Abstract
A convergent, transition-metal-free synthesis of 2-aryla-zaindoles has been developed. The interception of a reactive aza-ortho-azaquinone methide intermediate by an acyl anion equivalent generated through carbene catalysis provides high yields, a wide substrate scope, and the synthesis of previously inaccessible azaindoles.
Graphical Abstract
A convergent, transition-metal-free synthesis of 2-aryl-azaindoles enabled by carbene catalysis is reported with high yields and a wide substrate scope featuring previously inaccessible azaindoles.
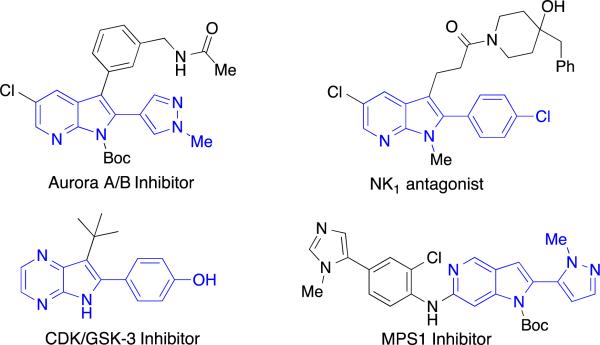
The azaindole and diazaindole hetereoaromatic scaffolds possess medically significant biological activity despite limited presence in known natural products.1 In particular, C2-aryl azaindole compounds show potent and selective inhibition of a variety of kinases over-expressed in virulent human-cancer cell lines (Figure 1).1,2 Additionally, specific C2-aryl azaindoles selectively inhibit anthelmintic activity, agonize melatonin receptors, and downregulate expression of inflammatory cytokines.3,4 In many cases, azaindoles can have superior pharmacokinetics when compared to other heterocyclic compounds.4b From an industrial standpoint, more than 100 patents containing bioactive C2-aryl azaindoles exemplify the pharmacophore potential of this scaffold. The continued interest in developing these molecules underscores the need for robust synthetic methods to access these structures in order to drive current and future research efforts.
Figure 1.
Selected biologically active 2-aryl-azaindoles
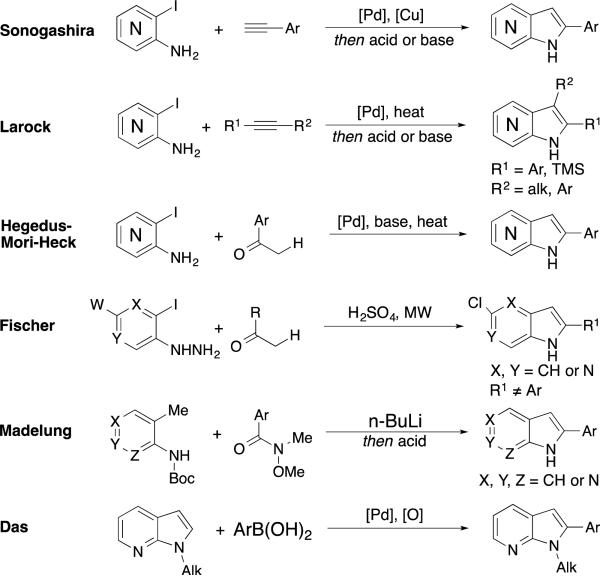
Several classic methods for the synthesis of this heterocycle currently exist and are analogous to established indole technology. The most common is the Sonogashira-type synthesis,2cef,5 which shows a broad scope for controlled C2-arylation in excellent to poor yields.6 While generally dependable, this method struggles or fails to generate azaindoles with hetero-, bromo-, or densely functionalized aromatic C2 substituents.6c, 7 The Larock synthesis (Figure 2b) yields 2,3-disubstituted azaindoles in a moderately reliable fashion but can provide limited yields and regiocontrol.2d, 8 Both methods depend on transition metal catalysts and alkyne-containing starting materials.
Figure 2.
