Abstract
As one of the key nutrient sensors, insulin signaling plays an important role in integrating environmental energy cues with organism growth. In adult organisms, relative insufficiency of insulin signaling induces compensatory expansion of insulin-secreting pancreatic beta (β) cells. However, little is known about how insulin signaling feedback might influence neogenesis of β cells during embryonic development. Using genetic approaches and a unique cell transplantation system in developing zebrafish, we have uncovered a novel role for insulin signaling in the negative regulation of pancreatic progenitor cell differentiation. Blocking insulin signaling in the pancreatic progenitors hastened the expression of the essential β cell genes insulin and pdx1, and promoted β cell fate at the expense of alpha cell fate. In addition, loss of insulin signaling promoted β cell regeneration and destabilization of alpha cell character. These data indicate that insulin signaling constitutes a tunable mechanism for β cell compensatory plasticity during early development. Moreover, using a novel blastomere-to-larva transplantation strategy, we found that loss of insulin signaling in endoderm-committed blastomeres drove their differentiation into β cells. Furthermore, the extent of this differentiation was dependent on the function of the β cell mass in the host. Altogether, our results indicate that modulation of insulin signaling will be crucial for the development of β cell restoration therapies for diabetics; further clarification of the mechanisms of insulin signaling in β cell progenitors will reveal therapeutic targets for both in vivo and in vitro β cell generation.
Keywords: β cell, Regeneration, Pdx1, IRS2, Insulin, Pancreas, Zebrafish, Development, Pancreatic progenitor
1. Introduction
Insulin is a crucial gluco-regulatory peptide hormone that is produced by pancreatic beta (β) cells and released in proportion to levels of circulating glucose. Under conditions of fluctuating metabolic demands and energy availability, the effective functional β cell mass (insulin releasing capacity of the pancreas) is regulated to match physiological demands through β cell compensation. The failure of β cell compensation to meet insulin demand results in diabetes mellitus, a metabolic disease of insufficient insulin signaling that is characterized by uncontrolled hyperglycemia and its associated morbid complications. While compensation can be transiently mediated in part via increased insulin production and release from existing β cells, long term β cell compensation involves the expansion of β cell mass by multiple mechanisms (Asghar et al., 2006; Wang et al., 2015; Weir and Bonner-Weir, 2004). For instance, in mice physiological stresses like over-nutrition and pregnancy can accelerate β cell replication (Kim et al., 2010; Lee and Nielsen, 2009; Tanaka and Wands, 1996). However, the replicative capacity of β cells varies widely among vertebrates by species and age. The capacity of human β cells is lower than that of most model organisms used to study replication (Kulkarni et al., 2012). Furthermore, β cell replicative capacity diminishes sharply after adolescence (Kulkarni et al., 2012; Linnemann et al., 2014). In addition, using model organisms, β cells have been shown to arise via neogenesis from non-β cell sources, including differentiation from facultative progenitor cells and conversion from other pancreatic endocrine cells (Bonner-Weir et al., 2010; Chera et al., 2014; Chung et al., 2010; Thorel et al., 2010; Xu et al., 2008; Ye et al., 2015). Stimulating β cell neogenesis and replication in humans will be crucial for restoring β cell function and ultimately curing diabetes. Thus, a comprehensive understanding of the molecular mechanisms that sense insulin insufficiency and translate it into β cell compensatory responses will impact the design of better diabetes therapies.
Several growth factors and cytokines have been shown to regulate β cell replication in response to metabolic demand. In the adult islet, insulin secreted by β cells has been shown to feedback upon β cells to regulate islet size and β cell mass (Withers et al., 1999). Activation of the insulin receptor triggers its autophosphorylation, which is followed by downstream signal propagation via the key effector Insulin Receptor Substrate 1/2 (IRS1/2)) to the Akt and Mitogen Activated Protein Kinase (MAPK) pathways; these pathways mediate many growth and metabolic responses (Siddle, 2011). Deletion of the insulin receptors in β cells (βIRKO) abolished compensatory β cell mass expansion in adult mice and resulted in hyperglycemia (Okada et al., 2007). Further, this suggests that reported influences of glucose on β cell replication (Otani et al., 2004) may be due in part to the indirect effects of augmenting insulin secretion. Although much is known about replication-mediated β cell mass compensation, little is known regarding the cellular and molecular mechanisms of de novo neogenesis-mediated β cell mass compensation.
It is likely that some mechanisms regulating β cell compensation via neogenesis are common to both the mature and developing pancreas, and the embryo is an especially amenable system in which to study β cell formation. However, while the intrinsic developmental programs regulating endocrine differentiation have been very well characterized (Pan and Wright, 2011), the extrinsic signals that control induction and differentiation of β cells, as well as those signals that match β cell mass to the needs of the embryo are less well understood. Among the pathways studied are fibroblast growth factor and Notch signaling, which suppress differentiation of pancreas progenitors (Apelqvist et al., 1999; Jensen et al., 2000; Norgaard et al., 2003) and epithelial growth factor signaling, which influences β cell neogenesis (Cras-Méneur et al., 2001; Miettinen et al., 2008; Suarez-Pinzon et al., 2005). Surprisingly, the roles of the pancreatic hormones have not been extensively studied during islet development. While glucagon signaling has been shown to regulate alpha (α) cell mass by proliferation, neogenesis, and cell fate switching mechanisms (Ye et al., 2015; Gelling et al., 2003; Hayashi et al., 2009; Prasadan et al., 2002), it is not clear whether other islet hormones like insulin have a significant role in the acquisition and stability of cell fates in the developing islet. Even though the insulin signaling pathway has been studied using mouse knockout models, the results from previous developmental studies appear contradictory. Mice lacking the insulin receptor exhibit severe hyperglycemia at birth despite the presence of normal islets (Accili et al., 1996; Joshi et al., 1996; Kitamura et al., 2003). However, deletion of either or both of the mouse insulin orthologues (Duvillié et al., 1997) or downstream effectors such as Akt lead to marked islet hyperplasia (Buzzi et al., 2010). Therefore, further investigation is required to resolve how insulin signaling regulates β cell neogenesis during development as well as in pathologies like diabetes.
Zebrafish are a relevant and powerful system for the study of β cell formation and homeostasis: they share key features of both carbohydrate metabolism and their β cell differentiation program with mammalian systems (Kinkel and Prince, 2009) while also affording many experimental advantages (Grunwald and Eisen, 2002). As in mice and humans, the zebrafish pancreas arises from two discrete endodermal progenitor domains that fuse to establish the architecture of the pancreas (Field et al., 2003; Jørgensen et al., 2007; Pauls et al., 2007). In zebrafish, the dorsal bud appears at approximately 14 hours post fertilization (hpf) and gives rise exclusively to differentiated endocrine cell types, which then cluster to form the principal islet by 24 hpf. Emerging around 34 hpf, the ventral bud engulfs the principal islet while differentiating into both exocrine and endocrine cell lineages. In this study, we have used zebrafish to explore the role of insulin signaling during embryonic β cell formation. Using genetic approaches in zebrafish that either inhibit insulin production or impair transduction through the insulin signaling pathway, we have shown that insulin signaling has an inhibitory role during early pancreas development: loss of insulin signaling drove the precocious differentiation of pancreatic progenitors into β cells. Using chimera analysis we found that insulin signaling within the endoderm itself suppresses β cell differentiation. Moreover, using a novel blastomere-to-larva transplantation strategy, we found that loss of insulin signaling in endoderm-committed blastomeres fostered their differentiation into β cells, and that the extent of this differentiation was dependent on the function of the host β cell mass. Taken together, our data suggest that manipulation of the insulin signaling pathway will be crucial for regenerative medicine approaches to diabetes therapies, including β cell differentiation from in situ progenitors during regeneration, and from stem cells in vitro.
2. Results
2.1. Intracellular blockade of insulin signaling promotes embryonic β cell formation
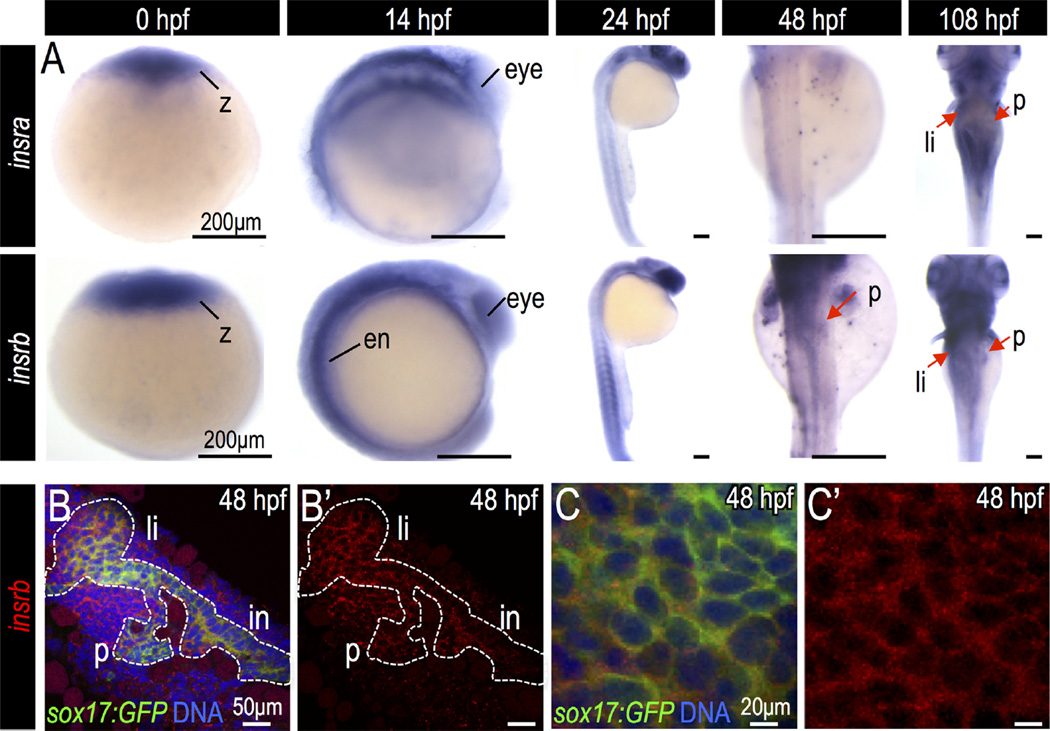
To determine whether pancreatic progenitor cells are competent to receive insulin signals, we performed whole mount in situ hybridization and quantitative PCR to evaluate the expression of insulin receptors at key time points during pancreas development. There are two isoforms of the zebrafish insulin receptor, insulin receptor a (insra) and insulin receptor b (insrb) (Toyoshima et al., 2008). Both were strongly expressed in zygotes, indicating that maternal contribution may affect early embryonic development (Fig. 1A, Fig. S1A–B). Only insrb was expressed in the embryonic pancreatic endoderm during early pancreas development, as visualized by co-localization with endoderm marker sox17 at 48 hours post fertilization (hpf) (Fig. 1B–C). In 108 hpf larvae, both insra and insrb were expressed in the pancreas, liver and intestine, which may reflect a metabolic role for insulin signaling during later developmental stages (Fig. 1A, Fig. S1C–D′).
Fig. 1.
Expression of insulin and insulin receptors during zebrafish endoderm development. (A) In situ hybridization for insra and insrb in the developing zebrafish embryos. (B–C) Fluorescent in situ hybridization of insrb (red) in Tg(sox17:GFP) endoderm (green) at 48 hpf. Abbreviations: z, zygote; ed, endoderm; p, pancreas; li, liver; in, intestine.
