Abstract
Estrogens are neuroprotective and, thus, potentially useful for the therapy of Alzheimer’s disease; however, clinical use of hormone therapy remains controversial due to adverse peripheral effects. The goal of this study was to investigate the benefits of treatment with 10β,17β-dihydroxyestra-1,4-dien-3-one (DHED), a brain-selective prodrug of 17β-estradiol, in comparison with the parent hormone using APPswe/PS1dE9 double transgenic mice to model the pathology of the disease. Ovariectomized and intact females were continuously treated with vehicle, 17β-estradiol, or DHED via subcutaneous osmotic pumps from 6 to 8 months of age. We confirmed that this prolonged treatment with DHED did not stimulate uterine tissue, whereas 17β-estradiol treatment increased uterine weight. Amyloid precursor protein decreased in both treatment groups of intact, but not in ovariectomized double transgenic females in which ovariectomy already decreased the expression of this protein significantly. However, reduced brain amyloid-β peptide levels could be observed for both treatments. Consequently, double-transgenic ovariectomized and intact mice had higher cognitive performance compared to untreated control animals in response to both estradiol and DHED administrations. Overall, the tested brain-selective 17β-estradiol prodrug proved to be an effective early-stage intervention in an Alzheimer’s disease-relevant mouse model without showing systemic impact and, thus, warrants further evaluation as a potential therapeutic candidate.
Keywords: estradiol, brain-selective prodrug, Alzheimer’s disease, transgenic mice, amyloid-β, radial arm water maze
INTRODUCTION
The current abandonment of late-stage clinical trials intended to test interventions that would cure Alzheimer’s disease (AD) through inhibiting the formation of the toxic amyloid-β (Aβ) species (Blennow et al., 2013) or passive immunotherapy (Sarazin et al., 2013) has increased the burden on researchers in the field to deliver disease-modifying therapies showing even marginal benefits. While a recent proposal to attack AD pathology with repurposing a drug already in use in the clinic for other diseases now invites skepticism (Tesseur and DeStrooper, 2013), encouraging results have been shown in patients with mild to moderate AD taking an N-methyl-D-aspartate (NMDA-) receptor antagonist together with vitamin E (Dysken et al., 2014). The delay of functional decline in the latter trial may be considered surprising after challenges and diminished enthusiasm involving the use of antioxidants in the clinic to ameliorate neurodegenerative diseases having been pointed out earlier (Kamat et al., 2008). This could prompt reconsideration of other, “non-classical” neurotherapeutic approaches whose potential have been downgraded due to similar pitfalls (Bajic et al., 2015), especially treatments aimed at the early stage of the disease. On the other hand, with the growing recognition of the importance to avoid undesirable impacts of drugs outside the organ to be treated (Kamat et al., 2008; Sayin et al., 2014), the introduction of novel strategies addressing this requirement could hold the key to the success of revisiting potential AD therapies proposed previously.
Age is the leading risk factor for the development of sporadic AD that occurs at a higher rate in women compared to men (Irvine et al., 2012). In addition, there is evidence that 17β-estradiol (E2) is neuroprotective in the brain; however, the underlying mechanisms to treat AD with this hormone remain unclear (Correia et al., 2010; Wise et al., 2009). Potential neural targets of E2 include activation of the mitogen activated protein kinase/extracellular signal-regulated protein kinase pathway, and estrogen receptor (ER) modulation of amyloid precursor protein (APP), presenilin-1 (PS1) and a disintegrin- and metalloproteinase domain-containing protein (ADAM10)—all pertinent to the neuropathology of AD (Wise et al., 2009). Furthermore, E2 may act as an antioxidant (Prokai et al., 2006) to ameliorate some effects of oxidative stress-related injury and redox dysregulation that have been implicated in the development and progression of the disease (Perry et al., 2002; Von Bernhardi and Eugenin, 2012). Estrogens also have been shown to provide numerous non-AD-related cognitive benefits (Bimonte-Nelson et al., 2010).
The efficacy of estrogen therapy on AD has been debated, nevertheless. The Women’s Health Initiative clinical trials have revealed improved cognition (Howell et al., 2006; Maki and Henderson 2012), although they relied on conjugated equine estrogen instead of E2, the main human estrogen, used in most basic science investigations. However, adverse side effects of the currently available estrogen therapies such as an increased incidence of breast cancer and circulatory system impacts, have been well known (Rossouw et al., 2002). Therefore, the benefits of any estrogen-based intervention can only be utilized as a potential remedy for AD with concomitantly eliminating or significantly reducing its adverse peripheral actions, especially when seeking usage beyond merely short-term treatments (Wharton et al., 2011). Present hormone therapies (HTs), including conjugated equine estrogen plus a synthetic progestin (medroxyprogesterone acetate) used in the Women’s Health Initiative study, do not meet this requirement. Also, a critical period or “window of opportunity” for initiating estrogen therapy has been widely recognized, because the brain and cognition become insensitive to exogenously administered estrogens following long-term ovarian hormone deprivation (Sherwin 2015; Daniel 2013). However, if estrogens are administered starting near the time of cessation of ovarian function, they will exert beneficial effects (Sherwin 2015; Daniel 2013).