Common approaches to C2-substituted azaindoles
Other existent methods emanating from Hegedus-Mori-Heck,9 Fischer,10 or Madelung11 approaches use palladium and strongly basic or acidic conditions with high temperatures. Many syntheses do not provide access to substitution of the C2-aryl moiety or the full scope of azaindole regioisomers. Various milder, non-traditional synthetic routes have been reported, but afford multiple azaindole regioisomers and/or display poor regiocontrol.12 While current methods allow access to a moderately diverse array of C2-aryl azaindoles, complimentary organocatalytic approaches with an emphasis on convergency and substitution variability would be important to improve access to these essential molecular scaffolds.
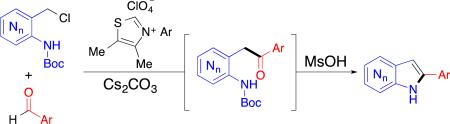
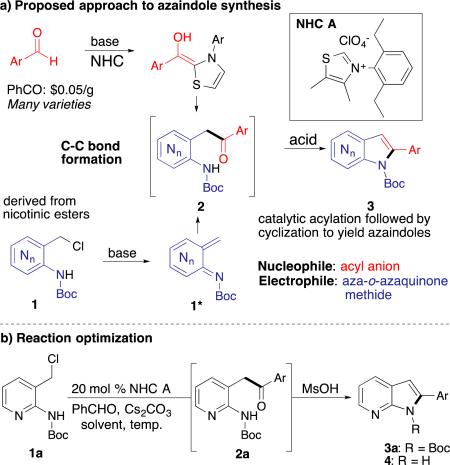
In our program to explore the versatility and power of N-heterocylic carbenes13 (NHCs) for new catalytic reactions, we recently discovered a novel and efficient synthesis of C2-aryl indoles through reaction of a transient aza-ortho-quinone methide14, 15 with an acylanion equivalent. We hypothesized that the analogous aza-ortho-aza-quinone methide intermediate 1* could be intercepted to form the related azaindole core 3 (Table 1a). As benzaldehyde derivatives are diverse and widely available, the success of this method could broaden the scope of C2-aryl substitution in a concise and efficient manner. Additionally, this proposed organocatalytic approach would compliment the known transition-metal catalysed syntheses.
Table 1.
Reaction approach and optimization
| |||||||
|---|---|---|---|---|---|---|---|
| Entrya | Solvent | % cat. | Equiv MsOH | Temp. (°C) | 2 (%)b | 3 (%)b | 4 (%)b |
| 1 | THF | 20 | - | rt | 78 | - | - |
| 2 | Dioxane | 20 | - | rt | 96 | - | - |
| 3 | Diethyl ether | 20 | - | rt | 59 | - | - |
| 4 | MTBE | 20 | - | rt | 64 | - | - |
| 5 | 2-methyl THF | 20 | - | rt | 66 | - | - |
| 6c | Dioxane | 10 | - | rt | 68 | - | - |
| 7d | Dioxane | 5 | - | rt | trace | - | - |
| 8c | Dioxane | 5 | - | 45 | trace | - | - |
| 9 | THF | 20 | 6.5 | rt | 70 | 0 | 0 |
| 10 | Dioxane | 20 | 6.5 | rt | 30 | 36 | 38 |
| 11e | Dioxane | 20 | 7.5 | rt, then 40 | 0 | 0 | 90 |
optimization reactions conducted on a 0.04 mmol scale (0.1 M) using 20% catalyst, 1.2 equivalents of aldehyde, and 1.2 equivalents of Cs2CO3 unless otherwise noted.
yield of isolated material
conducted at 0.2 M concentration
conducted at 0.4 M concentration
conducted on a 0.4 mmol scale; cyclization reaction went to completion overnight.