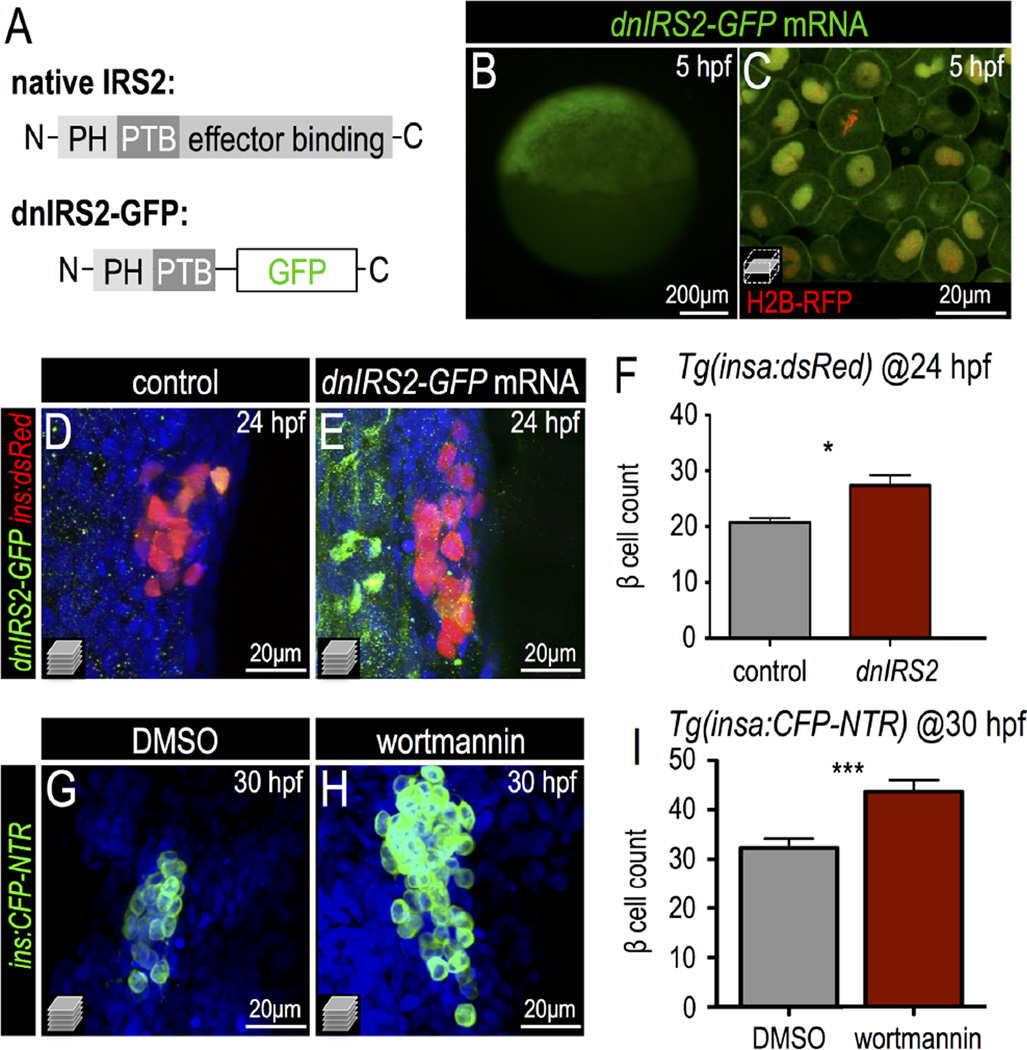
To investigate the influence of insulin signaling on β cell differentiation, we generated a truncated mutant form of zebrafish IRS2 in which GFP is substituted for the C-terminal SH2-binding domains, while retaining the N-terminal pleckstrin homology (PH) and phosphotyrosine binding (PTB) domains (Fig. 2A; Fig. S2A). As reported with similar constructs (Tanaka and Wands, 1996), this mutant protein (hereafter dnIRS2-GFP) is expected to non-productively bind to the insulin receptor and act as a dominant negative regulator of insulin signaling. Indeed, when dnIRS2-GFP mRNA was injected into zygotes, we observed that dnIRS2-GFP protein was enriched at the plasma membrane in 5 hpf embryos (Fig. 2B,C) and remained expressed in the embryos through 24 hpf (Fig. S2B). To determine whether this mode of insulin signaling blockade affected the formation of β cells, we counted cells expressing insulin in dnIRS2-GFP mRNA-injected embryos at 24 hpf. Relative to controls, we observed a 32% increase in the number of ins+ β cells marked by dsRed in Tg(insa:dsRed) embryos (Fig. 2D–F). Previous studies have shown that homeodomain transcription factor Pdx1 is essential for β cell differentiation and fate maintenance (Ahlgren et al., 1998; Jonsson et al., 1994; Offield et al., 1996). These roles of Pdx1 are conserved in zebrafish during β cell formation (Kimmel et al., 2011; Yee et al., 2001). We therefore checked the expression of pdx1 in dnIRS2-GFP injected embryos; consistent with the increased β cell quantity, we observed a marked increase in pdx1 expression in the pancreatic region at 24 hpf (Fig. S2C,D). Concordantly, blockade of the Akt branch of insulin signaling via treatment with the PI3 kinase inhibitor wortmannin (Standaert et al., 1996) also resulted in increased β cell formation at 30 hpf as marked by CFP in Tg(insa:CFP-NTR) embryos (Fig. 2G–I). In contrast, blockade of the MAPK pathway via the Erk inhibitor U0126 (Favata et al., 1998) did not significantly influence β cell quantity at this stage (Fig. S2E–G). Taken together, these data indicate that the excess β cell differentiation induced by insulin signaling deficiency may be primarily regulated via the Akt/PI3K branch of the insulin signaling pathway.
Fig. 2.
Insulin signaling blockade increases embryonic β cell formation. (A) Schematic of a dominant negative IRS2 construct designed to block transmission of insulin signaling; C-terminal effector binding domains are replaced with GFP. (B,C) 5 hpf embryo injected with dnIRS2-GFP mRNA (B) Photomicrograph of epifluorescence shows ubiquitous distribution of GFP. (C) Confocal plane shows the localization of dnIRS2-GFP to the plasma membrane while co-injected H2B-RFP mRNA labels cell nuclei red. (D, E) Confocal projections of control (D) and dnIRS2-GFP mRNA-injected islets in 24 hpf Tg(ins:dsRed) embryos. (F) Quantification of insa:dsRed+ β cells in 24 hpf control (n = 12) and dnIRS2-GFPmRNA injected embryos (n = 10). (G,H) Confocal projections of DMSO-treated control (G) and 1 µM wortmannin-treated islets in 30 hpf Tg(ins:CFP-NTR) embryos. (I) Quantification of β cells in DMSO-treated control (n = 18) and 1 µM wortmannin-treated (n = 17) islets in 30 hpf embryos. Student t-test was used to determine significance in F and I.
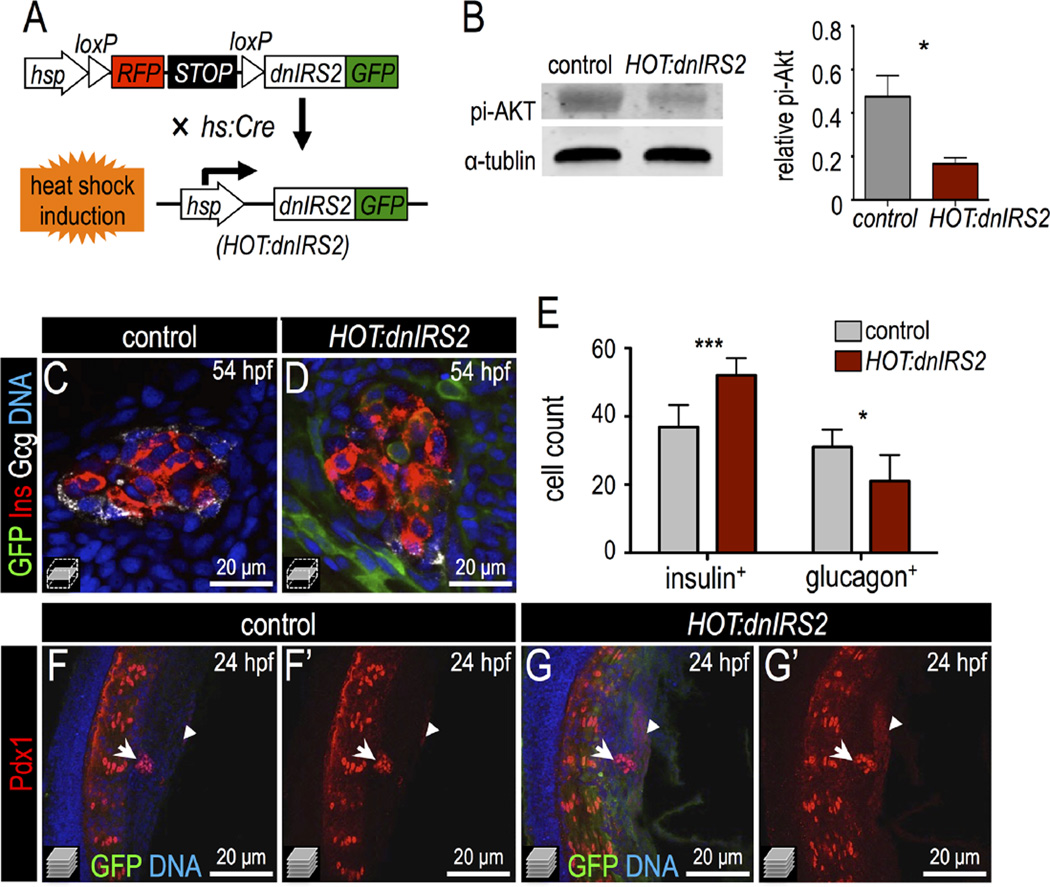
Next, we generated a transgenic model based on the HOT-Cre system (Hesselson et al., 2009) that conditionally mis-expresses dnIRS2-GFP; this permits inducible and sustained knockdown of insulin signaling. This transgenic line, Tg(hs:loxp-mcherry-stop-loxp-dnIRS2-GFP;cryaa:CFP), was crossed with Tg(hs:Cre) (Le et al., 2007) to generate double transgenic embryos in which heat shock induces ubiquitous expression of dnIRS2-GFP (Fig. 3A); these double transgenic fish are hereafter termed HOT:dnIRS2 for simplicity. Embryos bearing Tg(hs:loxp-mcherry-stop-loxp-dnIRS2-GFP; cryaa:CFP) alone, without Cre, were used as the control group. We verified the efficiency of insulin signaling blockade in HOT:dnIRS2 embryos at 54 hpf with an immuno-blot for phospho-Akt protein: after multiple heat shocks at 10, 24, 28, 32, 36, and 48 hpf the HOT: dnIRS2 embryos showed a 65% relative decrease in phosphorylated Akt as compared with controls (Fig. 3B). Importantly, we detected GFP fluorescence only after heat shock induction, and the induced dnIRS2-GFP appeared ubiquitous (see Fig. 3C,D,F,G; Fig. S3A″,B″). Next, we used HOT:dnIRS2 transgenic zebrafish to analyze islet development. As with both prior approaches—dnIRS2 mRNA injection and PI3K inhibitor treatment—the HOT:dnIRS2 double transgenic embryos showed increased β cell quantity at 54 hpf when heat shocked as above (Fig. 3C–E). Interestingly, we also noted a significant decrease in the number of glucagon+ α cells (Fig. 3C–E). We also examined Pdx1 protein distribution after misexpression of dnIRS2. In control 24 hpf embryos, Pdx1 was expressed predominantly in dorsal endocrine cells and weakly in adjacent pancreatic endoderm (Fig. 3F,F′); however, in HOT:dnIRS2 embryos that were heat shocked at 10 hpf, Pdx1 expression was strongly increased in both the principal islet (arrows) and the adjacent pre-pancreatic endoderm (arrowheads, Fig. 3G,G′). This result suggests that an increased Pdx1 expression domain in the ventral pancreatic endoderm precedes the early differentiation of ventral progenitor cells into β cells. Taken together, our genetic and pharmacological data indicate that insulin signaling blockade results in the following phenotypes: increased Pdx1 expression, increased formation of β cells, and decreased generation of α cells.
Fig. 3.
Inducible genetic blockade of insulin signaling promotes β cell formation. (A) Schematic of genetic insulin signaling blockade strategy. In double transgenic embryos, heat shock induces Cre expression and recombination of the mCherry-switch-dnIRS2-GFP transgene as well as expression of the recombined dnIRS2-GFP transgene. (B) Immunoblot of pi-Akt in Cre-negative control versus Cre-positive embryos showing that induction of dnIRS2-GFP effectively blocks insulin signaling. (C,D) Confocal planes of 54 hpf control and HOT:dnIRS2 islets stained for GFP (green), Insulin (red), and Glucagon (white). Embryos were heat shocked repeatedly, at 10, 24, 28, 32, 36, and 48 hpf; dnIRS2-GFP was induced ubiquitously, but only in Cre-positive HOT:dnIRS2 embryos. (E) Quantification of Insulin+ β cells and Glucagon+ α cells in control (gray, n = 8) and dnIRS2-expressing HOT:dnIRS2 (red, n = 6) groups. (F–G′) Merged and single channel confocal projections of 24 hpf control and HOT:dnIRS2 pancreatic endoderm stained for GFP (green) and Pdx1 (red). dnIRS2-GFP is induced ubiquitously in HOT:dnIRS2 embryos, which show expanded Pdx1 protein expression in the principal islet (arrows) and adjacent ventral endoderm (arrowheads). Student t-test was used in B and two-way ANOVA was used in E to determine significance.
