Abstract
The molecular and cellular mechanisms that underlie the many roles of macrophages in health and disease states in vivo remain poorly understood. The purpose of this Review is to present and discuss current knowledge on the developmental biology of macrophages, as it underlies the concept of a layered myeloid system composed of ‘resident’ macrophages that mostly originate from yolk sac progenitors and of ‘passenger’ or ‘transitory’ myeloid cells that originate and renew from bone marrow hematopoietic stem cells, and to provide a framework to investigate the functions of macrophages in vivo.
The current ‘mononuclear phagocyte system’ model 1 has guided many investigators studying the functions of macrophages over the past 30 years. It postulates that tissue macrophages are maintained from a constant supply of bone marrow derived circulating blood monocytes that extravasate into tissues 2. This model was initially based on the observation that blood leucocytes recruited in the inflamed peritoneum differentiate into macrophages. Subsequent transplantation experiments in irradiated recipients demonstrated that bone marrow hematopoietic stem cells (HSC) and HSC-derived myeloid progenitors can indeed give rise to tissue macrophages or dendritic cells in mice and humans 3, 4, 5, 6, 7. Therefore, investigations designed to study the molecular and cellular basis for macrophage phenotypic diversity and functions have largely focused on the activation, or polarization, of monocyte-derived macrophage by external cues, such as cytokines and microbe-associated signals 8, 9,10. Nevertheless, it remains currently unclear whether polarization stages of monocytes are stable or represent transient states of activation, whether they account for the phenotypic and functional heterogeneity of macrophages in vivo in health or disease states, and how many such polarized states exist 10, 11.
A new model of macrophage development is emerging based on a number of independent observations that two distinct populations of macrophages, which can be distinguished by their progenitors, developmental history, turnover and mechanisms of maintenance, coexist within the tissues of an adult mouse. The macrophage system of a mouse can now be described as a ‘layered’ system where ‘resident’ macrophages that develop in embryos independently of HSC 12 persist in adult tissues independently of adult HSC 12, 13, 14, 15, 16, 17, 18, and coexist with ‘passenger’ leucocytes such as monocytes and classical dendritic cells that originate and renew from bone marrow HSCs and myeloid progenitors 4, 6, 19. Resident macrophages include spleen red pulp macrophages, lung alveolar macrophages, epidermal Langerhans cells, brain microglia, liver Kupffer cells, large peritoneal macrophages, and F4/80bright pancreatic, kidney and cardiac macrophages. Many ‘resident’ macrophages are long-lived in mice and can proliferate within their tissue of residence, a mechanism involved in their maintenance in adults 20, 21, 22, 23, 24, 25, 26, 27. Nevertheless, bone marrow-derived progenitors also contribute to resident subsets in the lamina propria, spleen, brain, skin, heart, liver and kidney in a proportion that varies with the tissue considered, the age of the mice, and pathological processes12, 13, 14, 15, 16, 17, 18, 28, 29.
Therefore, the purpose of this Review is to present and discuss current knowledge on the developmental biology of resident macrophages, as it underlies the concept of a layered myeloid system composed of resident macrophages distinct from passenger macrophages and myeloid cells, and provides a new framework and experimental tools to characterise the functions of macrophages in vivo, in health and disease settings.
The original concept of ‘primitive macrophages’
During ontogeny, macrophages were found in the mouse yolk sac and the embryo before the initiation of HSC-derived hematopoiesis and before monocytes could be detected in the fetal blood. These ‘primitive macrophages’ were described in a series of seminal works by M. Naito and K. Takahashi in the 1980's 20, 30, 31 that reported the presence of large cells with macrophage morphology in blood islands of the yolk sac at E9 and in the fetal liver at E10. They were described as immature because of the lack of detectable phagocytic activity and a weak immunoreactivity for F4/80, as detected by electron microscopy 20, 30. In addition to the ‘immature macrophages’, these early studies also reported the existence of ‘fetal macrophages’ from E10 to E17, as slightly smaller cells with pseudopodia, phagocytic activity and frequent mitotic figures 31. At E17, the ‘fetal macrophages’ were reported as more differentiated, as they acquired ultrastructural features similar to those observed in adult tissue macrophages 30, 32. These early studies noted that the remaining difference between fetal and adult tissue macrophages was the presence of numerous mitotic figures in fetal macrophages throughout development. It was also noted that poorly differentiated myeloid cells are detected in the blood vessels of the yolk sac at E10 20, where immature stages of the monocyte and neutrophil differentiation series can be detected from E12 onwards, and bona-fide neutrophils and monocytes, based on ultrastructural features and PO-cytochemistry, are present in the fetal liver at E15 and E16 respectively 30. They therefore proposed that fetal macrophages develop without a monocytic intermediate, and in parallel with the monocyte and granulocyte lineage.
