Salomon O, Mikovic D, Peyvandi F. Rare bleeding disorders - bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemophilia. 2014;20(Suppl 4):71–5. doi: 10.1111/hae.12402. An excellent summary of assessment tools for evaluating rare bleeding disorders, the optimal management of fXI deficiency, and the correlation between clinical severity and laboratory diagnosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton-Maggs PH. Factor XI deficiency – resolving the enigma? Hematology Am Soc Hematol Educ Program. 2009:97–105. doi: 10.1182/asheducation-2009.1.97. [DOI] [PubMed]
- 8.Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders: A United Kingdom Haemophilia Centre Doctors’ Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2014;167:304–26. doi: 10.1111/bjh.13058. [DOI] [PubMed] [Google Scholar]
- 9.Gailani D, Neff AT. Rare coagulation factor deficiencies. In: Hoffman R, Nenz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, editors. Hematology: basic principles and practice. 6. Saunders-Elsevier; 2010. pp. 1939–52. [Google Scholar]
- 10*.Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325:153–8. doi: 10.1056/NEJM199107183250303. This seminal paper demonstrated that fXI deficiency in Jewish patients is due primarily to two types of mutations (type II and type III mutations), and that fXI levels were lower in homozygotes for the type II mutation than the type III mutation. It also correlated genotype with bleeding, and was one of the earliest studies to separate bleeding events into those involving tissues with high fibrinolytic activity and other tissues. [DOI] [PubMed] [Google Scholar]
- 11.Kadir RA, Economides DL, Lee CA. Factor XI deficiency in women. Am J Hematol. 1999;60:48–54. doi: 10.1002/(sici)1096-8652(199901)60:1<48::aid-ajh8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Berliner S, Horowitz I, Martinowitz U, et al. Dental surgery in patients with severe factor XI deficiency without plasma replacement. Blood Coag Fibrinol. 1992;3:465. [PubMed] [Google Scholar]
- 13.Livnat T, Tamarin I, Mor Y, et al. Recombinant activated factor VII and tranexamic acid are haemostatically effective during major surgery in factor XI-deficient patients with inhibitor antibodies. Thromb Haemost. 2009;102:487–8. doi: 10.1160/TH09-03-0172. [DOI] [PubMed] [Google Scholar]
- 14*.Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006;12:490–3. doi: 10.1111/j.1365-2516.2006.01304.x. Patients with severe fXI deficiency do not inevitably bleed with surgery. This paper describes clinical situations in which factor replacement can be avoided in fXI deficient patients because of the low risk of bleeding. [DOI] [PubMed] [Google Scholar]
- 15.Ragni MV, Sinha D, Seaman F, et al. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood. 1985;65:719–24. [PubMed] [Google Scholar]
- 16.Bolton-Maggs PH, Patterson DA, Wensley RT, Tuddenham EG. Definition of the bleeding tendency in factor XI-deficient kindreds: a clinical and laboratory study. Thromb Haemot. 1995;73:194–202. [PubMed] [Google Scholar]
- 17.Guéguen P, Galinat H, Blouch MT, et al. Biological determinants of bleeding in patients with heterozygous factor XI deficiency. Br J Haematol. 2012;156:245–51. doi: 10.1111/j.1365-2141.2011.08945.x. [DOI] [PubMed] [Google Scholar]
- 18.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 19.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–2. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 20.Al Dieri R, de Laat B, Hemker HC. Thrombin generation: what have we learned? Blood Rev. 2012;26:197–203. doi: 10.1016/j.blre.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 22.Luchtman-Jones L, Broze GJ., Jr The current status of coagulation. Ann Med. 1995;27:47–52. doi: 10.3109/07853899509031935. [DOI] [PubMed] [Google Scholar]
- 23.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets of antithrombotic therapy. J Thromb Haemost. 2015;13:1383–95. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–8. [PubMed] [Google Scholar]