2.2. Knockdown of insulin drives the early differentiation of β cells
To further delineate the mechanisms of insulin signaling in pancreatic progenitor differentiation and β cell development, we used splice-blocking morpholinos (MOs) to knockdown insulin during embryogenesis. First, a MO targeting the exon 2-intron 2 boundary was used to disrupt zygotic insulin a (hereafter insa) pre-mRNA splicing (Fig. S4A). Injection of insa MO (insaMO) resulted in two aberrant splicing products of insa pre-mRNA, demonstrating its efficacy (Fig. S4B–D); however, no general developmental defects were observed in insaMO-injected embryos (Fig. S4E). Using immunofluorescent staining, we observed a sustained absence of insulin protein in insaMO-injected β cells at 72 hpf and 96 hpf (Fig. S5). Finally, to confirm the specificity of our insaMO-mediated knockdown, we co-injected an mRNA encoding Insulin a protein with the insaMO. In embryos at 24 hpf, we found that the expanded β cell phenotype caused by insaMO (Fig. S6A–B′) was “rescued” to normal levels (Fig. S6C–D).
Due to genomic duplication, zebrafish have a second orthologue of insulin, insulin b (insb) (Papasani et al., 2006). In contrast to the two murine Insulin paralogues that are expressed nearly identically (Duvillié et al., 1997; Leroux et al., 2001; Soares et al., 1985), the expression patterns of zebrafish insulin paralogues differ significantly. Specifically, while insa is expressed only in pancreatic β cells, insb is widely expressed, particularly in the head and somatic musculature; this suggests that in zebrafish the two insulin genes execute distinct developmental functions (Papasani et al., 2006). To test this hypothesis, we knocked down insb using a MO that targeted the exon 4-intron 4 boundary (Fig. S7A). In insbMO-injected embryos we observed efficient disruption of insb splicing (Fig. S7B) and severe developmental defects, including microcephaly and shortening of the antero-posterior axis (Fig. S7C). We observed no change in insa splicing in insbMO-injected or insb splicing in insaMO-injected embryos (Fig. S7D,E). Furthermore, insbMO injection affected neither the expression of insulin protein in the islet, nor the number of pancreatic β cells (Fig. S7F–H).
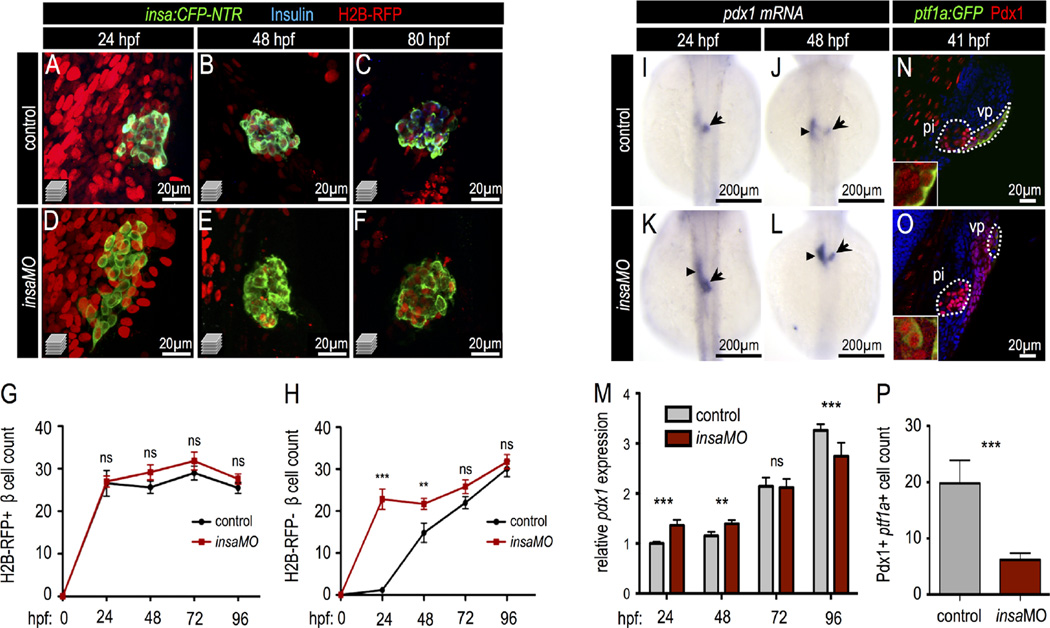
Next, we examined β cell formation during development in insa-deficient embryos. For this, we analyzed the differentiation of β cells from both progenitor cell sources: the dorsal (early) and ventral (late) pancreatic buds. β cells derived from each pancreatic bud can be distinguished using a label retaining cell (LRC) assay (Hesselson et al., 2009) in which embryonic cells are initially uniformly labeled by zygotic injection of H2B-RFP mRNA. As development proceeds, the fluorescence intensity of the encoded fluorescent protein is diluted by mitosis. Differences in H2B-RFP signal strength among cell populations reflect differences in their proliferative histories. The dorsal bud-derived β cells (DBCs), which differentiate early and become quiescent, remain H2B-RFP positive. In contrast, ventral bud-derived progenitor cells lose H2B-RFP signal via extensive proliferation before ventral bud-derived β cells (VBC) differentiate. Using this approach we quantified the number of DBCs and VBCs in insaMO-injected pancreata throughout development. We found that only DBCs were present in the principal islet of control embryos at 24 hpf—consistent with our previous findings (Ye et al., 2015; Hesselson et al., 2009)—and that insaMO did not influence DBC number (Fig. 4A–G). In contrast, H2B-RFP-negative VBCs were detected after 48 hpf and continued to increase throughout development (Fig. 4H). Strikingly, a significant number of precocious H2B-RFP-negative VBCs were detected in insaMO-injected embryos at 24 hpf (Fig. 4D,H). Similarly, we found that treatment with the PI3K inhibitor wortmannin also increased VBC but not DBC formation (Fig. S8). We excluded the possibility that these precocious VBCs were derived from hyper-proliferative DBCs since both phospho-histone H3 (PHH3) immunofluorescence and EdU DNA incorporation assays revealed no proliferation in control or insaMO-injected pancreata at 24 hpf (Fig. S9A–D″). These data support the interpretation that the excess VBCs in insaMO-injected embryos arose by neogenesis from ventral bud sources. Concordantly, in 24 hpf insaMO-injected pancreata, but not in control pancreata, ectopic Pdx1+ cells were detected in the ventral endoderm (Fig. S10A–D′).
Fig. 4.
Insulin knockdown increases Pdx1 expression and drives early pancreatic progenitor differentiation into β cells. (A–F) Confocal projections of 24 hpf, 48 hpf, and 80 hpf Tg(insa:CFP-NTR) control (A–C) and insaMO-injected (D–F) islets that were co-injected with H2B-RFP mRNA to distinguish dorsal (H2B-RFP-positive DBCs) and ventral (H2B-RFP-negative VBCs) bud-derived β cells. Islets were immuno-stained for CFP (β cells, green) and insulin (blue). Insulin protein was not detectable in insaMO-injected islets. (G,H) Quantification of H2B-RFP-positive DBCs (G) and H2B-RFP-negative VBCs (H) in control (black line) and insaMO-injected (red line) islets from 24 hpf to 96 hpf (n ≥ 8 islets at each stage). VBC count is increased early at 24 and 48 hpf, but normalizes to control levels by 72 hpf. (I–L) In situ hybridization of pdx1 expression in 24 hpf and 48 hpf control (I,J) and insaMO-injected (K,L) embryos. pdx1 expression was increased in the principal islet (arrow) and adjacent endoderm (arrow heads). (M) Real time PCR to detect Pdx1 expression in both control and insaMO-injected embryos from 24 hpf to 96 hpf (n = 3). (N,O) Confocal planes of 41 hpf Tg(ptf1a:GFP) control (N) and insaMO-injected (O) endoderm stained for GFP (green) and Pdx1(red). Pdx1 expression was increased while ptf1a:GFP expression was decreased in insaMO-injected embryos. (P) Quantification of double positive Pdx1+ ptf1a+ pancreatic progenitor cells in both control (n = 6) and insaMO (n = 6) injected embryos. Abbreviations: pi, principal islet; vp, ventral pancreas. ANOVA was used in G,H, and O and Student's t-test was used in P to determine significance.
However, despite the early increase in VBCs, we found that the total β cell quantity in insaMO-injected embryos had normalized and was indistinguishable from controls at 72 and 96 hpf (Fig. 4H) even though Insulin protein was still undetectable (Fig. S5). Similarly, HOT:dnIRS2 embryos that were received daily heat shocks also normalized their β cell mass by 96 hpf (Fig. S3C). Together, these results suggest that normalization of β cell mass after an early differentiation of β cells is not as a result of a particular experimental mode of insulin signaling blockade, but rather is likely to be biologically relevant. These data support the interpretation that insulin expression and release from the early pancreatic islet feeds back to inhibit precocious differentiation of β cells from the ventral bud. Additional non-insulin dependent mechanisms further may influence the total β cell mass between 24 and 72 hpf.
To analyze the mechanisms underlying insulin-directed pancreatic progenitor differentiation, we analyzed the expression of Pdx1 in both control and insaMO-injected embryos. In 24 hpf embryos, Pdx1 expression was strongly expressed in the principal islet; at 48 hpf after the induction of the ventral pancreatic bud, Pdx1 was expressed in both the principal islet and adjacent endoderm (Fig. 4I,J). After insaMO knockdown, we observed increased Pdx1 expression in the principal islet region (Fig. 4K,L, arrow), which is consistent with our interpretation that a reduction of insulin drives excess β cell differentiation from the ventral pancreas. In addition, insaMO also promoted ectopic pdx1 expression in the adjacent endoderm from which the ventral pancreatic progenitor cells are derived (Fig. 4K–L, arrowheads). An increase in Pdx1 expression at early stages of pancreas development (24 and 48 hpf), but not during later stages (72 and 96 hpf) was confirmed by quantitative PCR (Fig. 4M).
Expression of Pdx1—together with the transcription factor Ptf1a—defines the pancreatic progenitor domain in mammals (Burlison et al., 2008). During zebrafish early ventral bud development we performed immunofluorescent staining of Pdx1 in Tg (ptf1a:EGFP)jh1 embryos at 41 hpf. We found that all Ptf1a-positive cells in the ventral pancreatic endoderm were also Pdx1-positive (Fig. 4N, inset); suggesting that these are the equivalent of mammalian multipotent pancreatic progenitor cells. This interpretation is concordant with ptf1a lineage tracing studies in zebrafish (Wang et al., 2015). We next asked how loss of insulin affected development of this progenitor domain in our model. In sharp contrast to the expansion of the Pdx1+ domain observed with insaMO knockdown, pancreatic ptf1a:GFP expression was decreased after insaMO knockdown, as was the number of double positive Pdx1+ ptf1a:GFP+ pancreatic progenitors (Fig. 4O,P). These findings indicate that knockdown of insulin induces early differentiation of progenitor cells into β cells, which consequently diminishes the ventral pancreatic progenitor cell pool. In accord with this finding, we also observed decreased exocrine pancreas size in insaMO-injected larvae (data not shown). Overall, our findings suggest that insulin signaling is part of a feedback loop through which newly formed β cells govern the ongoing differentiation of the pancreatic progenitor cells. As such, the loss of insulin induces a compensatory increase of β cell differentiation during early islet development.