Our initial investigations took aza-ortho-azaquinone methide precursor, picolyl chloride 1a, benzaldehyde and NHC A with cesium carbonate as base (Table 1b). This mixture yielded arylketone 2a, analogous to the ketone intermediate observed in our indole-synthesis.15 A range of ethereal solvents were found to support the NHC-catalyzed acylation in good to excellent yields with a catalyst loading of 20 mol% (entries 1–5). Attempts to lower catalyst loading proved unproductive even at increased concentration or temperature (entries 6–8). With optimized conditions for ketone formation, we turned our focus to optimizing the dehydrative cyclization to afford the azaindole core in a one-pot operation. A survey of cyclization conditions revealed that methanesulfonic acid enables cyclization of ketone 2a in dioxane (entry 10). This cyclization fails in other more Lewis-basic ethereal solvents, as is illustrated in entry 9. Further optimization revealed an additional equivalent of acid and mild heating promotes complete conversion of ketone 2a to deacylated azaindole 4 in excellent yield. Attempts to selectively deliver boc-protected azaindole 3a by varying reaction conditions revealed de-acylation occurs concomitantly with the dehydrative cyclization.
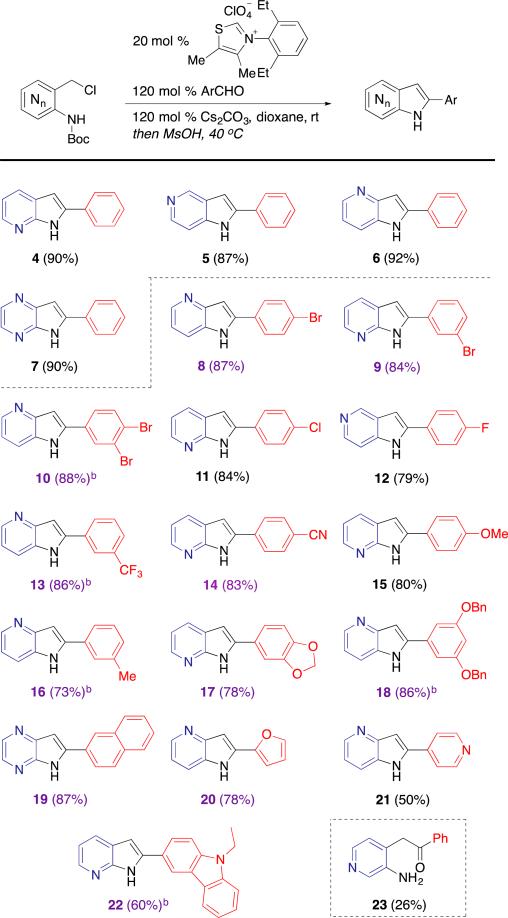
With optimized conditions in hand, we turned our focus to surveying the reaction scope with variation of the azaindole core (Table 2). We found that 4-, 5-, 7-, and 4,7-di- azaindoles with 2-phenyl substitution (4–7) could be delivered in excellent yields. Attempts to synthesize 2-phenyl-6-azaindole were unsuccessful and only produced; the acylation reaction was found to proceed in poor yield, and treatment with acid in dioxane or CH2Cl2 led only to removal of the carbamate protecting group to yield 23. Further investigation into the reduced yield of 23 revealed competing decomposition of the picolyl chloride starting material to reaction conditions.
Table 2.
Exploration of reaction scope
[a] purple denotes a previously unreported azaindole [b] denotes an unreported azaindole with substitution never before reported on any (di-)azaindole regiosiomer16
We then turned our focus to survey the scope of substituted benzaldehydes. Aryl aldehydes with p-, m-, and di-p,m-bromine substitution afforded azaindoles 8–10 in excellent yields. Of note is the synthesis of azaindole 10; to the best of our knowledge, it has never been reported and would be difficult to synthesize using transition-metal catalyzed methods due to competing insertion reactions. The developed method also functions well with aldehydes bearing chlorine (11) and fluorine (12) substitution. Use of other aldehydes with ortho substitution besides hydrogen groups proved unproductive, putatively due to steric clash of the substitution with the catalyst.15a,17 Benzaldehydes containing other electron-withdrawing groups, such as those with m-trifluoromethyl and pcyano substitution, resulted in the efficient synthesis of azaindoles 13 and 14, respectively. The use of aryl aldehydes with electron donating substituents in the meta and para positions afforded azaindoles 15–18 in high yields. Finally, polycyclic aromatic aldehydes (19) and, in fair to good yields, heteroaromatic aldehydes (20–22) were competent substrates. Further expansion of aldehyde scope towards use of non-aryl aldehydes have proved unproductive to date. Additionally, the use of secondary alkyl aldehydes afforded trace amounts of acylated product and a complex mixture of unknown reaction products. The use of cinnamyl or crotyl aldehydes yielded no detectable product.