- 25.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–12. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 26.Matafonov A, Sarilla S, Sun MF, et al. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–45. doi: 10.1182/blood-2010-10-312983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissey JH, Smith SA. Polyphophate as a modulator of hemostasis, thrombosis and inflammation. J Thromb Haemost. 2015;13(Suppl 1):S92–97. doi: 10.1111/jth.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng Y, Verhamme I, Smith SB, Sun MF, Matafonov A, Cheng Q, Smith SA, Morrissey JH, Gailani D. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121:3962–3969. doi: 10.1182/blood-2012-12-473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doolittle RF. Step-by-step evolution of vertebrate coagulation. Cold Spring Harb Symp Quant Biol. 2009;74:35–40. doi: 10.1101/sqb.2009.74.001. [DOI] [PubMed] [Google Scholar]
- 31.Ponczek MB, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008;6:1876–83. doi: 10.1111/j.1538-7836.2008.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Li-Ling J, Huang H, Ma F, Li Q. Phylogenetic analysis of vertebrate kininogen genes. Genomics. 2008;91:129–41. doi: 10.1016/j.ygeno.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14:427–37. doi: 10.1111/jth.13235. [DOI] [PubMed] [Google Scholar]
- 34.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–77. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papagrigoriou E, McEwan PA, Walsh PN, Emsley J. Crystal structure of the factor XI zymogen reveals a pathway for transactivation. Nat Struct Mol Biol. 2006;13:557–8. doi: 10.1038/nsmb1095. [DOI] [PubMed] [Google Scholar]
- 36.Geng Y, Verhamme IM, Sun MF, Bajaj SP, Emsley J, Gailani D. Analysis of the factor XI variant Arg184Gly suggests a structural basis for factor IX binding to factor XIa. J Thromb Haemost. 2013;11:1374–84. doi: 10.1111/jth.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shpilberg O, Peretz H, Zivelin A, et al. One of the two common mutations causing factor XI deficiency in Ashkenazi Jews (type II) is also prevalent in Iraqi Jews, who represent the ancient gene pool of Jews. Blood. 1995;85:429–32. [PubMed] [Google Scholar]
- 38.Peretz H, Mulai A, Usher S, et al. The two common mutations causing factor XI deficiency in Jews stem from distinct founders: one of ancient Middle Eastern origin and another of more recent European origin. Blood. 1997;90:2654–9. [PubMed] [Google Scholar]
- 39.Meijers J, Mulvihill E, Davie E, Chung D. Apple 4 in human blood coagulation factor XI mediates dimer formation. Biochemistry. 1992;31:4680–4. doi: 10.1021/bi00134a021. [DOI] [PubMed] [Google Scholar]
- 40.Meijers JC, Davie EW, Chung DW. Expression of human blood coagulation factor XI: characterization of the defect in factor XI type III deficiency. Blood. 1992;79:1435–1440. [PubMed] [Google Scholar]
- 41.Zivelin A, Bauduer F, Ducout L, et al. Factor XI deficiency in French Basques is caused predominantly by an ancestral Cys38Arg mutation in the factor XI gene. Blood. 2002;99:2448–54. doi: 10.1182/blood.v99.7.2448. [DOI] [PubMed] [Google Scholar]
- 42.Bolton-Maggs PH, Peretz H, Butler R, et al. A common ancestral mutation (C128X) occurring in 11 non-Jewish families from the UK with factor XI deficiency. J Thromb Haemost. 2004;2:918–24. doi: 10.1111/j.1538-7836.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 43.Brenner B, Laor A, Lupo H, et al. Bleeding predictors in factor-XI-deficient patients. Blood Coag Fibrinol. 1997;8:511–5. doi: 10.1097/00001721-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kravtsov DV, Wu W, Meijers JC, et al. Dominant factor XI deficiency caused by mutations in the factor XI catalytic domain. Blood. 104:128–134. doi: 10.1182/blood-2003-10-3530. [DOI] [PubMed] [Google Scholar]
- 45.Kravtsov DV, Monahan PE, Gailani D. A classification system for cross-reactive material-negative factor XI deficiency. Blood. 2005;105:4671–3. doi: 10.1182/blood-2004-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kravtsov DV, Matafonov A, Tucker EI, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–8. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Livnat T, Shenkman B, Martinowitz U, et al. The impact of thrombin generation and rotation thromboelastometry on assessment of severity of factor XI deficiency. Thromb Res. 2015;136:465–73. doi: 10.1016/j.thromres.2015.06.025. This paper describes a thrombin generation assay that distinguishes fXI deficient patients with a propensity to bleed from patients without a history of abnormal bleeding. [DOI] [PubMed] [Google Scholar]