2.3. Knockdown of insulin induces ectopic Pdx1 expression in α cells and destabilizes α cell fate
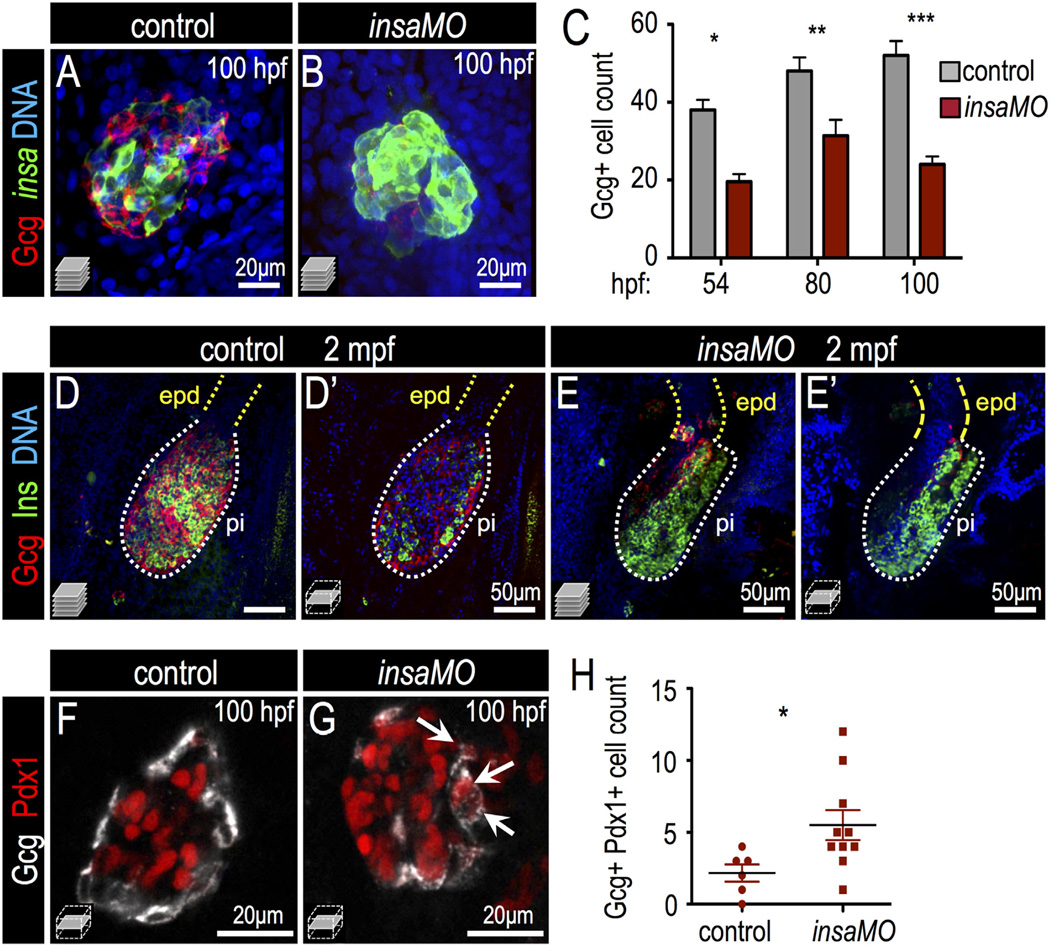
We next investigated how insulin signaling impacts specification of other endocrine cell types during development. First, α cells were examined by immunofluorescent staining of glucagon in insaMO-injected islets. Characteristically, α cells are located in the islet mantle and β cells are clustered in the islet core in both zebrafish and mouse islets. However, We noted a striking disorganization of islet cells in the insaMO-injected larvae: in insaMO-injected islets, the islet architecture was inverted such that there was a core of glucagon+ α cells was surrounded by insulin+ β cells (Fig. 5A,B; Fig. S11). As observed in HOT:dnIRS2 embryos (Fig. 3E), insaMO-injected islets also contained a decreased number of α cells at 54–100 hpf (Fig. 5C). Moreover, we found that the impaired α cell development in the insaMO-knockdown embryos persisted in adulthood at 2 months post fertilization (Fig. 5D–E′). In contrast to the α cell population, there was no significant change in the number of somatostatin+ delta (δ) cells (Fig. S12A–C). Also, the total endocrine cell number, as marked by Tg(neurod: GFP), only slightly increased in 24 hpf insaMO-injected embryos, but was unchanged at 2, 3, and 4 dpf (Fig. S12D–L). Together, these data indicate that part of the increased β cell mass may come at the expense of α cell fate in insulin-deficient embryos.
Fig. 5.
Insulin knockdown impairs α cell development and destabilizes α cell fate. (A,B) Confocal projections of 100 hpf Tg(insa:CFP-NTR) control (A) and insaMO-injected (B) islets stained for Glucagon (red) and CFP (β cells, green). α cell count is decreased and α/β cell architecture is inverted. (C) Quantification of Glucagon-positive cells in 54 hpf, 80 hpf and 100 hpf control (gray) and insaMO-injected (red) islets (n ≥ 5 for all time points except 54 hpf control, n = 3). (D–E′) Confocal projections (D,E) and confocal planes (D′,E′) of 2 months post fertilization (mpf) control (D,D′) and insaMO-injected (E,E′) islets stained for Glucagon (red), Insulin (green) and DNA (blue). Reduction of α cell mass was sustained into adult stages. (F,G) Confocal planes of 100 hpf control (F) and insaMO-injected (G) islets immunostained for Glucagon (white) and Pdx1 (red). Double positive Glucagon+ Pdx1+ cells (arrows) were increased with insaMO-injection. (H) Quantification of double positive Glucagon+ Pdx1+ cells in 100 hpf control (n = 6) and insaMO-injected (n = 10) islets. Two way ANOVA was used in C and Student's t-test was used in H to determine significance.
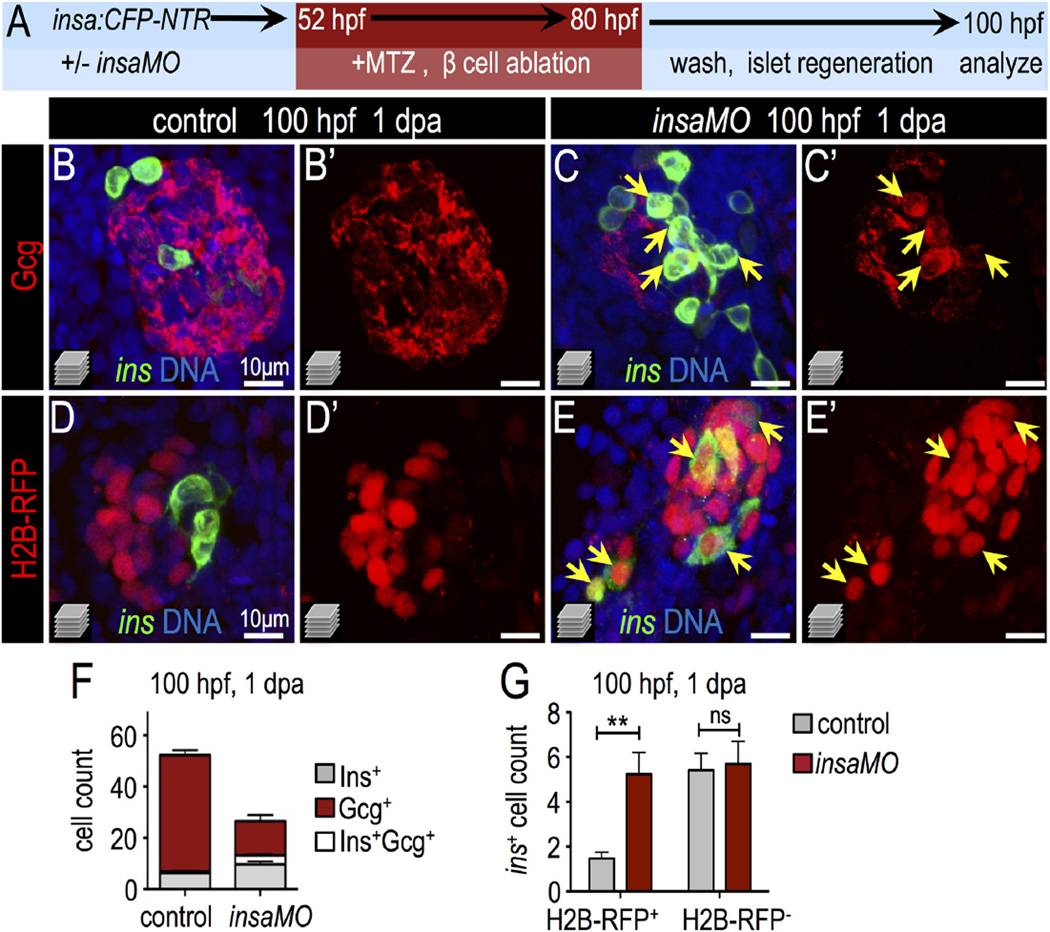
To test this interpretation, we first examined the expression of Pdx1 in the α cells of control and insaMO-injected islets. In insaMO-injected islets Pdx1 expression was often aberrantly co-expressed in glucagon+ α cells (Fig. 5F–H and S13A–E); this suggests that α cell maturation fails and that α cell identity is destabilized. Indeed α cells exhibit a degree of plasticity during development and regeneration. For instance, in zebrafish (Ye et al., 2015) and mouse (Chung et al., 2010; Thorel et al., 2010) models of extreme β cell ablation, α cells can spontaneously transdifferentiate into β cells through an intermediate Pdx1+ Glucagon+ stage. Furthermore, mis-expression of Pdx1 induces α cell fate instability and results in α to β transformation in some contexts (Yang et al., 2011). Based on this, we hypothesized that ectopic Pdx1 expression in α cells of insaMO-injected islets would result in the instability of α cell identity, particularly during β cell regeneration. To test this, we ablated β cells in Tg(insa:CFP-NTR)s892 embryos using metronidazole (Curado et al., 2007; Pisharath et al., 2007) and allowed islets to recover for one day (Fig. 6A). In regenerating control and insaMO-injected islets we quantified cells that were either CFP-NTR+ (β cells), Glucagon+ (α cells), or bihormonal CFP-NTR+ Glucagon+. In insa knockdown islets we observed increased β cell regeneration, and many regenerated β cells were co-labeled with Glucagon (Fig. 6B–C′,F). To distinguish between the possibilities that these double positive cells had formed by (1) de novo neogenesis, or (2) the transdifferentiation of Glucagon+ α cells, we used the LRC assay, in which all early differentiated endocrine cells (<40 hpf) in the principal islet are labeled. In this experiment, a cell of any endocrine subtype that transdifferentiated into a β cell during regeneration would have been labeled with H2B-RFP, while those that arose by de novo neogenesis would have been unlabeled (Ye et al., 2015). Consistent with our interpretation of α to β cell transdifferentiation, we found that the β cell ablated insaMO-injected islets showed a 2.5-fold increase of regenerated H2B-RFP-positive β cells, but no change in the quantity of regenerated H2B-RFP-negative β cells (Fig. 6D–E′,G). As a second test of α cell transdifferentiation, we also performed genetic α cell lineage tracing using the HOTcre system. Specifically, through combination of the transgenic lines Tg(gcga:Cre)s962 and Tg(hsp70l:loxp-mcherry-stop-loxp-H2B-GFP;cryaa:CFP)s923, α cells were labeled with H2B-GFP by heat-shock before β cell ablation (Ye et al., 2015). In these islets, if α cell identity was lost during β cell regeneration, then pre-labeled α cells would be detectable as both glucagon-negative and H2B-GFP-positive. Indeed, in insaMO-injected embryos we found that a significant population of α cells pre-labeled in this fashion lost glucagon expression during β cell regeneration (Fig. S13F–H); thus α cell plasticity appears to increase after insulin knockdown. The loss of glucagon expression could indicate that α cells have de-differentiated, or have been converted into another endocrine cell type; however, we hypothesize that α to β cell conversion predominates after loss of insulin signaling, as observed in previously published studies of transdifferentiation after β cell ablation in zebrafish (Ye et al., 2015). In summary, we have demonstrated that blocking insulin signaling via insaMO decreases α cell formation and induces Pdx1 expression in α cells. Furthermore, the increased β cell regeneration via α to β cell conversion in insaMO embryos may result from instability of α cell fate and α to β cell conversion consequent to diminished insulin signaling.
Fig. 6.