In addition to the current substrate scope, multiple recrystallization techniques to yield pure azaindoles have been developed. Initially, aqueous work-up and silica gel chromatography successfully yielded 2-phenyl-7-azaindole 4. However, we observed poor solubility of most unprotected azaindoles in common chromatographic solvents, rendering standard isolation difficult. Further investigation showed that a basic workup followed by recrystallization from DCM through slow addition of toluene or hexanes afforded the azaindole products in high purity and yield. This purification method takes advantage of the limited solubility of the synthesized compounds and the minimal formation of insoluble side-products/byproducts. The general cleanliness of the reaction was studied by NMR spectroscopy and LCMS for azaindole 4, revealing nearly full conversion of azaindole precursor to product. For this reaction, the only discernable impurity in greater than 5% yield in NMR spectra of the unpurified material after basic work up was benzoin.
In conclusion, we have developed an efficient method for the transition-metal-free synthesis of azaindoles. This method provides access to a range of 2-aryl azaindole regioisomers as well as to diazaindoles. A broad scope of meta- and para- functionalization on the C2 aryl substituent is tolerated, including halogens, other electron-withdrawing groups, and electron-donating groups. The azaindole products were purified through an aqueous workup followed by recrystallization or column chromatography. To the best of our knowledge, this is the first synthesis of the full regioisomeric family of azaindoles employing mild temperature and conditions without transition metal catalysis. Further expansion of NHC-catalyzed dual activation strategies to access functionalized heterocycles are underway.
Supplementary Material
Acknowledgments
HAS, MTH, and KAS acknowledge financial support generously provided by the NIH (NIGMS R01-GM073072). HAS thanks Northwestern University for a Summer Undergraduate Research Grant.
Notes and references
- 1.Pertinent reviews on azaindole synthesis and medicinal activities: Mérour J-Y, Buron F, Plé K, Bonnet P, Routier S. Molecules. 2014;19:19935–19979. doi: 10.3390/molecules191219935. Mérour J-Y, Routier S, Suzenet F, Joseph B. Tetrahedron. 2013;69:4767–4834.
- 2.a Mettey Y, Gompel M, Thomas V, Garnier M, Leost M, Ceballos-Picot I, Noble M, Endicott J, Vierfond J-M, Meijer L. J. Med. Chem. 2003;46:222–236. doi: 10.1021/jm020319p. [DOI] [PubMed] [Google Scholar]; b Pin F, Buron F, Saab F, Colliandre L, Bourg S, Schoentgen F, Le Guevel R, Guillouzo C, Routier S. Med. Chem. Commun. 