Abstract
Given the recent National Institutes of Health proposal for balanced use of male and female cells and animals in preclinical studies, we explored whether sex bias exists in skin research. We surveyed 802 dermatological research articles from 2012 through 2013. No information about the sex of studied cells or animals was provided in 60% of papers. Among keratinocytes of known sex, 70% were male. Few studies compared male versus female cells or animals. Disclosure of sex and comparative studies contribute to our understanding of the biologic basis of sex differences. Addressing sex-specific differences in preclinical research informs subsequent clinical trial design and promotes individualized therapy.
More than 20 years ago, the US National Institutes of Health established the Office of Research on Women’s Health, aimed in part at increasing representation of women in clinical trials. A parallel call to action, however, for male and female sex representation in cell- and animal-based research was only raised in October 2014, when the National Institutes of Health announced its intention to develop guidelines requiring investigators to report plans for balancing male and female cells and animals in preclinical studies (Clayton and Collins, 2014; Sandberg et al., 2015).
More women than men seek dermatological consultation for skin disease; our analysis of 149,614 patients seen for cutaneous issues (based on ICD-9 diagnosis codes) in outpatient clinics at Northwestern Medicine revealed a female to male ratio of 1.8:1. Although social behavior may influence seeking consultation, biological differences may underpin this imbalance. For example, women exhibit a greater prevalence of rosacea, lupus, and scleroderma with a different distribution of androgenetic alopecia in comparison with men. In addition, melanoma incidence rates are higher in younger females and older males (Liu et al., 2013), with a higher death rate from melanoma in males (De Giorgi et al., 2011; Joosse et al., 2011).
Comparative studies are needed to discover and elucidate differences in disease prevalence, impact, and response to interventions, which could have a genetic, epigenetic, hormonal, and/or behavioral (e.g., sun protection habits) basis. Several results from in vitro and in vivo comparative research show that differences originate from intrinsic cellular differences and are further dichotomized by the different hormonal environments to which they are exposed. For example, CD4+ T cells from women produce more IFNγ (Th1 bias) and from men produce more IL-17A (Zhang et al., 2012). Male gut microbiota transferred to female nonobese diabetic mice protects against the development of autoimmunity in an androgen receptor-dependent manner (Markle et al., 2013). These differences begin to explain the predominance of autoimmune disorders in women.
In cutaneous biology, comparative studies are beginning todefine the distinct action of estrogen on estrogen alpha and beta receptors and their relationships with the IGF-1 receptor in understanding wound re-epithelialization, macrophage polarization, and why skin wound healing is slower in men than in women (Campbell et al., 2010; Emmerson et al., 2012, 2013; Markiewicz et al., 2013; Campbell et al., 2014). The impact of estrogen on keratinocyte differentiation and cutaneous SCC progression in mice (Brooks et al., 2014), as well as the lower catalase levels in the skin and ultraviolet B-induced myeloid cells of male mice (Sullivan et al., 2012) may contribute to the two- to threefold increase in nonmelanoma skin cancers in men and the larger, histologically advanced, more numerous, and less inflamed UVB-induced SCCs in male hairless mice (Thomas-Ahner et al., 2007). Finally, sex-specific differences in the expression of glucocorticoid receptors exist in the liver, central nervous system, and immune system, suggesting that male skin may also be more sensitive to corticosteroids (Chrousos, 2010; Duma et al., 2010; Quinn et al., 2014).
To explore how often discovery in cutaneous biology stems from the study of one sex and whether sex is disclosed, we evaluated a cohort of research publications from 1 January 2012 to 31 December 2013 that included all “Original Articles” in three dermatological science-focused journals (Journal of Investigative Dermatology, Journal of Dermatological Science, and Experimental Dermatology). Of 802 original articles reviewed, one or more animal model or cell type was studied in 549 (Table 1). Both animals and cells were studied in 143 papers, more than one cell type in 126, and more than one animal type in 74 (generally more than one mouse strain).
Table 1.
Published reports using cells and animals in skin research
| Journal | No sex stated | Male only | Female only | Both sexes | Total | |
|---|---|---|---|---|---|---|
| Cells1 | J Invest Dermatol | 119 | 112 | 14 | 9 | 254 |
| J Dermatol Sci | 104 | 26 | 4 | 0 | 134 | |
| Exp Dermatol | 100 | 34 | 4 | 9 | 147 | |
| Total | 323 | 172 | 22 | 18 | 535 | |
| Mice2 | J Invest Dermatol | 124 | 11 | 44 | 5 | 184 |
| J Dermatol Sci | 21 | 12 | 19 | 3 | 55 | |
| Exp Dermatol | 14 | 8 | 15 | 4 | 41 | |
| Total | 159 | 31 | 78 | 12 | 280 |
Cells included primarily normal human keratinocytes, melanocytes, and fibroblasts.