Insulin knockdown enhances regeneration of β cells. (A) Schematic of β cell ablation and regeneration experiments. Control and insaMO-injected insa:CFP-NTR embryos were treated with MTZ from 52 to 80 hpf, recovered in fresh egg water for 1 day, and analyzed at ~100 hpf (1 day post ablation; dpa). (B–C′) Merged (B,C) and single channel (B′,C′) confocal projections of regenerated 100 hpf control (B,B′) and insaMO-injected (C,C′) islets immunostained for Gcg (red), CFP (β cells, green), and DNA (blue). Arrows indicate the expansion of strongly double positive Ins+ Gcg+ cells. (D–E′) Merged (D,E) and single channel (D′,E′) confocal projections of 100 hpf control (D,D′) and insaMO-injected (E,E′) islets immunostained for CFP (green) and DNA (blue). All islets expressed zygotically-injected H2B-RFP (red) to mark DBCs. Arrows indicate the expansion of double positive H2B-RFP+ Ins+ cells; this indicates conversion of non-β endocrine cells into β cells. (F) Quantification of insa+, Gcg+, and insa+ Gcg+ cells in 1 dpa control (n = 17) and insaMO-injected (n = 22) islets. (G) Quantification of H2B-RFP+ insa+ and H2B-RFP− insa+ regenerating β cells in 1 dpa control (n = 17) and insaMO-injected (n = 13) islets. Student's t-test was used in G to determine statistical significance.
2.4. Loss of insulin signaling in transplanted blastomeres promotes β cell formation
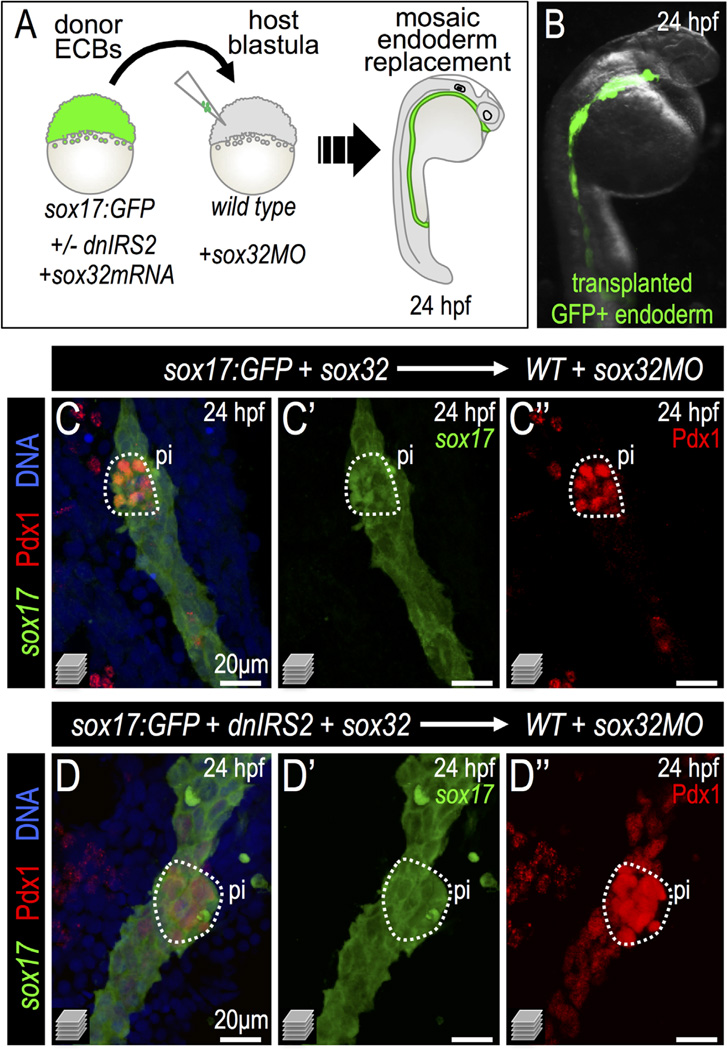
To distinguish whether the precocious β cell differentiation observed in our insulin signaling-knockdown models was due specifically to loss of insulin signaling in endodermal lineages, we generated endodermal chimeras via blastomere transplantation. Specifically, sox32 mRNA was injected into donor zygotes to drive endoderm formation while sox32 MO was used to block host embryo endoderm formation (Stafford et al., 2006). In these chimeras, transplanted donor cells (endoderm-committed blastomeres; ECBs) replaced nearly all endodermal tissues in host embryos (Fig. 7A–B). Thus, we generated chimeric embryos in which endodermal gene expression could be independently manipulated. When we blocked insulin signaling in the transplanted ECBs—by injection of dnIRS2-GFP mRNA into donor zygotes—we found that the differentiating donor endoderm exhibited an expanded Pdx1+ domain identical to that seen with global dnIRS2 over-expression (Fig. 7C–D″). This indicates that endodermal progenitor cells directly respond to insulin signaling by limiting Pdx1 expression.
Fig. 7.
Insulin signaling blockade in transplanted endodermal committed blastomeres promotes Pdx1 expression. (A) Scheme of endoderm replacement transplantation experiments. sox32-injected blastomeres termed “Endoderm Committed Blastomeres” (ECBs) were isolated from donor Tg(sox17:GFP) blastulae with or without injected dnIRS2-GFP mRNA. ECBs were transplanted into endoderm deficient, sox32MO-injected host blastulae. (B) 24 hpf chimera shows extensive replacement of endogenous endoderm with transplanted Tg(sox17:GFP) donor cells. (C–D″) Merged and single channel confocal projections of 24 hpf embryos with endoderm transplants from sox32 mRNA (C–C″, n = 8) or sox32 and dnIRS2 mRNA (D–D″, n = 7) injected donors. Embryos were immunostained for GFP (green), Pdx1 (red), and DNA (blue). dnIRS2-GFP-expressing endoderm showed increased Pdx1 expression.
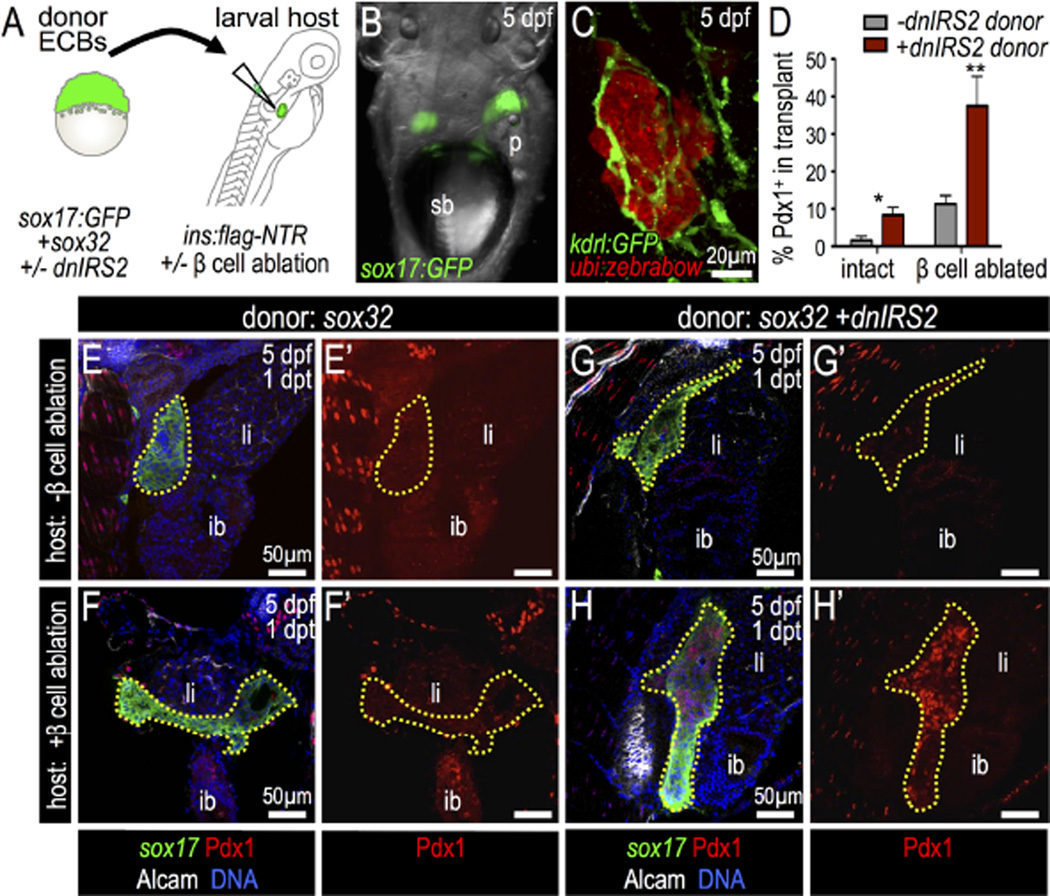
Overall, our data indicate that insulin signaling acts as a negative regulator in a developmental context, inhibiting the early differentiation of ventral pancreatic progenitors. We next asked whether blocking insulin signaling in transplanted multipotent progenitor cells could promote their differentiation into β cells in a more mature pancreatic setting. To approach this in a manner that might have relevance to therapies involving stem cell transplantation, we developed a novel blastula to larvae cell transplantation assay (Fig. 8A,B). For this, blastomeres were collected from sox32 mRNA-injected donor blastula stage embryos; these ECBs were transplanted into the pancreatic region in larval stage hosts, and the survival and integration of donor endoderm into host tissues was verified. RFP-expressing ECBs from Tg(ubi:zebrabow) embryos were transplanted into Tg(kdrl:GFP) hosts with GFP-expressing vasculature; blood vessels pervaded the transplanted donor tissue in chimeras (Fig. 8C), demonstrating a close interaction between the donor and host tissues. Approximately 80% of host embryos (33 of 41) at one day post transplantation (dpt) had successfully integrated the transplanted ECBs.
Fig. 8.
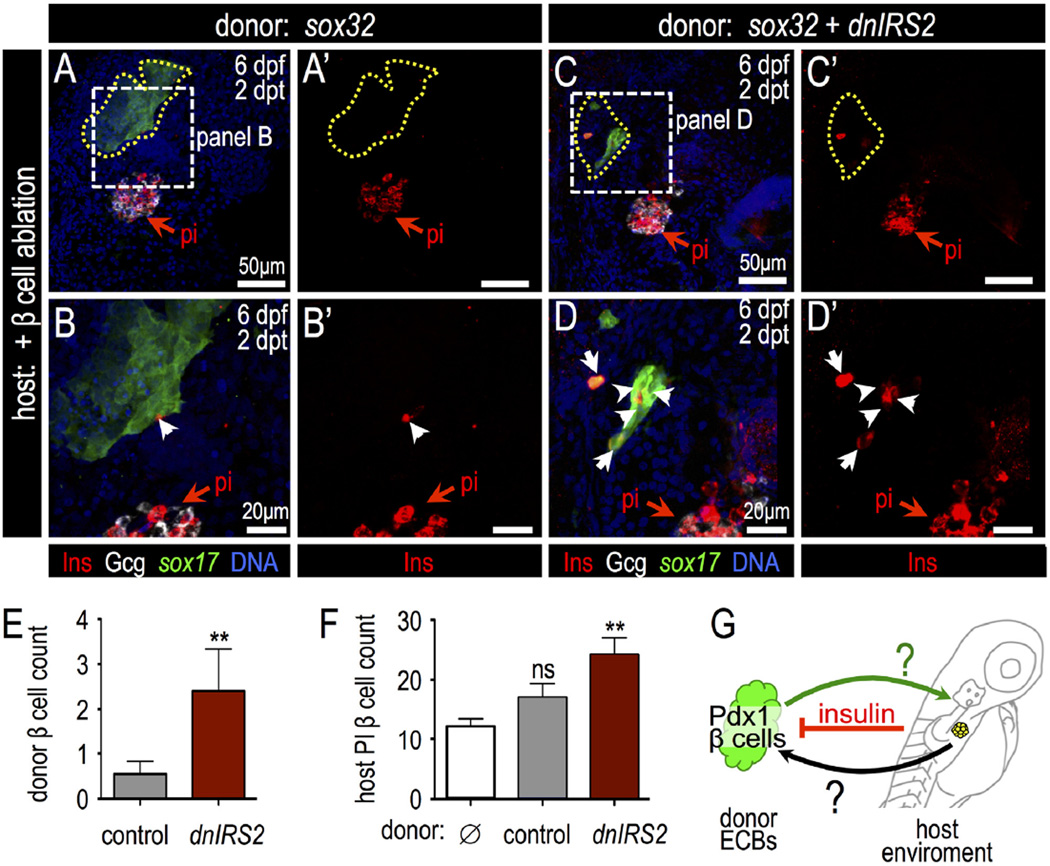
Insulin signaling blockade in endodermal committed blastomeres promotes Pdx1 expression after transplantation into β-cell ablated hosts. (A) Blastula ECB to 4 dpf Tg (insa:flag-NTR) larva cell transplantation scheme. (B) 5 dpf host shows Tg(sox17:GFP) donor cells at 1 day post transplantation (dpt). (C) Confocal projection of Tg(kdrl:GFP) (green) host with transplanted Tg(ubi:zebrabow) donor cells (red) at 1 dpt shows engraftment. (D) Percentage of transplanted ECBs that are Pdx1-positive. Chimeras were generated with the following four combinations: (1) wild type ECBs (−dnIRS2) into non-ablated host (n = 4); (2) ECBs +dnIRS2 into non-ablated host (n = 6); (3) wild type ECBs (−dnIRS2) into β ablated host (n = 6); and (4) ECBs +dnIRS2 into β ablated host (n = 8). (E–H′) Merged and single channel confocal planes of chimeras showing Tg(sox17: GFP) donor ECBs (outlined) at 1 dpt, stained for GFP (green), Pdx1 (red), Alcam (white), and DNA (blue). (E–F′) sox32 mRNA-injected blastomeres transplanted into β intact (E′ E′) and β cell-ablated hosts (F–F′). Few transplanted ECBs expressed Pdx1. (G–H′) sox32 and dnIRS2 mRNA-injected blastomeres transplanted into β cell-intact (G–G′) and β cell-ablated (H–H′) hosts. Loss of β cells increases Pdx1 expression in transplanted ECBs. Student t-test was used for statistical analysis in D. Abbreviations: sb, swim bladder; p, pancreas; ib, intestine bulb; li, liver.