This study is focused on the AD-therapeutic potential of a novel brain-selective E2 prodrug, 10β,17β-dihydroxyestra-1,4-dien-3-one (DHED), that spares the periphery from estrogenic stimulation (Prokai et al., 2015). Specifically, we aimed at determining whether DHED treatment would slow the progression of the onset of AD characteristics, including the reduction of Aβ formation and protection against cognitive impairment, by directly comparing with the efficacy of E2 treatment in a well-characterized double-transgenic (DTG) mouse model of the disease (Jankowsky et al., 2004). This particular model expresses behavioral deficiencies at 6–7 months of age that correlate temporally with the appearance of amyloid plaques (Jankowsky et al., 2005; Reiserer et al., 2007). Therefore, our experimental design focusing on evaluation scheduled at 8 months of age and, also considering that early initiation of estrogen therapy is required to obtain beneficial outcome (Sherwin 2006; Daniel 2013), with treatments starting at 6 months of age in DTG female mice. Our specific hypothesis is that DHED treatment proves as effective as E2 against early-stage disease progression in the DTG AD mouse model, but without concomitant peripheral uterotrophic side-effects that are unavoidable with E2 and any currently available HT.
Materials and methods
Chemicals
DHED was prepared according to previously published procedures (Prokai et al., 2015). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Animals
Transgenic APPswe/PS1dE9 mice (The Jackson Laboratory, ME) were obtained at 3–4 months of age from an established colony at the Veterans Affairs Maryland Health Care System, Baltimore, MD, where the APPswe/PS1dE9 hemizygote genotype was maintained by crossing a female wild-type (C57BL/6, Jackson) with a male APPswe/PS1dE9. Founder animals were transferred to the University of Maryland, College Park, for breeding on site according to Institutional Animal Care and Use Committee (IACUC) approved protocols. Animals were weaned at 21 to 25 days of age, then tail snipped and genotyped at 30 to 35 days of age. They were group housed by gender in an environmentally controlled animal facility on a 12-hour light/dark schedule. To minimize any confounding factor of estrogenic compounds in the diet, animals were placed on a phytoestrogen free diet one week prior to experimental treatment (Bio-Serv, Frenchtown, NJ) to eliminate dietary estrogen sources. Food and water were provided ad libitum. All experimental procedures were conducted under the University of Maryland, College Park IACUC approved protocols. Animals were individually housed following ovariectomy and osmotic pump implantation (see below).
Treatments and monitoring
Experiments started at 5.5 months of age with ovariectomy or sham surgery (intact groups) with 7 to 9 animals assigned to each treatment group. We used Alzet osmotic minipumps (model number 2004 for 28-day delivery at 0.025 μL/min, Durect Corp., Cupertino, CA) to treat the mice starting at 6 months of age. They were lightly anesthetized using isoflurane; a small patch of hair was removed from the upper back, the area was disinfected, and the osmotic pump was implanted subcutaneously between the scapulae via a small incision. The incision was closed, sutured and allowed to heal. E2 and DHED were dissolved in USP-grade propylene glycol (Fisher Scientific, Pittsburgh, PA), in which both E2 and DHED are freely soluble, to provide compatibility with the Alzet pumps (Prokai et al., 2015). The concentration of the experimental agent was 56 μg/mL, which resulted in a delivery rate of 2 μg/day. The minipumps were replaced once after 4-weeks to continuously deliver this dose over the 8-week treatment period and, therefore, starting behavioral evaluations were done at 8 months of age followed by evaluation of APP and Aβ post mortem immediately after testing behavior. Release of the compound was confirmed for the E2 treatment group by measuring circulating levels using an E2 immunoassay (EIA; Cayman Chemicals, Ann Arbor, MI). We confirmed low plasma E2 concentrations after ovariectomy and restoration of E2 levels by the E2-filled osmotic pump, while ovariectomized (OVX) females treated with vehicle continued to have low E2 verifying successful ovariectomy (Figure S1). Due to the unavailability of a sensitive and/or specific DHED assay, it was presumed that DHED would be delivered similarly. Following surgery, animals recovered on a surgical grade heating pad (Kent Scientific Instruments, Torrington, CT) under continuous observation before being placed in a cage containing clean, dry bedding. They were monitored daily and no special postoperative care was generally required. During treatment, vaginal smears were assessed as a measure of estrogenic stimulation in the periphery (Jefferson et al., 2009).