In the recent literature, this early work was sometimes reported as evidence for the early colonization of the fetus by ‘yolk-sac macrophages’ before their replacement by monocyte-derived macrophages. However, this body of work described the identification of proliferating non-phagocytic macrophage precursors (described as ‘immature macrophages’) that originate in the yolk sac, colonize the embryo and finish their differentiation in situ within the fetal tissues 20, 30, 31. Their description did not correspond to a particular wave of hematopoietic progenitors (‘primitive’ or ‘definitive’), as discovered subsequently. However, the description of erythro-myeloid progenitors (EMP) 33, 34, 35, 36 (see below) and recent fate mapping data are in accordance with the authors original interpretation of their morphological data. Indeed, genetic pulse labeling of Csfr1+ Kit+ CD45lo AA4.1+ EMP present in the yolk sac at E8.5 is followed by the detection of labeled CD11b+ cells with a negative or faint immunoreactivity for F4/80 at E9.5 in both the yolk sac and embryo and then by the detection of F4/80bright cells starting from E10.5 18.
More recent works have identified three successive but overlapping waves of hematopoietic progenitors during development, all of which have the potential to give rise to fetal macrophage 34. They can be distinguished by the anatomical site (extra-embryonic yolk sac versus intra-embryonic) where they are generated, expand and differentiate, their differentiation potential and their transcription factor dependency (Table 1 and Fig. 1). Here we will focus on the myeloid lineages. Erythroid lineages have been reviewed in 37.
Table 1.
phenotype, differentiation potential, and molecular features of hematopoietic progenitors of
| Primitive | Definitive | |||
|---|---|---|---|---|
| Progenitor name | Mp | EMp | HSC | |
| Origin | Posterior plate mesoderm | hemogenic endothelium | Arterial hemogenic endothelium | |
| Progenitor surface phenotype | Kitlo CD41lo | Kit+ CD41+ VE-Cad+ CD16/32+ AA4.1+ Tie2+ CD45lo Sca1− | Lin− Kit+ Sca1+ CD41+ VE− Cad+ Tie2+ CD45+ | |
| time window of generation | E7.0-? | E8.25-E10.5 | E9.5-E12.5 | |
| Anatomical location of | Generation | YS (blood islands) | YS | AGM (and large arteries) |
| Expansion | YS | FL | FL | |
| Differentiation/committement | YS | YS and FL | FL, bone marrow | |
| Differentiation potential | Erythroid | Erythroid | ||
| Myeloid | Myeloid | Myeloid | ||
| Lymphoid | ||||
| Macrophage lineage | Pu.1-dependent | Pu.1-dependent | Pu.1-dependent | |
| Runx1-independent | Runx1 -dependent | Runx1 -dependent | ||
| Myb-independent | Myb-independent | Myb-dependent | ||
| Notch1 independent | Notch1-dependent | |||
| Macrophage self-renewal | ? | yes | ||
| progenitor long-term self-renewal | no (transient) | no (transient) | yes | |
| TF expression | Pu.1 (spi1) | n/a | + | + |
| Myb | n/a | + | + | |
| Gata2 | n/a | + | + | |
| Gata3 | n/a | - | + | |
| Lmo2 | n/a | - | + | |
| TF requirement | Pu.1 (spi1) | yes | yes | no (**) |
| Myb | no | no (*) | yes | |
| Runx1 | no | yes | yes | |
| CBFb | no | yes | yes | |
| Scl/tal-1 | yes | yes | yes | |
| Notch1 | no | no | yes | |
red blood cell differentiation is compromised
macrophage differentiation is compromised
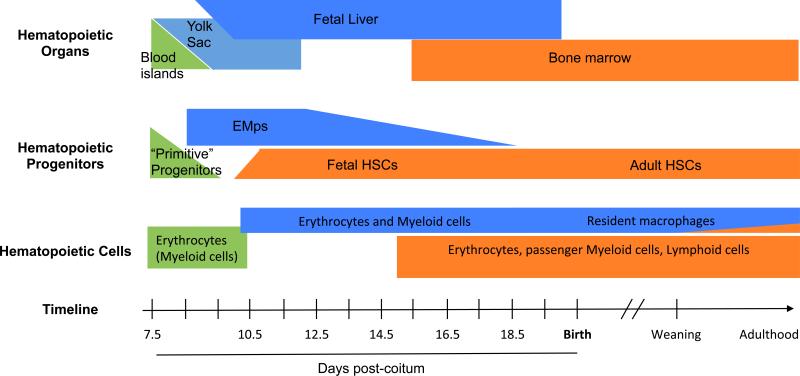
Figure 1.