2011;2:899–903. [Google Scholar]; c Bavetsias V, Faisal A, Crumpler S, Brown N, Kosmopoulou M, Joshi A, Atrash B, Pérez-Fuertes Y, Schmitt JA, Boxall KJ, Burke R, Sun C, Avery S, Bush K, Henley A, Raynaud FI, Workman P, Bayliss R, Linardopoulos S, Blagg J. J. Med. Chem. 2013;56:9122–9135. doi: 10.1021/jm401115g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Koolman H, Heinrich T, Böttcher H, Rautenberg W, Reggelin M. Bioorg. Med. Chem. Lett. 2009;19:1879–1882. doi: 10.1016/j.bmcl.2009.02.069. [DOI] [PubMed] [Google Scholar]; e Power DP, Lozach O, Meijer L, Grayson DH, Connon SJ. Bioorg. Med. Chem. Lett. 2010;20:4940–4944. doi: 10.1016/j.bmcl.2010.06.024. [DOI] [PubMed] [Google Scholar]; f Naud S, Westwood IM, Faisal A, Sheldrake P, Bavetsias V, Atrash B, Cheung K-MJ, Liu M, Hayes A, Schmitt J, Wood A, Choi V, Boxall K, Mak G, Gurden M, Valenti M, de Haven A, Henley BA, Baker R, McAndrew C, Matijssen B, Burke R, Hoelder S, Eccles SA, Raynadu FI, Linardopoulos S, van Montfort RLM, Blagg J. J. Med. Chem. 2013;56:10045–10065. doi: 10.1021/jm401395s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher MH, Schwartzkopf G, Hoff DR. J. Med. Chem. 1972;15:1168–1171. doi: 10.1021/jm00281a019. [DOI] [PubMed] [Google Scholar]
- 4.a Zlotos DP, Jockers R, Cecon E, Rivara S, Witt-Enderby PA. J. Med. Chem. 2013;57:3161–3185. doi: 10.1021/jm401343c. [DOI] [PubMed] [Google Scholar]; b Trejo A, Arzeno H, Browner M, Chanda S, Cheng S, Comer DD, Dalrymple SA, Dunten P, Lafargue JA, Lovejoy B, Freire-Moar J, Lim J, Mcintosh J, Miller J, Papp E, Reuter D, Roberts R, Sanpablo F, Saunders J, Song K, Armando V, Warren SD, Welch M, Weller P, Whiteley PE, Zeng L, Goldstein DM. J. Med. Chem. 2003;46:4702–4713. doi: 10.1021/jm0301787. [DOI] [PubMed] [Google Scholar]
- 5.Rai R, Kolesnikov A, Sprengler PA, Torkelson S, Ton T, Katz BA, Yu C, Hendrix J, Shrader WD, Stephens R, Cabuslay R, Sanford E, Young WB. Bioorg. Med. Chem. Lett. 2006;16:2270–2273. doi: 10.1016/j.bmcl.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 6.a McLaughlin M, Palucki M, Davies IW. Org. Lett. 2006;8:3307–3310. doi: 10.1021/ol061232r. [DOI] [PubMed] [Google Scholar]; b Leboho TC, van Vuuren SF, Michael JP, de Koning CB. Org. Biomol. Chem. 2014;12:307. doi: 10.1039/c3ob41798k. [DOI] [PubMed] [Google Scholar]; c Simpson I, St-Gallay SA, Stokes S, Whittaker DTE, Wiewiora R. Tetrahedron Lett. 2015;56:1492–1495. [Google Scholar]
- 7.Spergel SH, Okoro DR, Pitts W. J. Org. Chem. 2010;75:5316–5319. doi: 10.1021/jo100623d. [DOI] [PubMed] [Google Scholar]
- 8.a Cooper LC, Chicchi GG, Dinnell K, Elliott JM, Hollingworth GJ, Kurtz MM, Locker KL, Morrison D, Shaw DE, Tsao K-L, Watt AP, Williams AR, Swain CJ. Biorg. Med. Chem. Lett. 2001;11:1233–1236. doi: 10.1016/s0960-894x(01)00182-2. [DOI] [PubMed] [Google Scholar]; b Ujjainwalla F, Warner D. Tetrahedron Lett. 1998;39:5355–5358. [Google Scholar]; c Livecchi M, Calvet G, Schmidt F. J. Org. Chem. 2012;77:5006–5016. doi: 10.1021/jo300481s. [DOI] [PubMed] [Google Scholar]
- 9.Lachance N, April M, Joly M-A. Synthesis. 2005;15:2571–2577. [Google Scholar]
- 10.Thomae D, Jeanty M, Coste J, Guillaumet G, Suzenet F. Eur. J. Org. Chem. 2013;16:3328–3336. [Google Scholar]
- 11.a Hands D, Bishop B, Cameron M, Edwards JS, Cottrell IF, Wright SHB. Synthesis. 1996:877. [Google Scholar]; b Norman MH, Chen N, Chen Z, Fotsch C, Hale C, Han N, Hurt R, Jenkins T, Kincaid J, Liu L, Merona O, Santora VJ, Sonnenberg JD, Karbon W. J. Med. Chem. 2000;43:4288–4312. doi: 10.1021/jm000269t. [DOI] [PubMed] [Google Scholar]