Other animal models used included rat (N=8), zebrafish (N=5), rabbit (N=4), pig (N=2), guinea pig (N=2), horse (N=1), hamster (N=1), chicken (N=1), and sheep (N=1).
Among 535 papers utilizing cultured cells, 60.4% disclosed no information about the sex of origin, whereas 32.1% used only male cells and 4.1% only female cells (Figure 1a). Only 3.3% of studies examined male and female cells but rarely in the same experiments. Of 139 studies utilizing normal human keratinocytes (most common cell type), sex was unstated in 60.4%, only male in 29.5%, only female in 4.3%, and both sexes in 5.8% (Figure 1b). Forty-four publications utilized the HaCaT keratinocyte line, which originated from a male patient (Boukamp et al., 1988). The preponderance of studies with male cells is not surprising, given the easy availability of male foreskins as a source of cultured cells. However, our knowledge from these male sex-biased studies may not be generalizable if inherent cell differences related to sex exist.
Figure 1. Underreporting of cell and animal sex in basic skin research.
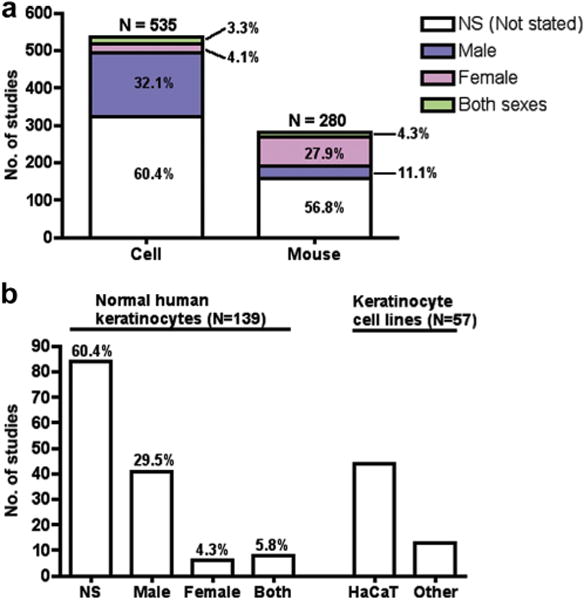
(a) N = total number of publications with cells or mice. “Both sexes” largely represents different sex used in different experiments, rather than comparative research. (b) Sex distribution in normal human keratinocytes and keratinocyte cell lines. HaCaT cells, a spontaneously transformed keratinocyte line, are of male origin.
Animal models were studied in 305 papers, with mice used in 92.1% (Table 1). Mouse sex was unspecified in 56.8% of the papers, but 27.9% reported only females and 11.1% only males (Figure 1a). This relatively greater use of female mice may reflect the greater aggressiveness of male mice. Mice of both sexes were used in 4.3% of papers, but few compared male and female mice in the same experiments.
Our findings of sex bias in basic science skin research are echoed by studies in other fields (Beery and Zucker, 2011; Taylor et al., 2011; Yoon et al., 2014), in which the sex of the origin of cells and animals was largely unreported and, if known, was primarily male. Tissue from noncutaneous sources is no more accessible in males than females; hence, this sex bias most likely originates from the historical convention of using male-only models in biomedical research. Concerns about hormonal effects and added financial costs to adequately power both male and female cohorts, coupled with an insufficient appreciation for potential differences in outcome, may further exacerbate the bias. Moreover, the age of the source of cells (i.e., neonatal versus adult) makes a difference biologically (Gilchrest, 1983; Gilchrest et al., 1982; Gilchrest et al., 1984). Thus, testing hypotheses in both male and female adult skin-derived cells not only addresses sex bias but also more closely models downstream clinical applications. Male and female skin for culture is currently available from abdominoplasties, reduction mammoplasties, rhytidoplasties (face lifts), and normal volunteers.
Why is it important to perform comparative analyses? Limiting studies to only one sex may lead to discoveries that are only relevant for one sex. Furthermore, having preclinical studies in mice of a single sex increases the risk of designing clinical trials that are inappropriate or even harmful for the non-studied sex. Of the 10 prescription drugs withdrawn from the US market between 1997 and 2000, eight “posed greater health risks for women than for men,” and half of these were widely prescribed to both sexes (http://www.gao.gov/products/GAO-01-286R). Perhaps the most widely publicized case of sex differences in medication response is the use of zolpidem (Ambien) for insomnia. More than 20 years after its initial approval, the recommended dose for women was decreased after new data revealed slower medication metabolism among females (Greenblatt et al., 2000). At least some of these serious adverse effects, which required withdrawal or dosing modification, might have been discovered preclinically if comparative testing had been performed.