Time scale of hematopoietic progenitors development in the mouse embryo.There are three successive but overlapping waves of hematopoietic progenitors during development, all of which have the potential to give rise to fetal macrophages. They can be distinguished by the hematopoietic niches exist where the progenitors expand and differentiate during development (upper panel), the yolk sac (primitive progenitors in green and EMP in blue), the fetal liver (EMP and HSC), and finally the bone marrow (HSC, orange). While primitive progenitors are only described in a small time window (middle panel), definitive progenitors (including EMPs and HSCs) co-exist during most fetal development in the fetal liver. Only HSC-derived hematopoiesis then shifts to the bone marrow niche. The three waves of progenitor can also be distinguished by their differentiation potential in vivo (lower panel). Primitive progenitors are restricted to the erythroid or the myeloid lineages, while EMP have both erythroid and myeloid potential. EMP-derived hematopoiesis gives rise to erythrocytes, macrophages, monocytes, granulocytes and mast cells and is sufficient to support survival of embryos lacking HSCs until birth. EMP-derived macrophages persist after birth in most tissues as resident macrophages, with the exception of the intestine lamina propria. HSCs are distinguished from other progenitors by their capacity to perform long-term repopulation of all leukocytes lineages when transplanted into a conventional irradiated recipient mouse.
Primitive hematopoiesis is a source of macrophages in embryos
Primitive hematopoiesis is the source of the first hematopoietic cells detected in the developing embryo. It is is characterized by the emergence of progenitors with limited potential for erythrocytes and macrophages and which can be found in the extra-embryonic yolk-sac blood islands in mice 36. These progenitors are thought to arise directly from the posterior plate mesoderm, and clonal analysis of the blood islands reveals that the endothelial cells lining the blood islands and the hematopoietic cells they contain do not share a common origin 38. This distinguishes them from the subsequent ‘definitive’ hematopoietic waves, which are thought to arise from a hemogenic endothelium. Accordingly, primitive progenitors and their daughter cells are not affected when transcription factors required for the endothelial-hematopoieitic transition, such as RUNX1, are absent (Table 1).
In zebrafish embryos, the ‘primitive’ wave can give rise to erythrocytes and macrophages, as well as neutrophils, in the absence of Runx1 39, 40, 41. In the mouse embryo, the in vivo characterization of the macrophage progeny of the primitive wave is still hampered by experimental constrains. However, (rare) progenitors with restricted macrophage potential in vitro can be found in the yolk sac at the neural plate stage between E7.5 and E8 36,34.