We next analyzed the differentiation of these transplanted ECBs in the context of wild type or β cell-ablated hosts. First, we found that control donor ECBs differentiated into sox17:GFP+ endodermal cells by 1 dpt in wild type hosts; however, these ECBs did not further differentiate into Pdx1+ cells. In contrast, β-cell-ablated host larvae promoted the expression of Pdx1 in control donor ECBs (Fig. 8D,E–F′), which demonstrates that the host β cell mass influences the differentiation of transplanted donor ECBs at a distance. Next, to test whether insulin was essential for this action, dnIRS2 mRNA-injected donor cells—with impaired insulin signaling—were transplanted. dnIRS2 ECBs in control hosts showed a clear increase in Pdx1+ cell quantity; this increase was even more striking in dnIRS2 donor ECBs that had integrated into β cell-ablated chimeras (Fig. 8D,G–H′). Together, these results indicate that insulin signaling intrinsically regulates Pdx1 expression in the endodermal progenitor-like ECBs. Furthermore, these ECBs are also influenced by β cell function at a distance.
Next, to determine whether donor ECBs can differentiate into pancreatic endocrine cells, at 2 dpt we examined Insulin and Glucagon protein expression. Neither hormone was detected in donor cells that were transplanted into non-ablated host larvae (data not shown); this is consistent with the minimal Pdx1 induction observed in ECBs in a non-ablated host environment. In contrast, when ECBs were transplanted into β cell-ablated host larvae, these donor cells differentiated into both insulin+ cells and glucagon+ cells (Fig. 9A–B‴, Fig. S14A–B′). Furthermore, when dnIRS2 mRNA-injected ECBs were transplanted into β cell-ablated hosts, there was a marked increase in the number of insulin-expressing cells in the transplant; in contrast, no glucagon+ cells were detected (Fig. 9C–E, Fig. S14C,C’). This is concordant with our earlier results that showed that inhibition of insulin signaling impairs α cell differentiation. Interestingly, in addition to an increase of β cell differentiation in dnIRS2 donor ECBs, we observed transplanted dnIRS2 ECBs can influence host β cell regeneration as well. We observed a significant increase of β cells in the principal islets of host larvae when they were transplanted with dnIRS2 donor ECBs (Fig. 9F, Fig. S15). In summary, we have demonstrated that insulin signaling in transplanted endoderm-committed blastomeres regulates Pdx1 expression and β cell formation both in donor and host tissues.
Fig. 9.
Insulin signaling blockade in ECB donor cells promotes Insulin expression and host β cell regeneration. (A–D′) Merged and single channel confocal projections of transplanted Tg(sox17:GFP) donor ECBs in β cell-ablated host larvae stained for GFP (green), Insulin (red) and Glucagon (white) at 2 days post transplantation (dpt). (B–B′, D–D′) Higher magnification of boxed region in panels A–A′ and C–C′, respectively. In (A–B′) donor cells were injected with sox32 mRNA only; in (C–D′) donor ECBs had been zygotically-injected with sox32 and dnIRS2 mRNAs. Cells derived from the ECB transplant are bounded by a dotted yellow line and the principal islet (pi) of the host is indicated by a red arrow. (E) Quantification of Insulin+ β cells in donor ECBs that were transplanted into β cell ablated hosts; −dnIRS2 (n = 11), +dnIRS2 (n = 10). Blockade of insulin signaling with dnIRS2 increased the production of insulin-positive β cells. (F) Quantification of host-derived regenerated β cells in the principal islet (pi) after transplantation with no cells (sham control, n = 12), donor ECBs injected with sox32 mRNA alone (n = 11), or ECBs injected with sox32 plus dnIRS2 mRNAs (n = 11). (G) Model for the role of insulin signaling in regulating β cell differentiation and reciprocal interactions between transplanted endoderm-committed blastomeres and the host environment. Student's t-test was used in E and one-way ANOVA was used in F to determine significance. Abbreviation: pi, host larvae principal islet at 2 days post β cell ablation.
3. Discussion
The coordinated growth and development of the pancreatic β cell mass involves many phases, including specification and expansion of the pancreatic progenitor cell population, β cell differentiation, and β cell proliferation. Here, we provide evidence that insulin signaling acts as a negative feedback signal to regulate pancreatic progenitor cell differentiation during development. We have used multiple approaches to block insulin signaling—including mis-expression of dominant negative pathway effectors, knock down of insulin ligand with morpholinos, and small molecule inhibitors—to show that this pathway regulates the neogenesis of both β cells and α cells. With loss of insulin signaling during embryonic development, we observed increased Pdx1 expression, a precocious differentiation of β cells, and a coordinated loss of α cell identity. In addition, using a novel blastomere-to-larva transplantation strategy, we found that loss of insulin signaling promoted the differentiation of endoderm committed blastomeres (ECBs) into β cells. Taken together, our data suggest that appropriate modulation of insulin signaling may be important for the optimal generation β cells in vitro and in vivo, and thus may facilitate β cell replacement strategies for the treatment of diabetes.
3.1. A conserved role for insulin signaling in islet development
Our results in zebrafish are consistent with a role for insulin as a physiologically relevant signal that feeds back to regulate pancreas progenitor differentiation during the expansion of the β cell mass. The release of active insulin from nascent functional β cells would provide a direct means for progenitors to sense and interpret embryonic requirements for β cells: matching β cell number with changing physiological needs of the growing embryo. Consistent with this, a comparable response was also observed in the β cell mass of mutant mice lacking insulin expression. Although loss of both mouse Insulin orthologues is required to induce hyperglycemia, islet hyperplasia was observed in the single Ins1 and Ins2 knockouts, and double null mice (Duvillié et al., 1997). Indeed, islet size is significantly increased in Ins1−/−Ins2−/− mice at birth, and the β cell mass in Ins2−/− mice was increased almost three-fold at 7 weeks of age, indicating that increased β cell mass expands to compensate for low insulin production (Duvillié et al., 1997; Leroux et al., 2001). This is further supported by the finding that expression of human INSULIN in the Ins1−/−; Ins2−/− mouse reverses the compensatory β cell hyperplasia (Karaca et al., 2007). These findings, together with our results, support the hypothesis that insulin acts as a negative regulator for β cell formation during development (Duvillié et al., 1997; Leroux et al., 2001).
In contrast to Insulin gene mutants, Insr−/− mice initially appear unaffected as neonates, showing a normal β cell mass (Accili et al., 1996; Joshi et al., 1996). Moreover, immediately after feeding severe insulin resistance in these mutants results in hyperglycemia, hyperinsulinemia and neonatal death (Accili et al., 1996; Joshi et al., 1996). On the surface, these data appear contrary to our hypothesis. However, this contradictory result may be due to compensatory/redundant activation of IGF-1 receptors in the absence of insulin receptors (Kitamura et al., 2003). Local production of IGF-1 in the pancreas may act as negative regulatory factor of β cell mass, as knockout of Igf1 in the pancreas increases β cell differentiation and a 2.3-fold enlarged islet cell mass (Lu et al., 2004). Thus, any de-repression of β cell differentiation resulting from loss of insulin signaling through InsR is likely countered by redundant activation of IGF1R signaling. On the other hand, our data does not exclude the possibility that insulin regulates pancreatic progenitor cell differentiation in part via activation of IGF1R, given the complex interaction between the insulin and IGF1 signaling pathways (Siddle, 2011; Kitamura et al., 2003). However, given that Insulin binds the IGF1R and InsR/IGF1R heterodimers with much lower affinity than InsR (Pandini et al., 2002; Soos et al., 1993), it is likely that our manipulations affect insulin receptor signaling rather than IGF1R signaling. In further support of this interpretation, knockdown either of igf1 or igf1r in zebrafish results in severe neural and gross developmental defects (Eivers et al., 2004; Zou et al., 2009), which are significantly different from the insulin knockdown phenotypes reported here. This supports our assertion that the insulin receptor is the major member of the insulin receptor family that suppresses the differentiation of pancreas progenitor cells.
In addition to a direct effect of insulin signaling on the differentiation of pancreas progenitors, additional indirect actions are also possible. For instance, in the absence of insulin signaling, we expect that free glucose levels will be increased in developing zebrafish, as we have previously shown after β cell ablation (Ye et al., 2015; Andersson et al., 2012). Glucose can act both as a β cell mitogen (reviewed in Oh (2015)) and as a factor promoting de novo β cell neogenesis/differentiation (Soggia et al., 2012; Guillemain et al., 2007; Ninov et al., 2013). However, our transplantation experiments intimate that glucose levels cannot mediate the differentiation effect alone. First, in our endodermal chimeras, insulin signaling, and thus glucose disposal, should be unaffected in all non-endodermal tissues, including skeletal muscle; yet Pdx1 expression was clearly increased in dnIRS2-injected endoderm. The heterochronic ECB transplants provide more convincing evidence: in these, dnIRS2 induced the expression of Pdx1 even when host β cells, and also carbohydrate metabolism, were unperturbed. These data support a model in which insulin ligand signals the undifferentiated endoderm directly. There are at least two explanations for the additive effects of dnIRS2 expression in ECBs and host β cell-ablation upon the differentiation of β cells. Most simply, the loss of insulin release via β cell ablation could further depress the insulin signaling knock down elicited by dnIRS2. Alternately, it could result from the combined effects of decreased insulin signaling and increased free glucose levels. The coordinated actions of glucose and insulin would provide a integrated mechanism to regulate differentiation of β cells, under varied physiological conditions. Both insulin signaling (Nakae et al., 2002; Martinez et al., 2006) and glucose sensation (Housley et al., 2008) can modulate the activity of the Foxo1, a transcription factor that regulates the expression of β cell characteristics (Talchai et al., 2012). Whether molecular integration of both insulin and glucose sensing pathways is mediated by Foxo1 during β cell development and regeneration is an exciting hypothesis that requires more study.
When we transplanted dnIRS2-expressing ECBs into zebrafish larvae, we also found that these pluripotent “insulin resistant” blastomeres promoted β cell regeneration in host tissues. This may reveal a bidirectional interchange between the ECB-derived progenitors and their environment. This proposition is consistent with previous studies that show that transplanted stem cells can secrete angiogenic factors and growth factors (Gnecchi et al., 2008; Horie et al., 2011). In fact, some functional benefits observed after stem cell transfer might be due in part to the secretion of soluble factors that act as paracrine or endocrine fashion to promote tissue regeneration (Gnecchi et al., 2008; Kono et al., 2014; Mirotsou et al., 2007). Thus, we propose that in our model that the transplanted donor blastomeres interact through the release of secreted signaling factors that act as paracrine or systemic signals (Fig. 9G). Additional studies are needed to test this hypothesis and to identify whether there are additional signaling factors besides insulin and glucose; unveiling these mechanisms will be essential for understanding the deficiencies of β cell compensation that are seen in insulin-resistant and diabetic states.