Radial-arm water maze (RAWM) behavioral testing
The RAWM tests were performed after the end of treatments. This water maze had 6 arms radiating out from the central pool area, with a platform at the end of the goal arm (specific for each mouse) (Alamed et al., 2006). A large plastic tub was spray-painted black and visual cues (three-dimensional objects) placed at the end of each arm on the wall of the pool. Mice received one day of training (Day 1) in 12 trials that alternated between a hidden and visible platform followed by 3 trials of all hidden platforms. Days 2 and 3 consisted of 15 hidden platform test trials. Each mouse was assigned one goal arm, which remained constant throughout the three test days. The start arm changed for each trial and if the mouse did not locate the platform within 60 seconds, the mouse was gently directed to the platform and allowed to rest there for 10 seconds before being removed from the pool. A 60-second cutoff time was chosen to ensure endurance and stamina for the animals, as well as to mimic a previous RAWM paradigm (Alamed et al., 2006). An error was recorded as an entry (all four paws) into an incorrect arm or entry into the goal arm without successful location of the platform. An error score did not require that the animal swim to the back of the arm entirely before turning around. When measured, latency was recorded as the time (in seconds) for the animal to locate the platform correctly. All animals were scored by the same observer from a consistent site in the testing facility.
Tissue collection
Animals were euthanized by cervical dislocation. The brain was immediately removed and half of each brain was flash frozen and stored at −80°C for later analysis. Samples were homogenized using 1 mL of homogenization buffer [225 mM ultra-pure mannitol, 75 mM ultra-pure sucrose, 5 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), pH to 7.4 at 4°C] for biochemical and molecular analyses. Protein levels were measured using the Lowry protein assay method (Lowry et al., 1951). Assay reagents were purchased from Bio-Rad (Hercules, CA). The other half of each brain was postfixed in 4% paraformaldehyde, transferred to 30% sucrose after 24 hours, and allowed to sink before being processed for histology. Uterus was also harvested and weighed according to a procedure adopted from the literature (Jefferson et al., 2009). Body weight did not differ across treatment groups to the extent that it would have justified adjustment for statistical analysis of uterine wet weights.
APP immunochemistry
Serial sections of 50-μm thickness were collected using a freezing microtome (American Optical AO, Buffalo, NY) and sections stored in cryobuffer [in water: 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol (Fisher Scientific)] until staining. Sections were washed in 1x tris buffered saline (TBS) to remove cryobuffer residue and treated with 1% hydrogen peroxide (30 min, Fisher Scientific) to block endogenous peroxidase activity, washed, treated with 0.3% Triton X-100 (Fisher Scientific) for 10 min, and incubated with 5% normal goat serum (30 min, Vector Labs, Burlingame, CA). Tissue was incubated with primary antibody overnight at 4°C; amyloid isoforms and soluble APP were localized using an antibody against APP (6E10) (1:300, Covance). Vectastain ABC kit and biotinylated secondary antibody (Vector Labs) were used to reveal antibody immunostaining by 3,3′-diaminobenzidine tetrahydrochloride. Tissue was mounted on subbed slides and counterstained with cresyl violet, dehydrated, and coverslipped.
Semi-quantitative measurement of immunostaining
Images were captured using an AxioCam MR camera and AxioVision image capture software (Zeiss, Houston, TX). Quantification of plaque number and size were performed using ImageJ software (NIH, Bethesda, MD), with analysis of five sequential tissue slices ranging from early hippocampal to late hippocampal tissue in each animal. Measurements were taken at 2.5x magnification in hippocampal areas (CA1, CA2, CA3, and dentate gyrus), arcuate nucleus, motor cortex, and somatosensory cortex. Color de-convolution was used to distinguish staining from 3,3′-diaminobenzidine or cresyl violet. Images were then transformed to 8-bit gray scale and threshold adjusted to reveal plaques. For each animal, the number of plaques was averaged from 5 to 6 brain sections for each animal.