Erythro-Myeloid Progenitors constitute the first wave of definitive hematopoiesis and give rise to most resident macrophages
The first ‘definitive’ progenitors emerge in the yolk sac of mouse embryos at E8.25 34, 36. Termed erythro-pyeloid progenitors (EMP), these progenitors are phenotypically defined as Kit+, AA4.1+ (CD93), CD41+, VE-cadherin+ (VE-Cad), CD16/32+ (FCγII/III receptors) and CD45low 33, 35 (Table 1). Examination of their erythroid progeny led to their classification as ‘definitive’ progenitors 42. However, they can be distinguished from HSCs by the lack of lymphoid potential, both in vitro and in vivo, the lack of long-term repopulating potential 35 and the lack of cell-surface expression of Sca-1 35. The EMP-derived hematopoiesis is sufficient to support survival of embryos lacking HSCs until birth 43. EMP emerge from the yolk sac hemogenic endothelium in a Runx1-dependant endothelial to hematopoietic transition 44. Yolk sac-EMP emergence does not require blood flow, as evidenced by NCX1-deficient and VE-CAD-deficient embryos 35, 45, 46. They are generated from Tie2+ yolk sac ancestors from E7.5 to E10.5 18. EMP numbers in the yolk sac peak between E9.5 and E10.5 and they seed the fetal liver as soon as E9 47. Circulating EMP are still detected in peripheral blood at E14.5 35 and EMP-derived Kit+ cells are found in the fetal liver at 16.5 18. EMP expand in the fetal liver and differentiate into erythrocytes, megakaryocytes, macrophages, monocytes, granulocytes and mast cells 18. Yolk sac-EMP isolated from the E8.5 yolk sac and the E12.5 fetal liver have the same in vitro differentiation potential, but erythroid, monocyte and granulocyte and mast cell potential is only observed in vivo after seeding of the fetal liver 18. Thus, the fetal liver niche provides critical cues or an environment for EMP to reach their full potential. Yolk sac-EMP express Myb 48 but their commitment and differentiation into the myeloid fate is unaltered in Myb-deficient embryos, although their erythroid potential is blocked 12, 18, 49, 50 (Table 1). Therefore Myb is required for the commitment and differentiation of EMP into the erythroid fate 50, but is dispensable for myeloid differentiation, and possibly redundant, because EMP express Mybl2 (also known as b-Myb), a gene that can rescue hematopoiesis in Myb-deficient Drosophila 51.
HSCs, the second wave of definitive hematopoiesis, are developmentally distinct from EMPs
HSCs are distinguished from other progenitors by their capacity to perform long-term repopulation of all leukocytes lineages when transplanted into a conventional irradiated recipient mouse. HSC-derived hematopoiesis is described elsewhere 52, 53. HSC and EMP arise from distinct hemogenic endothelial cells 43. HSC emerge from the hemogenic endothelium of the aorto-gonado-mesonephros (AGM) region (starting at E10.5 in mice) 39, 54, 55 and of the umbilical and vitelline arteries 56 and migrate to the fetal liver, where they expand and differentiate from E12.5 till definitive hematopoiesis begins to shift to the BM. HSC colonize the embryonic bone marrow at E15 and active hematopoeisis starts there at E17 47, 57. Fetal and adult HSC require the transcription factor Myb for their self-renewal and maintenance, and loss of Myb expression leads to rapid HSC-derived hematopoiesis failure 12, 58, 59, 60. In addition, HSC also require NOTCH1 for their emergence, in contrast to EMP, as Notch1−/− embryos have normal numbers of hematopoietic progenitors in the yolk sac but very few in the body of the embryo 61. Several groups have attempted to pinpoint the specific site and time when HSC can be first detected within the embryo proper. Pioneering work by Dzierzak has demonstrated that the AGM region contained the progenitors endowed with LTR ability at E10.5 62. However, immature HSC have been detected at E9-9.5 in the para-aortic splanchnopleura (P-SP), a structure that later evolves into the AGM region. Such immature HSC migrate into the fetal liver at E10, mature into HSC and contribute to the adult HSC pool 47.
Genetic models distinguish the three progenitor waves
As described above, the emergence of different waves of hematopoietic precursors partially overlap in a short timeframe, which has made fate-mapping difficult. However these precursors can be distinguished genetically, and overall the layering of the hematopoietic system and in particular of macrophage development appears to stand on a robust genetic basis. Primitive hematopoiesis occurs in the absence of Runx1 and Myb 63, 64, while EMP are Runx1-dependent for their emergence 64 but Myb-independent for their myeloid differentiation 12, 18, 49 and Notch1-independent 61. In contrast, fetal and adult HSC require Runx1, Myb and Notch1 (Table 1) 39, 40, 58, 59, 61. Based on these data, several reports have investigated the lineage of origin of fetal (primitive) and adult macrophages and the mechanisms that may account for their persistence in adults.