3.2. Insulin signaling regulates endocrine subtype differentiation during development and transdifferentiation during regeneration
In addition to regulating pancreatic progenitor cell differentiation into β cells, our data also indicate that insulin plays a crucial role in the specification of the α cell endocrine subtype. After blockade of insulin signaling we found an increase of β cell formation from the pancreatic progenitor cells, and a decrease of α cell fate. This function of insulin signaling is likely to be mediated in part by its repressive actions on Pdx1, as our results show that loss of insulin signaling promotes Pdx1 expression in glucagon positive α cells as well as pancreas progenitor cells and transplanted endoderm-committed blastula cells. In mice, enforcing expression of Pdx1 in pancreatic endocrine progenitor cells results in increased β cell number and decreased α cell number during embryonic stages (Yang et al., 2011). As one of the key transcription factors required for β cell specification and function, Pdx1 enhances transcription of β cell-expressed genes such as Insulin, Glut2, Glucokinase, and Mafa but inhibits α cell-expressed genes such as Arx and Glucagon (Gao et al., 2014; Waeber et al., 1996; Wang et al., 2001). Besides regulating pancreatic progenitor cell differentiation, Pdx1 has also been shown to directly drive an α to β cell fate switch. In mouse models, mis-expressing Pdx1 in α cells drove postnatal conversion of these cells into β cells (Yang et al., 2011). Moreover, we have previously shown that in zebrafish models, Pdx1 mis-expressing immature “α-like” cells are unstable, and can transdifferentiate into β cells (Ye et al., 2015). In the current study, we show that loss of insulin during development also induces the formation of Pdx1+ glucagon+ “α-like” cells. These “α-like” cells are likely to be the source of increased α to β cell transdifferentiation after loss of insulin signaling.
3.3. Insulin signaling maintains pluripotency in multiple contexts
Insulin has been classically viewed as a mitogen (Siddle, 2011); however, insulin also plays key roles in maintaining stem cell fate and regulating stem cell differentiation (Bendall et al., 2007; Choi et al., 2011; Shim et al., 2012; Wang et al., 2007). For example, self-renewal of human embryonic stem (ES) cells requires the activation of insulin and IGF signal pathways; blocking these signals promotes ES cell differentiation (Bendall et al., 2007; Wang et al., 2007). Also, in Drosophila larvae, starvation reduces the number of hematopoietic progenitor cells by increasing their differentiation, and these starvation effects are mediated by the insulin signaling pathway (Shim et al., 2012; Benmimoun et al., 2012). Furthermore, reduced systemic insulin signaling and down-regulation of the downstream insulin signaling component Akt/PI3K promotes hematopoietic and skin stem cell differentiation (Shim et al., 2012; Sadagurski et al., 2006).
Our study clearly supports a role for insulin in regulating pancreatic cell differentiation that is similar to the well-described role of the Notch signaling pathway. Notch sustains Ptf1a expression in early pancreatic progenitors, stimulates progenitor self-renewal and blocks endocrine cell differentiation via repression of the bHLH transcription factor Neurog3 (Lee et al., 2001; Shih et al., 2012; Ahnfelt-Rønne et al., 2012). Accordingly, disruptions of Notch signaling induce premature endocrine cell differentiation and diminish the size of the pancreatic progenitor cell pool (Shih et al., 2012; Fujikura et al., 2006). The strong phenotypic similarities between the loss of insulin signaling and the loss of Notch signaling suggest that these pathways interact—that insulin signaling may act as a positive regulator of Notch signaling in pancreatic progenitor cells. Indeed, there is evidence that supports that PI3K/Akt and Notch pathways cross talk: during megakaryocyte development the PI3K/AKT pathway is activated by Notch stimulation, which in turn enhances Notch-dependent differentiation (Cornejo et al., 2011). Cooperation between Notch and insulin signaling may therefore integrate environmental and metabolic cues to regulate progenitor cell maintenance and differentiation. Therefore, it is plausible that insulin signaling in pancreatic progenitor cells maintains a positive circuit between PI3K/Akt and Notch that blocks the endocrine differentiation program and prevents the premature differentiation of β cells. Clearly it will be important to further clarify the interaction of the notch and insulin signaling pathways during progenitor differentiation.
Lastly, recent studies have indicated that endogenous pancreatic multipotent progenitor cells are present in adult pancreas both in human (Meier et al., 2006; Patti et al., 2005; Phillips et al., 2007) and mouse (Xu et al., 2008; Razavi et al., 2015), and that metabolic stress can influence their proliferation and differentiation into β cells (Razavi et al., 2015). Maintaining the balance of self-renewal and differentiation in these progenitor cells is likely to be crucial for long-term β cell compensation in response to metabolic stresses. Given our findings that insulin signaling plays an important role in regulating progenitor cell differentiation during development, understanding the roles and mechanisms of insulin signaling in adult pancreatic progenitors, and how to appropriately manipulate insulin signaling in these populations, may prove to be critical for developing new regenerative therapies for diabetes.
4. Materials and methods
4.1. Zebrafish maintenance and strains
Zebrafish were raised under standard laboratory conditions at 28 °C. In order to induce dnIRS2-GFP overexpression via the HotCre system, heat shock activations were performed at 38.5 °C for 20 min at the stages indicated in the text. All animal procedures were conducted in accordance with OLAW guidelines and were approved by the Indiana University Institutional Animal Care and Use Committee. The following transgenic lines were used: Tg (sox17:GFP)s870 (Sakaguchi et al., 2006), Tg(insa:CFP-NTR)s892 (Curado et al., 2007), Tg(insa:dsRed)m1018 (Anderson et al., 2009), Tg (insa:Flag-NTR;cryaa:mCherry)s950 (Andersson et al., 2012), Tg (neurod:GFP) (Obholzer et al., 2008), Tg(gcga:GFP)ia1 (Pauls et al., 2007), Tg(gcga:cre; cryaa:YFP)s962 (Ye et al., 2015), Tg(hs:loxp-mcherry-loxp-H2B-GFP)s925 (Hesselson et al., 2009), Tg(kdrl:GFP)s844 (Jin et al., 2005), Tg(ubi:zebrabow)a131 (Pan et al., 2013), Tg(hs:cre) (Le et al., 2007) and Tg(hs:loxp-mcherry-loxp-dnIRS2-GFP). To construct the hsp70L-loxP-mCherry-STOP-loxP-dn-irs2a-GFP transgene, the N-terminus of irs2a was amplified from BAC DKEYP-24D6 using the oligos: 5′-GGCGC GCCAC CATGG CGAGT CCGCC GCCG and 5′-CTCGC CCTTG CTCAC CATGG CTGCC ATGCT GTCAG T and GFP was amplified from using: 5′-ACTGA CAGCA TGGCA GCCAT GGTGA GCAAG GGCGA G and 5′-CGAGC TGTAC AAGTA AAGCG GCCGC. The two resulting products were fused by PCR and cloned into plasmid hsp70L-loxP-mCherry-STOP-loxP-H2B-GFP_cryaa-cerulean (Addgene #24334) using AscI and NotI; Transgenesis was performed as described (Hesselson et al., 2009).
4.2. Detection of protein, mRNA and proliferation
Whole mount immunofluorescent staining and western blotting was performed as described before (Anderson et al., 2009). In situ hybridization and quantitative PCR were used to detect mRNA expression. In order to detect cell proliferation, an EdU incorporation assay was performed as described (Anderson et al., 2009). The following antibodies were used for immunofluorescence staining: anti-GFP (Aves Labs); anti-insulin (Biomeda); anti-glucagon (Sigma); anti-dsRed (Clontech); anti-PHH3 (Cell Signaling); anti-Alcam (Zn-8); anti-Pdx1 (gift of Dr. C. Wright, Vanderbilt U.). Alexa Fluor-conjugated secondary antibodies were used for visualization (Life Technologies). For immunoblotting, anti p-Akt473 (Cell Signaling) was used. The relative expression level of p-Akt473 was determined by normalizing to α-tublin (Santa Cruz). For in situ hybridization, the primer sets in Table 1 were used to PCR amplify probe templates from cDNA which were then synthesized and labeled in vitro using the DIG RNA labeling kit T7 (Roche). in situ hybridization was performed as described (Anderson et al., 2009) and color was developed with BM purple AP Substrate (Roche). For fluorescent in situ hybridization, Vector Red (Vector Inc.) was used for color develoment. For quantitative PCR, mRNA was extracted with Trizol (Life Technologies) and reverse transcribed with iScript (BioRad). The Mastercycler Realplex PCR system (Eppendorf) was used with Sybr Green mix and Mytaq (BioLine) to generate Ct values. The relative expression of each sample was determined by normalizing to β-actin using the relative standard curve method (Hesselson et al., 2009). The primer sets in Table 2 were used for quantitative PCR analysis.
Table 1.
Primer sets used for synthesis of in situ hybridization probes.
| insra | 5′-GCTCG TGCGC GTGTT CATAT |
| 5′-GCTAA TACGA CTCAC TATAG GTTTC CGTGG CCTGA GTTC | |
| insrb | 5′-GGCTG GACAC ATCTG TGGTT G |
| 5′-GCTAA TACGA CTCAC TATAG GCGGT GGAGG ACAAT TATAT CGTAG | |
| pdx1 | 5′-GGGAG ACTGC AGGTA GAGCA |
| 5′-GCTAA TACGA CTCAC TATAG GGCCT TTTGC CAATC TGTTT GC |
Table 2.
Primer sets for qPCR analysis.
| insa | 5′-TCTGC TTCGA GAACA GTGTG |
| 5′-GGAGA GCATT AAGGC CTGTG | |
| insra | 5′-CGGCT CGCTG TGTTG TATTG |
| 5′-TACTG TCCCT CCTCT CACGG | |
| insrb | 5′-TCGCC TACAT CTTGT GCCTC |
| 5′-AGCTC AAGCC CCTGA AATCC | |
| pdx1 | 5′-ACACG CACGC ATGGA AAGGA CA |
| 5′-GCGGG CGCGA GATGT ATTTG TT | |
| β-actin | 5′-GGCAC GAGAG ATCTT CACTC CCC |
| 5′-GGGGA AAACA GCACG AGGG GC |
4.3. Microinjections
H2B-RFP mRNA, dnIRS2-GFP mRNA, insulin mRNA and sox32 mRNA were transcribed with SP6 mMessage machine kit (Invitrogen) in vitro. In order to label the differentiated dorsal pancreatic endocrine cells, 100 pg of H2B-RFP mRNA were injected into zygotes. Immunostaining with anti-dsRed (Clontech) and Alexa 568 (Life Technologies) antibodies was used to enhance signal strength. In order to block insulin signaling or to misexpress insulin, 200 pg dnIRS2-GFP mRNA or 200 pg insulin mRNA were injected into zygotes, respectively. Immunostaining with anti-GFP (Aves Labs) and Alexa 488 (Life Technologies) antibodies was used to enhance the dnIRS2-GFP signal strength. To induce endoderm differentiation, 200 pg sox32 mRNA were injected into zygotes. To knockdown gene expression, antisense morpholinos (Gene Tools, LLC) were injected into zygotes (Table 3): insaMO (4 ng, unless indicated), insbMO (2 ng, unless indicated) and sox32MO (4 ng). insaMO and insbMO were designed to bind the exon-intron junction of the target gene pre-mRNA and disrupt splicing. For assessment of insaMO and insbMO efficiency, mRNA was extracted from 2 dpf control and MO-injected embryos and cDNA was synthesized as described above. The PCR product was then run through a 1% agarose gel and imaged with Carestream Gel Logic system. The primers 5′-CATTC CTCGC CTCTG CTTC and 5′-GGAGA GCATT AAGGC CTGTG were used to assess insaMO efficiency and primer set 5′-CAGAC TCTGC TCACT CAGGA AA and 5′-GCGTG TAATG GTCAT TTATT GC were used for insbMO. The amplified cDNA products were purified from the gel and sequenced.
Table 3.