Amyloid-β levels
Aβ(1-40) and Aβ(1-42) peptides were quantified by enzyme-linked immunosorbent assay (ELISA; Invitrogen, Grand Island, NY). Brain homogenates were prepared as discussed above in 1 mL of homogenization buffer. After thorough homogenization samples were centrifuged at 10,000 rpm for 3 minutes to remove debris and separate out the supernatant. The supernatant collected after this centrifugation was used to perform the Aβ ELISA. Samples were measured using a monoclonal primary (rabbit) antibody specific for the NH2-terminus region of human Aβ(1-40) or Aβ(1-42) and bound primary antibody was revealed using horseradish peroxidase-labeled anti-rabbit antibody proportional to human Aβ in the sample. Absorbance was measured at 450 nm using a Victor microplate reader (Wallac 1420, Perkin Elmer, Waltham, MA). Final concentration of Aβ was expressed as pg/mg protein.
Statistical analyses
One-way analysis of variance (ANOVA) was performed considering 3x2 factorial design (Box et al., 2005) for all analyses involving DTG animals except for the evaluation of behavioral results. Post hoc analyses were performed using Tukey’s honestly significant differences (HSD) test to compare each treatment group to each other. Analyses of behavioral data were based on a method specific for the RAWM paradigm and Alzheimer’s transgenic mice (Morgan, 2009). We first performed repeated measures ANOVA to see a main effect of treatment and trials. We then made post hoc means comparisons using Fischer’s (LSD) test to identify group differences on specific trials or blocks. Statistical significance was accepted at p<0.05. Effect sizes were estimated as partial eta squared (ηp2) for ANOVA results (Brown, 2008) and Cohen’s d for pairwise comparisons (Cohen, 1988).
Results and Discussion
Our data provide supporting evidence for neurobiochemical and cognitive benefits from E2 treatment on AD-like disease in a well-characterized DTG AD mouse model (Heikkinen et al., 2004; Jankowsky et al., 2005). Despite proven neuroprotection, adverse peripheral effects remained; however presenting an issue to be addressed with any currently approved estrogen therapy. In our study, we used both OVX animals (where surgically removal of the main source of estrogen production in the body precipitates an estrogen-deprived state that is used often as one of the menopause models in rodents (Moiety et al., 2015)) and, also, intact female DTG mice. Our findings demonstrate that treatment with DHED, a unique bioprecursor prodrug of E2 (Fig. 1; Prokai et al., 2015), can provide the APP-/Aβ-lowering effects and cognitive benefits of E2 (Figures 2, 3, and 4) without uterotrophic side-effects in both OVX and intact DTG mice (Figure 5). Moreover, there was no evidence of stimulating peripheral estrogen-sensitive tissues by chronic DHED administration via subcutaneous Alzet osmotic pumps or any adverse impact throughout the 8-week upon continuous treatment, which we started at 6 months of age considering that early initiation of hormone therapy is necessary to improve neurobiological and cognitive deficits associated with neurodegenerative diseases (Sherwin 2005; Daniel 2013). This is especially important regarding the timing of therapy relative to the onset of menopause. Few, if any, estrogenic therapies provide the advantage of lowering APP and Aβ peptide levels, as well as improved cognition without concomitant uterine tissue hyperplasia and hypertrophy. Taken together with our recent assessment that, unlike E2, DHED treatment also did not stimulate the growth of breast cancer cells in vivo in a nude mouse xenograft model (Prokai et al., 2015), this brain-selective prodrug of the main human estrogen offers an inherently safe approach for the treatment of estrogen-responsive neurodegenerative disorders such as AD.
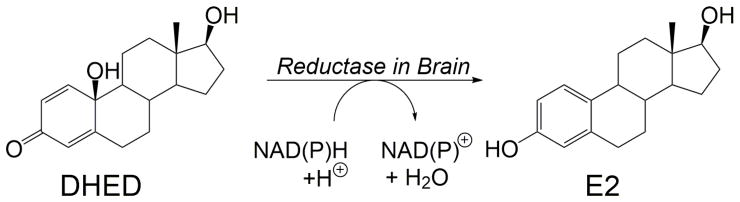
Fig. 1.
Conversion of the prodrug 10β,17β-dihydroxyestra-1,4-dien-3-one (DHED) to 17β-estradiol (E2) through enzyme-catalyzed reduction involving NAD(P)H as a cofactor.
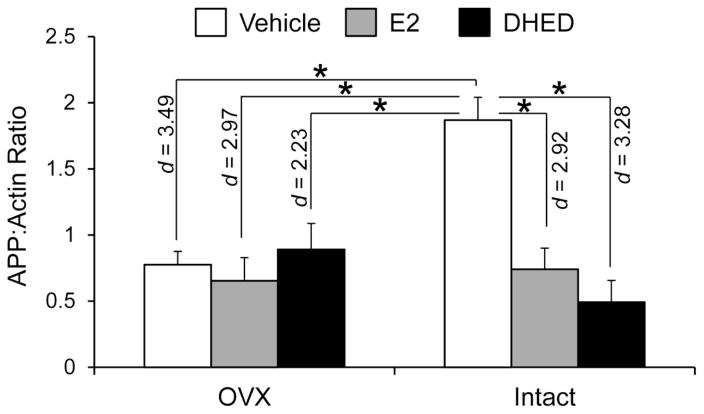
Fig. 2.