Resident macrophages originate in majority from yolk sac EMP
The development of fetal F4/80bright macrophages is unaltered in Myb-deficient mice and adult F4/80bright macrophages are largely maintained in adult mice in the absence of Myb, and independently of bone marrow progenitors 12. In addition, adult tissue resident F4/80bright macrophages were traced back to Myb-independent Csf1r-expressing (Csf1r+) precursors present in the early mouse embryo at E8.5 using Cre-mediated hydroxy-tamoxifen (OH-TAM)-induced pulse labeling 65, 66 in Csf1r-MeriCremer mice 12 (see below).
These data suggested that F4/80bright macrophages do not originate from HSC, but from yolk sac progenitors such as either primitive progenitors or EMP 48. In accordance, brain microglia develop from yolk sac progenitors14, later functionally identified as EMP 48. Following pulse-labeling of Csf1r-expresing cells with OH-TAM at E8.5, the labeling efficiency of F4/80bright macrophages was 30% at day 10.5, and remained high (~20%) in microglia, but progressively decreased from E12.5 onwards in other organs to reach a plateau at birth, maintained even in one year-old animals (5% for the liver Kupffer cells, 1% for epidermal Langerhans cells (12,18, 67 and unpublished data from E. Gomez Perdiguero and F. Geissmann). This may be explained by the observation that pulse labeling with OH-TAM at E8.5 only labels the very first EMP to emerge in the yolk sac, while EMP continue to emerge in the yolk sac until at least E10.5 18, and therefore the labeling percentage of the macrophages in embryonic tissues may reflect the time at which their colonization by fetal macrophages is completed. However, it is also possible that this “dilution” reflects a contribution of another progenitor, such as fetal HSC or HSC-derived monocytes to several fetal macrophage populations 17, 67, 68, 69. A contribution of fetal monocytes is unlikely, as they also derive from EMP until E16.5 18, 35. However, a contribution of fetal HSC is still debated 17, 68, 69, 70.
The main argument against a significant contribution of fetal HSCs come from experiments using a Tie2-MeriCreMer fate mapping model, where the expression of Cre recombinase is inducible in Tie2-expressing cells 18, 71. Tie2 (also known as Tek) is expressed in the hemogenic endothelium, endothelial cells and hematopoietic progenitors in the yolk sac 72, aorta-gonado-mesonephros region, fetal liver HSC and adult HSC 52. Pulse labeling with tamoxifen at E7.5, E8.5, E9.5 and E10.5 resulted in labeling of adult HSC, lymphocytes, monocytes and neutrophils with the same efficiency, as expected. In contrast, fetal macrophages and adult resident F4/80bright macrophages in all organs were labeled with a decreasing efficiency (high at E7.5 to very low at E10.5) indicating that fetal and adult F4/80bright macrophages originate in majority from a progenitor that does not express Tie2 after E10.5, with a minor contribution of progenitors (such as HSC) expressing Tie2 after E10.5.
Using fate mapping models where the expression of Cre recombinase is either inducible or constitutive in Csf1r-expressing cells (Csf1r-MeriCremer and Csf1r-iCre mice), the macrophage progenitor itself was identified as a CD45loKit+AA4.1+ EMP that appears in the yolk sac from E8.5 18. These EMPs migrate in the fetal liver, where they expand and persist as Kit+ progenitors until at least E16.5 18. These EMPs give rise to erythrocytes, monocytes, granulocytes and mast cells until late fetal development 18,35 in accordance with the earlier observation that HSC-independent hematopoiesis is not only necessary, but also sufficient to support survival until the birth of embryos lacking HSCs 43. They also give rise to F4/80bright fetal macrophages in all embryonic tissues from E10.5 onwards 18. An EMP origin of the majority of resident macrophages was also favoured in an independent report 67.