- 12.a Moustafa MMAR, Pagenkof BL. Org. Lett. 2010;12:3168–3171. doi: 10.1021/ol101078z. [DOI] [PubMed] [Google Scholar]; b Ortgies S, Breder A. Org. Lett. 2015;17:2748–2751. doi: 10.1021/acs.orglett.5b01156. [DOI] [PubMed] [Google Scholar]
- 13.a Nair V, Bindu S, Sreekumar V. Angew. Chem. 2004;116:5240–5245. doi: 10.1002/anie.200301714. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:5130–5135. doi: 10.1002/anie.200301714. [DOI] [PubMed] [Google Scholar]; b Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; c Marion N, D_ez-Gonz_lez S, Nolan SP. Angew. Chem. 2007;119:3046–3058. [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; d Nair V, Vellalath S, Babu BP. Chem. Soc. Rev. 2008;37:2691–2698. doi: 10.1039/b719083m. [DOI] [PubMed] [Google Scholar]; e Phillips EM, Chan A, Scheidt KA. Aldrichimica Acta. 2009;42:55–66. [PMC free article] [PubMed] [Google Scholar]; f Nair V, Menon RS, Biju AT, Sinu CR, Paul RR, Jose A, Sreekumar V. Chem. Soc. Rev. 2011;40:5336–5346. doi: 10.1039/c1cs15139h. [DOI] [PubMed] [Google Scholar]; g Bugaut X, Glorius F. Chem. Soc. Rev. 2012;41:3511–3522. doi: 10.1039/c2cs15333e. [DOI] [PubMed] [Google Scholar]; h Douglas J, Churchill G, Smith A. Synthesis. 2012:2295–2309. [Google Scholar]; i De Sarkar S, Biswas A, Samanta RC, Studer A. Chem. Eur. J. 2013;19:4664–4678. doi: 10.1002/chem.201203707. [DOI] [PubMed] [Google Scholar]; j Ryan SJ, Candish L, Lupton DW. Chem. Soc. Rev. 2013;42:4906–4917. doi: 10.1039/c3cs35522e. [DOI] [PubMed] [Google Scholar]
- 14.a Stetter H, Landscheidt A. Chem. Ber. 1979;112:2419–2422. [Google Scholar]; b Stetter H, Basse W, Nienhaus J. Chem. Ber. 1980;113:690–698. [Google Scholar]; c Mattson AE, Bharadwaj AR, Scheidt KA. J. Am. Chem. Soc. 2004;126:2314–2315. doi: 10.1021/ja0318380. [DOI] [PubMed] [Google Scholar]
- 15.Recent examples of aza-ortho-quinone methides interacting with NHC-generated nucleophiles see: Hovey MT, Check CT, Sipher AF, Scheidt KA. Angew. Chem. 2014;126:9757–9761. doi: 10.1002/anie.201405035. Angew. Chem. Int. Ed. 2014;53:9603–9607. doi: 10.1002/anie.201405035. Lee A, Price CK, Izquierdo J, Mishra RK, Scheidt KA. J. Am. Chem. Soc. 2014;136:10589–10592. doi: 10.1021/ja505880r. selected recent examples of NHC acyl anion equivalents reacting with unconventional electrophiles, see: Li Y, Shi F-Q, He Q-L, You S-L. Org. Lett. 2009;11:3182–3185. doi: 10.1021/ol9013238. Toh QY, McNally A, Vera S, Erdmann N, Gaunt MJ. J. Am. Chem. Soc. 2013;135:3772–3775. doi: 10.1021/ja400051d. for a related, yet noncatalytic, NHC-mediated acyl anion addition to primary benzyl halides, see: Lin L, Li Y, Du W, Deng W-P. Tetrahedron Lett. 2010;51:3571–3574. for the catalytic addition of NHC-mediated acyl anions to benzylic/activated alkyl halides, see: Padmanaban M, Biju AT, Glorius F. Org. Lett. 2011;13:98–101. doi: 10.1021/ol102626p.
- 16.As revealed by a thorough Reaxys search.
- 17.Piel I, Pawelczyk MD, Hirano K, Fröhlich R, Glorius F. Eur. J. Org. Chem. 2011;2011:5475–5484. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.