APP (6E10) protein levels. Protein levels expressed as APP to β-actin ratios ± SEM (n = 5–7/group). Statistically significant main effects of treatment (F(2,29)=8.82; p=0.001; ηp2=0.378) and ovarian status (F(1,29)=4.77; p=0.037; ηp2=0.141), as well as significant treatment × ovarian status interaction (F(2,29)=9.45; p=0.001; ηp2=0.394) were shown in DTG females by factorial analysis. By Tukey’s HSD test, following groups showed statistically significant differences, as shown here in Fig. 2 (Effect sizes were indicated by Cohen’s d values): E2 and DHED treatments significantly decreased APP in both OVX and intact mice compared to vehicle-treated intact animals. Also, there was a significant decrease of APP levels in vehicle-treated OVX mice compared to the vehicle-treated intact group. Therefore, E2-treated and DHED-treated OVX animals did not show statistically significant difference compared to the vehicle-treated OVX mice. *p < 0.05
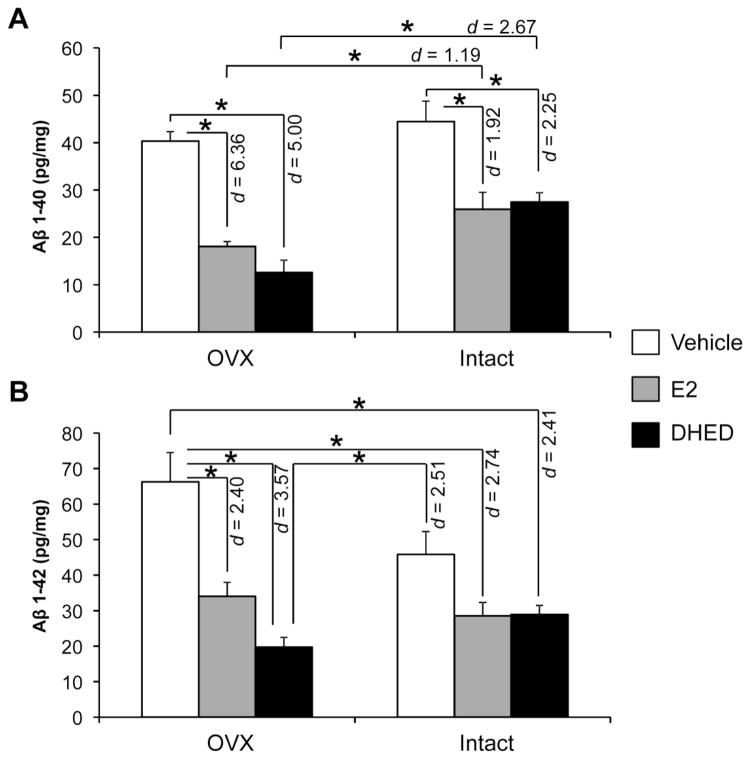
Fig. 3.
Aβ(1-40) and Aβ(1-42) levels in the brain. Data are expressed as pg peptide/mg ± SEM (n = 5–7/group). Statistically significant main effects of treatment (F(2,28)=36.4; p<0.001; ηp2=0.722) and ovarian status (F(1,28)=15.3; p=0.001; ηp2=0.353) were observed for Aβ(1-40). Statistically significant main effects of treatment (F(2,28)=22.6; p<0.001; ηp2=0.618) and ovarian status × treatment interaction (F(2,28)=4.48; p=0.021; ηp2=0.242) were observed for Aβ(1-42). Overall, there were statistically significant differences between E2-treated and vehicle-treated, as well as between DHED-treated and vehicle-treated DTG mice both in Aβ(1-40) and Aβ(1-42) levels, but not between E2-treated and DHED-treated animals. In addition, reduction of Aβ(1-40) was significantly lower in E2- and DHED-treated OVX mice, when compared to Aβ(1-40) measured in the corresponding intact animals (Effect sizes were shown by Cohen’s d values). *p < 0.05
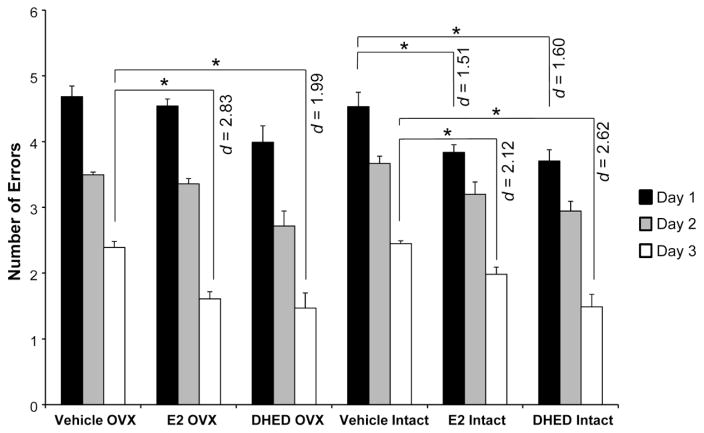
Fig. 4.