We consider further study of possible differences in biologic responses of male and female cells to be an important unmet need. Testing of cells and tissues in both sexes should be the norm. As a reasonable first step, we suggest that scholarly journals, and particularly dermatological journals, require authors to state the sex and age of animals and cells of origin, and encourage researchers to report observed sex- and age-based differences. Further, we recommend the increased commercial availability and utilization of well-characterized skin cells from varied sources (male and female, young and aging). Accounting for sex in research, whether by regulatory agencies or in grant applications, must be balanced against the increased cost of replicating studies in both sexes, particularly in animal studies. Nevertheless, preclinical research that considers sex- and age-based influences could prevent the later discovery of response differences in expensive clinical trials, making the incremental allocation of funding toward this goal likely to be cost-effective. Ultimately, addressing these questions in preclinical research can improve the clinician’s understanding of sex- and age-based influences on normal and pathological cellular responses and inform the choice of intervention, further individualizing therapy and preventing adverse events.
Acknowledgments
We appreciate the careful review of this manuscript by Teresa Woodruff, PhD, Melina Kibbe, MD, and Robert Lavker, PhD.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks YS, Ostano P, Jo SH, et al. Multifactorial ERβ and NOTCH1 control of squamous differentiation and cancer. J Clin Invest. 2014;124:2260–76. doi: 10.1172/JCI72718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Emmerson E, Davies F, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med. 2010;207:1825–33. doi: 10.1084/jem.20100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Emmerson E, Williams H, et al. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J Invest Dermatol. 2014;134:2447–57. doi: 10.1038/jid.2014.175. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3:pe36. doi: 10.1126/scisignal.3143pe36. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi V, Gori A, Grazzini M, et al. Estrogens, estrogen receptors and melanoma. Expert Rev Anticancer Ther. 2011;11:739–47. doi: 10.1586/era.11.42. [DOI] [PubMed] [Google Scholar]
- Duma D, Collins JB, Chou JW, et al. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3:ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E, Campbell L, Davies FC, et al. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/ERα cross talk. J Invest Dermatol. 2012;132:2838–48. doi: 10.1038/jid.2012.228. [DOI] [PubMed] [Google Scholar]
- Emmerson E, Rando G, Meda C, et al. Estrogen receptor-mediated signalling in female mice is locally activated in response to wounding. Mol Cell Endocrinol. 2013;375:149–56. doi: 10.1016/j.mce.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA. In vitro assessment of keratinocyte aging. J Invest Dermatol. 1983;81:184s–9s. doi: 10.1111/1523-1747.ep12541084. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Murphy GF, Soter NA. Effect of chronologic aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85–8. doi: 10.1111/1523-1747.ep12500031. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Vrabel MA, Flynn E, et al. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol. 1984;83:370–6. doi: 10.1111/1523-1747.ep12264638. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, van Moltke LL, et al. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293:435–43. [PubMed] [Google Scholar]
- Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131:719–26. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- Liu F, Bessonova L, Taylor TH, et al. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013;26:128–35. doi: 10.1111/pcmr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz M, Znoyko S, Stawski L, et al. A role for estrogen receptor-α and estrogen receptor-β in collagen biosynthesis in mouse skin. J Invest Dermatol. 2013;133:120–7. doi: 10.1038/jid.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci. 2014;1317:1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- Sandberg K, Umans JG, the Georgetown Consensus Conference Work Group Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29:1646–52. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Tober KL, Burns EM, et al. UV light B-mediated inhibition of skin catalase activity promotes Gr-1+ CD11b+ myeloid cell expansion. J Invest Dermatol. 2012;132:695–702. doi: 10.1038/jid.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KE, Vallejo-Giraldo C, Schaible NS, et al. Reporting of sex as a variable in cardiovascular studies using cultured cells. Biol Sex Differ. 2011;2:11. doi: 10.1186/2042-6410-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Ahner JM, Wulff BC, Tober KL, et al. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67:3468–74. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- Yoon DY, Mansukhani NA, Stubbs VC, et al. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156:508–16. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang MA, Rego D, Moshkova M, et al. Peroxisome proliferator-activated receptor (PPAR) α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109:9505–10. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]