- 48**.Pike GN, Cumming AM, Hay CR, Bolton-Maggs PH, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;12:397–405. doi: 10.1182/blood-2014-12-616565. This paper describes an assay in which thrombin generation is followed in platelet rich-plasma after stimulation with a low concentration of tissue factor. The assay is designed to enhance the importance of thrombin-mediated feedback activation of fXI, and was able to distinguish fXI deficient patients with a bleeding history from those without abnormal bleeding. [DOI] [PubMed] [Google Scholar]
- 49**.Zucker M, Seligsohn U, Salomon O, Wolberg AS. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–30. doi: 10.1111/jth.12600. This paper examines the importance of fXI to clot resistance to fibrinolysis. The assay may be useful in distinguishing bleeders from non-bleeders with fXI deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colucci M, Incampo F, Cannavò A, Menegatti M, Siboni SM, Zaccaria F, Semeraro N, Peyvandi F. Reduced fibrinolytic resistance in Patients with FXI deficiency. Evidence of a thrombin-independent impairment of the TAFI pathway. J Thromb Haemost. doi: 10.1111/jth.13342. in press. [DOI] [PubMed] [Google Scholar]
- 51.Bane CE, Ivanov I, Matafonov A, et al. Factor XI deficiency alters the cytokine response and activation of contact proteases during polymicrobial sepsis in mice. PLOS. 2016;11:e0152968. doi: 10.1371/journal.pone.0152968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salomon O, Zivelin A, Livnat T, et al. Prevalence, causes, and characterization of factor XI inhibitors in patients with inherited factor XI deficiency. Blood. 2003;101:4783–8. doi: 10.1182/blood-2002-09-2794. [DOI] [PubMed] [Google Scholar]
- 53.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84–7. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 54*.Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–40. doi: 10.1056/NEJMoa1405760. A phase 2 trial testing the ability of antisense-mediated reduction of plasma fXI to prevent thrombosis during knee replacement surgery. Patients underwent surgery with fXI levels as low as a few percent of normal, without experiencing abnormal intra-operative bleeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salomon O, Steinberg DM, Tamarin I, Zivelin A, Seligsohn U. Plasma replacement therapy during labor is not mandatory for women with severe factor XI deficiency. Blood Coagul Fibrinolysis. 2005;16:37–41. doi: 10.1097/00001721-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Kadir RA, Davies J, Winikoff R, et al. Pregnancy complications and obstetric care in women with inherited bleeding disorders. Haemophilia. 2013;19(Suppl 4):1–10. doi: 10.1111/hae.12269. [DOI] [PubMed] [Google Scholar]
- 57.O’Connell NM, Riddell AF, Pascoe G, Perry DJ, Lee CA. Recombinant factor VIIa to prevent surgical bleeding in factor XI deficiency. Haemophilia. 2008;14:775–81. doi: 10.1111/j.1365-2516.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 58.Riddell A, Abdul-Kadir R, Pollard D, Tuddenham E, Gomez K. Monitoring low dose recombinant factor VIIa therapy in patients with severe factor XI deficiency undergoing surgery. Thromb Haemost. 2011;106:521–7. doi: 10.1160/TH10-12-0816. [DOI] [PubMed] [Google Scholar]
- 59.Setty S, Reddell A, England A, Gomez K, Kadir R. The role of recombinant factor VIIa for obstetric block in women with severe factor XI deficiency. Haemophilia. 2011;17:906–9. doi: 10.1111/j.1365-2516.2011.02525.x. [DOI] [PubMed] [Google Scholar]
- 60.Pike GN, Bolton-Maggs PH. Factor XI-related thrombosis and the role of concentrate treatment in factor XI deficiency. Haemophilia. 2015;21:477–80. doi: 10.1111/hae.12678. [DOI] [PubMed] [Google Scholar]
- 61.Chtourou S, Poulle M. Factor XI. In: Bertoli IJ, Goss N, Curling J, editors. Production of plasma proteins for therapeutic use. John Wiley & Sons; New York, NY: 2012. [Google Scholar]
- 62.Bauduer F, de Raucourt E, Boyer-Neumann C, et al. Factor XI replacement for inherited factor XI deficiency in routine clinical practice: results of the HEMOLEVEN prospective 3-year postmarketing study. Haemophilia. 2015;21:481–9. doi: 10.1111/hae.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aledort LM, Goudemand J Hemoleven Study Group. United States’ factor XI-deficiency patients need a safer treatment. Am J Hematol. 2005;80:301–2. doi: 10.1002/ajh.20565. [DOI] [PubMed] [Google Scholar]
- 64.Key NS. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 65.Gailani D. Future prospects for contact factors as therapeutic targets. Hematology Am Soc Hematol Educ Program. 2014;2014:52–59. doi: 10.1182/asheducation-2014.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]