RAWM test performance of DTG mice. Number of errors are shown as averages for each day ± SEM (n = 7–9/group). Statistically significant main effect for treatment was shown in the experiment (F(6,70)=9.15; p<0.001; ηp2=0.44). All animals manifested learning over the 3 days of testing period, as they made fewer errors each day. However, E2- and DHED-treated intact animals showed improved ability to learn the task on day 1 compared to vehicle-treated controls. On day 3, both intact and OVX E2- and DHED-treated females made significantly less errors than the corresponding vehicle-treated animals (Effect sizes were indicated by Cohen’s d values). *p < 0.05
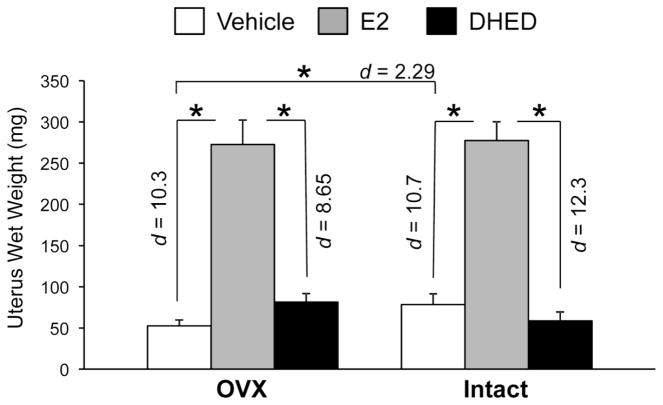
Fig. 5.
Uterotrophic effect of treatment. Data show wet tissue weight ± SEM (n = 5–7/group). Statistically significant main effects of treatment (F(2,24)=463; p<0.001; ηp2=0.975) and a treatment × ovarian status interaction (F(2,24)=4.74; p=0.018; ηp2=0.283) were shown in DTG females by factorial analysis. Uterine wet weights showed a significant increase in the E2-treated OVX and intact DTG mice, when compared to the vehicle and DHED-treated groups (Effect sizes were shown by Cohen’s d values). However, DHED-treated females did not differ from vehicle-treated animals. *p < 0.05.
Reducing APP may provide a pathway to lower Aβ production and associated subsequent plaque formation, which is plausibly linked to cognitive improvement observed in our experiments. Specifically, we report the reduction of full length APP protein as a result of both E2 and DHED treatments in intact DTG females (Fig. 2). However, no effect was obtained compared to vehicle controls for either E2 or DHED treatments in our OVX animals, presumably because ovariectomy itself reduced APP levels in the DTG mice. The different response in the early (5.5-month) OVX paradigm compared to that of the intact animals implies an involvement of other endogenous ovarian steroids such as progesterone (Carroll et al., 2007) in these experiments. Indeed, neuropathology in AD transgenic female mice has been shown to be regulated by both estrogen and progesterone. Nevertheless, a substantial APP loss would be detrimental, because this protein has endogenous roles important in synaptic function (Koike et al., 2012; Muller and Zheng 2012). Therefore, therapeutic strategies will require caution regarding reductions in endogenous APP.
Although immunohistochemistry showed no differences among the DTG groups involved in our study considering the number of amyloid plaques in the hippocampus and cortex (Figure S2A–D), Aβ peptide levels did change significantly (Fig. 3). Aβ(1-40) and Aβ(1-42), which are formed as a result of APP cleavage, also decreased however in both intact and OVX female DTG mice after E2 and DHED treatments. The decrease in Aβ peptide levels was generally more profound in OVX animals than in intact ones. Again, progesterone (which is present in the intact but absent in the OVX animals) has been shown to attenuate the beneficial effect of E2 on Aβ accumulation in AD transgenic female mice (Carroll et al., 2007). Nevertheless, DHED treatment showed benefits of decreasing toxic APP fragments even in our intact DTG mice.