Morpholino Sequences.
| Name | Target | Sequence | Dose (ng) |
|---|---|---|---|
| insaMO | insa (NM_131056.1) | 5′-CCTCT ACTTG ACTTT CTTAC CCAGA |
4 |
| insbMO | insb (NM_001039064.1) | 5′-AAGTT GGAGA CGTTG CTCAC CCAGC |
2 |
| Sox32MO | Sox32(NM_131851.1) | 5′-GCATC CGGTC GAGAT ACATG CTGTT |
4 |
4.4. Chemical treatments
For β cell ablations, Tg(insa:CFP-NTR)s892 embryos were incubated in 0.1% DMSO (Sigma) ± 10 mM Metronidazole (MTZ, sigma) in egg water. After 24 h of treatment, from 54 hpf to 78 hpf, embryos were washed extensively with fresh egg water, and recovered for 1 day. In the mosaic analyses, Tg(insa:Flag-NTR;cryaa: mCherry)s950 embryos were treated ± 10 mM MTZ from 3 dpf to 4 dpf and MTZ was washed out 2 h prior transplantation. For inhibition of the Akt pathway, Tg(insa:CFP-NTR)s892 embryos were incubated in 0.1% DMSO ± 1 µM wortmannin (Sigma) from 14 hpf and fixed at 30 hpf or 42 hpf. For inhibition of MAPK pathway, Tg (insa:CFP-NTR)s892 were incubated in 0.1%DMSO ± 100 µM U0126 (Sigma (Hawkins et al., 2008)).
4.5. Cell transplantations
To analyze the roles of insulin signaling in the endoderm, chimeric zebrafish embryos were generated by cellular transplantation (Anderson et al., 2013). sox32 mRNA was synthesized in vitro and injected (200 pg/embryo) into donor zygotes and 4 ng sox32MO was injected into host zygotes to promote the endoderm replacement by donor cells. Tg(sox17:GFP)s870 donor embryos were used to distinguish the transplanted endoderm. To analyze the differentiation of blastomeres in zebrafish host larvae, Tg(sox17: GFP)s870 donor embryos were injected with 200 pg sox32 mRNA alone or together with 200 pg dnIRS2-GFP mRNA. De-chorionated donor blastulae were immobilized in agarose molds and 4 dpf host larvae were immobilized with 2% methylcellulose. About 20–40 cells were extracted from the donor and injected into host larvae in the abdominal cavity behind the liver and above the pancreas. To examine blood vessel infiltration of transplanted donor tissue, Tg(ubi:zebrabow)a131 donor embryos were injected with 200 pg sox32 mRNA and transplanted into 4 dpf Tg(kdrl:GFP)s844 host larvae.
Supplementary Material
Acknowledgments
We thank Sucharat Tayarachakul, Meagan Wooten and Jessica Fettig for expert fish care, Dr. Carmella Evans-Molina for Wortmannin, Dr. Raghavendra Mirmira for U0126 and pi-Akt antibody, and Dr. Chris Wright for Pdx1 antibody.
Funding
This work was supported by the NIH via the Indiana University Diabetes and Obesity Research Training Program DK064466 (LY), JDRF 10-2010-100 (RMA), and March of Dimes #1-FY14-211 (RMA).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
LY and RMA conceived and designed the experiments. LY, RMA, MAR, and TLM performed the experiments. LY and RMA analyzed the data and wrote the paper. LY, TLM, MAR, and RMA edited the paper.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2015.12.003.
References
- Asghar Z, et al. Insulin resistance causes increased beta-cell mass but defective glucose-stimulated insulin secretion in a murine model of type 2 diabetes. Diabetologia. 2006;49:90–99. doi: 10.1007/s00125-005-0045-y. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Accili D, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, et al. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab. 2012;15:885–894. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnfelt-Rønne J, et al. Ptf1a-mediated control of Dll1 reveals an alternative to the lateral inhibition mechanism. Development. 2012;139:33–45. doi: 10.1242/dev.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, et al. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev. Biol. 2009;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, et al. Hepatocyte growth factor signaling in intrapancreatic ductal cells drives pancreatic morphogenesis. PLoS Genet. 2013;9:e1003650. doi: 10.1371/journal.pgen.1003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, et al. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi F, et al. Differential effects of protein kinase B/Akt isoforms on glucose homeostasis and islet mass. Mol. Cell. Biol. 2010;30:601–612. doi: 10.1128/MCB.00719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CVE, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- Chera S, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C-H, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- Cras-Méneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes. 2001;50:1571–1579. doi: 10.2337/diabetes.50.7.1571. [DOI] [PubMed] [Google Scholar]
- Curado S, et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA. 2011;108:18702–18707. doi: 10.1073/pnas.1109348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo MG, et al. Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood. 2011;118:1264–1273. doi: 10.1182/blood-2011-01-328567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillié B, et al. Phenotypic alterations in insulin-deficient mutant mice. Proc. Natl. Acad. Sci. USA. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eivers E, McCarthy K, Glynn C, Nolan CM, Byrnes L. Insulin-like growth factor (IGF) signalling is required for early dorso-anterior development of the zebrafish embryo. Int. J. Dev. Biol. 2004;48:1131–1140. doi: 10.1387/ijdb.041913ee. [DOI] [PubMed] [Google Scholar]
- Field HA, Dong PDS, Beis D, Stainier DYR. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev. Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fujikura J, et al. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 2006;3:59–65. doi: 10.1016/j.cmet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gelling RW, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish – emergence of a new model vertebrate. Nat. Rev. Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Guillemain G, Filhoulaud G, Da Silva-Xavier G, Rutter GA, Scharfmann R. Glucose is necessary for embryonic pancreatic endocrine cell differentiation. J. Biol. Chem. 2007;282:15228–15237. doi: 10.1074/jbc.M610986200. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, et al. Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet {alpha}-cells but not of intestinal l-cells. Mol. Endocrinol. 2009;23:1990–1999. doi: 10.1210/me.2009-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson D, Anderson RM, Beinat M, Stainier DYR. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc. Natl. Acad. Sci. USA. 2009;106:14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley MP, et al. O-GlcNAc regulates FoxO activation in response to glucose. J. Biol. Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins TA, Cavodeassi F, Erdélyi F, Szabó G, Lele Z. The small molecule Mek1/2 inhibitor U0126 disrupts the chordamesoderm to notochord transition in zebrafish. BMC Dev. Biol. 2008;8:42. doi: 10.1186/1471-213X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Joshi RL, et al. Targeted disruption of the insulin receptor gene in the mouse results in neonatal lethality. EMBO J. 1996;15:1542–1547. [PMC free article] [PubMed] [Google Scholar]
- Jørgensen MC, et al. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Jin S-W, Beis D, Mitchell T, Chen J-N, Stainier DYR. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Mizrachi E-B, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu. Rev. Physiol. 2003;65:313–332. doi: 10.1146/annurev.physiol.65.092101.142540. [DOI] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. BioEssays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Onder L, Wilfinger A, Ellertsdottir E, Meyer D. Requirement for Pdx1 in specification of latent endocrine progenitors in zebrafish. BMC Biol. 2011;9:75. doi: 10.1186/1741-7007-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca M, et al. Transgenic expression of human INS gene in Ins1/Ins2 double knockout mice leads to insulin underproduction and diabetes in some male mice. Front. Biosci. 2007;12:1586–1593. doi: 10.2741/2171. [DOI] [PubMed] [Google Scholar]
- Kono TM, et al. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32:1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Nielsen JH. Regulation of beta cell replication. Mol. Cell. Endocrinol. 2009;297:18–27. doi: 10.1016/j.mce.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Linnemann AK, Baan M, Davis DB. Pancreatic β-cell proliferation in obesity. Adv. Nutr. 2014;5:278–288. doi: 10.3945/an.113.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le X, et al. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc. Natl. Acad. Sci. USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux L, et al. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes. 2001;50(Suppl. 1):S150–S153. doi: 10.2337/diabetes.50.2007.s150. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes. 2004;53:3131–3141. doi: 10.2337/diabetes.53.12.3131. [DOI] [PubMed] [Google Scholar]
- Lee JC, et al. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- Miettinen P, Ormio P, Hakonen E, Banerjee M, Otonkoski T. EGF receptor in pancreatic beta-cell mass regulation. Biochem. Soc. Trans. 2008;36:280–285. doi: 10.1042/BST0360280. [DOI] [PubMed] [Google Scholar]
- Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes. 2006;55:1581–1591. doi: 10.2337/db05-0678. [DOI] [PubMed] [Google Scholar]
- Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC. Increased islet beta cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia. 2006;49:2689–2696. doi: 10.1007/s00125-006-0410-5. [DOI] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev. Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Ninov N, Hesselson D, Gut P, Zhou A, Fidelin K, Stainier DYR. Metabolic regulation of cellular plasticity in the pancreas. Curr. Biol. 2013;23:1242–1250. doi: 10.1016/j.cub.2013.05.037. 10.1016/j.cub.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- Okada T, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl. Acad. Sci. USA. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani K, et al. Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am. J. Physiol. Endocrinol. Metab. 2004;286:E41–E49. doi: 10.1152/ajpendo.00533.2001. [DOI] [PubMed] [Google Scholar]
- Offield MF, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Oh YS. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anat. Cell Biol. 2015;48:16–24. doi: 10.5115/acb.2015.48.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Prasadan K, et al. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–3236. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- Pauls S, Zecchin E, Tiso N, Bortolussi M, Argenton F. Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev. Biol. 2007;304:875–890. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Papasani MR, Robison BD, Hardy RW, Hill RA. Early developmental expression of two insulins in zebrafish (Danio rerio) Physiol. Genom. 2006;27:79–85. doi: 10.1152/physiolgenomics.00012.2006. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, et al. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- Patti ME, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- Phillips JM, O’Reilly L, Bland C, Foulis AK, Cooke A. Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes. 2007;56:634–640. doi: 10.2337/db06-0832. [DOI] [PubMed] [Google Scholar]
- Pan YA, et al. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development. 2013;140:2835–2846. doi: 10.1242/dev.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi R, et al. Diabetes enhances the proliferation of adult pancreatic multipotent progenitor cells and biases their differentiation to more β-cell production. Diabetes. 2015;64:1311–1323. doi: 10.2337/db14-0070. [DOI] [PubMed] [Google Scholar]
- Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J. Mol. Endocrinol. 2011;47:R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54:2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Avignon A, Yamada K, Bandyopadhyay G, Farese RV. The phosphatidylinositol 3-kinase inhibitor, wortmannin, inhibits insulin-induced activation of phosphatidylcholine hydrolysis and associated protein kinase C translocation in rat adipocytes. Biochem. J. 1996;313(Pt. 3):1039–1046. doi: 10.1042/bj3131039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MB, et al. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol. Cell. Biol. 1985;5:2090–2103. doi: 10.1128/mcb.5.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, et al. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem. J. 1993;290(Pt. 2):419–426. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soggia A, et al. Activation of the transcription factor carbohydrate-responsive element-binding protein by glucose leads to increased pancreatic beta cell differentiation in rats. Diabetologia. 2012;55:2713–2722. doi: 10.1007/s00125-012-2623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol. Cell. Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HP, et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Kikuchi Y, Kuroiwa A, Takeda H, Stainier DYR. The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Dev. Camb. Engl. 2006;133:4063–4072. doi: 10.1242/dev.02581. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J. Clin. Investig. 1996;98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y, et al. The role of insulin receptor signaling in zebrafish embryogenesis. Endocrinology. 2008;149:5996–6005. doi: 10.1210/en.2008-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Park JT, Parsons MJ, Leach SD. Fate mapping of ptf1a-expressing cells during pancreatic organogenesis and regeneration in zebrafish. Dev. Dyn. 2015;244:724–735. doi: 10.1002/dvdy.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl. 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- Withers DJ, et al. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol. Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J. Biol. Chem. 2001;276:25279–25286. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Ye L, Robertson MA, Hesselson D, Stainier DYR, Anderson RM. Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development. 2015;142:1407–1417. doi: 10.1242/dev.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee NS, Yusuff S, Pack M. Zebrafish pdx1 morphant displays defects in pancreas development and digestive organ chirality, and potentially identifies a multipotent pancreas progenitor cell. Genesis. 2001;30:137–140. doi: 10.1002/gene.1049. [DOI] [PubMed] [Google Scholar]
- Yang Y-P, Thorel F, Boyer DF, Herrera PL, Wright CVE. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Kamei H, Modi Z, Duan C. Zebrafish IGF genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One. 2009;4:e7026. doi: 10.1371/journal.pone.0007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.