Our results also concur with previous studies that demonstrated E2 inhibition of Aβ oligomer production, release, and plaque formation (Li et al., 2000; Morinaga et al., 2011; Xu et al., 1998). Due to the previously discussed reduction in APP protein by the treatments, one could link this to less Aβ oligomer production. The cleavage of APP and subsequent Aβ oligomer formation involve α-, β-, and γ-secretase enzymes, which create various products including the Aβ peptides (LaFerla 2002). Because of the different cleavage enzymes and products that are produced during the processing of APP, the decrease in Aβ may not be a direct result of the decrease in APP levels. Many enzymes are involved in the cleavage and the degradation process of APP and Aβ, respectively, such as insulin-degrading enzyme (IDE), a protein also known to break down Aβ oligomers (Jayaraman et al., 2012). Nevertheless, our results implicate potential pathways through which E2 could impact Aβ load. Due to our findings of decreased Aβ fragments, E2 may act at the level of Aβ production and clearance, reducing the amount of circulating Aβ and/or the formation of new plaques. However, the degradation pathway of Aβ should be further explored to determine the mechanism through which E2 is working to reduce Aβ burden in the brain, which was beyond the scope of our study focusing on a comparative evaluation of a potential novel approach via brain-selective E2 therapy against the progression of AD.
Overall plaque load in our study did not decrease significantly as a result of E2 or DHED treatment (Figure S2). Other studies have reported results that agree with ours, demonstrating that E2 treatment failed to impact amyloid deposition after plaque formation had already started (Heikkinen et al., 2004; Green et al., 2005). Plaque formation was apparent by 6 months in the DTG model employed. Therefore, while there was a decrease in APP and Aβ levels in our experiments, the mechanism through which estrogen acts on Aβ may be by reducing Aβ oligomer production and the formation of new plaques, not by affecting previously formed plaques.
A key symptom of Alzheimer’s disease is impaired cognitive function. Cognitive dysfunction in AD has been associated with aggregation of Aβ fragments, a result of APP processing (Cleary et al., 2004; Reddy and Beal, 2008). Impaired cognition in the APPswe/PS1dE9 versus wild-type mice has been documented as early as 6–7 months of age (Jankowsky et al., 2005; Savonenko et al., 2005), which we also confirmed for these DTG animals involved in our study that performed RAWM testing at 8 months of age using the number of errors as a measure of behavioral competence (Figure S3). RAWM testing has been developed to reveal learning and memory deficits (Diamond et al., 1999) and have been used to show significant cognitive decline in APP transgenic mice compared to wild-type animals (Gordon et al., 2001; Wilcock et al., 2004; Alamed et al., 2006). The paradigm also is reliable in measuring the effect of therapeutic interventions in APP transgenic animals (Wilcock et al., 2004). In our study, intact DTG E2- and DHED-treated females showed fewer errors compared to vehicle-treated OVX and intact animals on the first day, and the initial deficits were rescued both in the intact and OVX DTG E2- and DHED-treated females by the final day of behavioral testing (Fig. 4). Our key overall observation was that DHED treatment generally paralleled effects of E2 treatment as measured by the RAWM test. However, DHED treatment was not accompanied by the profound uterotrophic effect observed with the administration of E2 (Fig. 5).
In conclusion, we report positive neurobiochemical effects and consequent behavioral improvement faithfully replicating those of E2 as a result of DHED treatment in a mouse model of AD. Decreases of APP/Aβ levels and errors in the RAWM show that DHED treatment affected major AD-related characteristics of the DTG mouse model of the disease. These encouraging, therapeutically relevant effects, combined with the lack of uterine tissue stimulation, make DHED treatment a unique approach to provide the benefits of E2 in AD therapy while minimizing the hormone’s adverse impact on estrogen sensitive peripheral tissues. Further studies will aim at determining optimal dose, optimal timing and duration of the treatment period to maximize potential therapeutic benefits of DHED-based intervention to slow the progression of AD.
Supplementary Material
Highlights.
Treatment with 10β,17β-dihydroxyestra-1,4-dien-3-one (DHED), a brain-selective prodrug of 17β-estradiol, for 8 weeks decreased amyloid precursor protein in APPswe/PS1dE9 double-transgenic mice
DHED treatment reduced brain amyloid-β peptide levels
DHED-treated APPswe/PS1dE9 double-transgenic mice had higher cognitive performance compared to untreated control animals
DHED treatment faithfully replicated positive neurobiochemical effects and consequent behavioral improvement observed for 17β-estradiol
DHED did not stimulate uterine tissue, whereas 17β-estradiol treatment did.
Acknowledgments
This research funded by the National Institutes of Health grants AG031387 (MAO) and AG031535 (LP), the Robert A. Welch Foundation (endowment BK-0031, LP), the VA Research Service Rehabilitation R&D REAP (RAS), and the Biomedical R&D CDA02 (RAS), and was conducted in partial fulfillment of the Ph.D. requirements for the University of Maryland (AET).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alamed J, et al. Two-day radial-arm water maze learning and memory task, robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Bajic V, et al. Non-classical therapeutic approach in the treatment of Alzheimer’s disease: A mini review. Lett Drug Des Discov. 2015;12:158–164. [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: Mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, et al. Semagacestat’s fall: where next for AD therapies? Nat Med. 2013;19:1214–1215. doi: 10.1038/nm.3365. [DOI] [PubMed] [Google Scholar]
- Box GEP, Hunter SJ, Hunter WG. Statistics for Experimenters: Design, Innovation, and Discovery. 2. Wiley-Interscience; Hoboken, NJ: 2005. pp. 317–333. [Google Scholar]
- Brown JD. Effect size and eta squared. JALT Testing & Evaluation SIG Newsletter. 2008;12:38–43. [Google Scholar]
- Carroll JC, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, et al. Natural oligomers of the amyloid- β protein specifically disrupt cognitive function. Nat Neurosci. 2004;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Correia SC, et al. Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer’s disease? Curr Aging Sci. 2010;3:113–126. doi: 10.2174/1874609811003020113. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm Behav. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Dysken MW, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease. JAMA. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MN, et al. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Green PS, et al. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer’s disease. Endocrinology. 2005;146:2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, et al. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187:105–117. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Howell N, Dykens J, Moos WH. Alzheimer’s disease, estrogens, and clinical trials: A case study in drug development for complex disorders. Drug Dev Res. 2006;66:53–77. [Google Scholar]
- Irvine K, et al. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J Clin Exp Neuropsychol. 2012;34:989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, et al. 17β-estradiol and progesterone regulate expression of β-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–5479. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, et al. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–1889. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat CD, et al. Antioxidants in central nervous system diseases: Preclinical promise and translational challenges. J Alzh Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike MA, et al. APP knockout mice experience acute mortality as the result of ischemia. PLoS One. 2012;7:e42665. doi: 10.1371/journal.pone.0042665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Li R, et al. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;1:265–275. [PubMed] [Google Scholar]
- Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women’s Health Initiative ten years on. Climacteric. 2012;3:256–262. doi: 10.3109/13697137.2012.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiety FMS, et al. Comparative study on induction and effects of surgical menopause in a female rat model: a prospective case control study. Int J Clin Exp Med. 2015;8:9403–9411. [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Water maze tasks in mice: Special reference to Alzheimer’s transgenic mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2009. [PubMed] [Google Scholar]
- Morinaga A, et al. Effects of sex hormones on Alzheimer’s disease-associated beta-amyloid oligomer formation in vitro. Exp Neurol. 2011;228:298–302. doi: 10.1016/j.expneurol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Muller UC, Zheng H. Physiological functions of APP family proteins. Cold Spring Harb Perspect Med. 2012;2:a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci Transl Med. 2015;7:297ra113. doi: 10.1126/scitranslmed.aab1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, et al. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev Res. 2006;66:118–125. [Google Scholar]
- Reddy PH, Beal FM. Amyloid beta, mitochondrial dysfunction and synpatic damage: implications for cognitive decline in aging and Alzheimer’s disease. Cell. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiserer RS, et al. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sarazin M, et al. Immunotherapy in Alzheimer’s disease: Do we have all the pieces of the puzzle? Biol Psychiatry. 2013;74:329–332. doi: 10.1016/j.biopsych.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Sayin VI, et al. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tesseur I, De Strooper B. When the dust settles: what did we learn from the bexarotene discussion? Alzh Res Ther. 2013;5:54. doi: 10.1186/alzrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bernhardi R, Eugenin J. Alzheimer’s disease: Redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antiox Redox Sign. 2012;16:974–1031. doi: 10.1089/ars.2011.4082. [DOI] [PubMed] [Google Scholar]
- Wharton W, et al. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: Results of a randomized controlled trial. J Alzh Dis. 2011;26:495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, et al. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflamm. 2006;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Suzuki S, Brown CM. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dial Clin Neurosci. 2009;11:297–303. doi: 10.31887/DCNS.2009.11.3/pmwise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. Estrogen reduced neuronal generation of Alzheimer β-amyloid peptides. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.