Altogether these data support a major contribution from yolk-sac EMPs to fetal and adult resident macrophages (Fig.1 Fig.2). EMP-derived erythrocytes, monocytes, granulocytes and mast cells are ultimately replaced by HSC derived myeloid cells in the late embryo 18, while EMP-derived macrophages persist all through fetal and post-natal development as tissue-resident macrophages 12, 18 (Fig. 2). It was also speculated that a first wave of Myb-independent EMP give rise to microglia and a second wave of Myb-dependent EMPs give rise to other macrophages respectively 67. However, there is no available data to support the existence of two genetically distinct populations of EMP, and this hypothesis is not in accordance with previous reports that Myb is expressed in early yolk sac-EMP 36, 48 and with the persistence of fetal resident macrophages in all Myb-deficient embryonic tissues 12.
Figure 2.
Schematic of myeloid development and maintenance in the mouse embryo and adult (see text). Circular red arrows indicate self renewal potential. Primitive progenitors do not share a common origin with endothelial cells lining the blood islands in the yolk sac (YS) and they emerge the absence of Runx1 and Myb. Within definitive hematopoiesis, HSC and EMP arise from distinct hemogenic endothelial cells through a Runx1-dependent endothelial to hematopoetic transition. Both EMP and HSC express the transcription factor Myb, and while no fetal or adult HSC-derived hematopoiesis can occur in the absence of Myb, EMPs are Myb-independent for their myeloid differentiation. EMP-derived hematopoeisis gives rises to erythrocytes and short-lived myeloid cells (monocytes, granulocytes, mast cells) that are replaced by HSC-derived cells late during gestation. EMP-derived macrophages colonise all tissues during fetal development where they specialize to their tissue of residency after birth and can persist throughout adult life by local proliferation (red arrow). Depending on the age and environmental challenges, HSC-derived cells can contribute to adult tissue resident populations.
The contribution of “primitive” hematopoiesis to microglia
Primitive hematopoiesis could also contribute to the “primitive” and fetal macrophages. Early (E7.5) pulse-labelling of progenitors using several different reporters such as Runx1, Csf1r, Tie2 and Kit 12, 14, 18, 70, all result in a more efficient labelling of adult microglia in comparison with other adult tissue resident macrophages. As mentioned above, this may relate either to an earlier termination of brain colonization by macrophages in comparison to other organs, or the existence of several subsets of EMPs. However, it might also reflect the contribution of primitive hematopoiesis to microglia. It will be interesting to determine whether primitive progenitors are labelled in these models, and to investigate whether macrophages produced by primitive hematopoiesis can persist in adult mice. Myb-independency does not allow to genetically distinguish primitive from EMP-derived hematopoiesis and it is not yet known whether Runx1 is required for the differentiation of macrophages in mice. Altogether, the lack of genetic dissection and the low temporal resolution of classical fate mapping approaches in the mouse has not yet allowed independent tracking of the respective progeny of the two progenitor populations. A better understanding of the molecular control of primitive progenitors and EMP commitment and differentiation is required to develop better tools in order to characterize the possible contribution of “primitive” hematopoiesis to the development of fetal macrophages, and possibly adult macrophages in mice.
Contribution of HSCs to populations of resident macrophages in adult mice varies depending on organs and time
The analysis of HSC contribution to adult resident macrophages in the absence of bone marrow irradiation was performed by several groups using Flt3-Cre mice 12, 17, 18, 73 based on the observation that Flt3 (encoded by Flk2) is expressed by HSC-derived progenitors71, 74, but not by fetal macrophages 12. HSC contribution was also studied in several independent models of bone marrow transplantation without irradiation such as Mx-1--Cre Mybfl/fl and KitW/Wv Rag2−/− γc−/− 12, 18 and in parabiotic mice 15, 75. Overall, these analyses revealed that HSC contribution to resident macrophages was different between organs and frequently increased with age. HSC contribution to adult resident macrophages was either minor (<5%) in brain, liver, epidermis 12, 14, 48, small but increasing with age in lung, heart and spleen 18, 75, and sometimes predominant after weaning in the case of the gut lamina propria 28, 76 (Fig.1 and Fig. 2). It is not yet known whether the precursor(s) of the HSC-derived resident macrophages are monocytes or earlier myeloid progenitors, except in the case of the gut, where Ly6C+ monocytes are proposed to be the immediate precursor of gut lamina propria macrophages 28, 76.
Of note, a recent report investigated the origin of resident macrophages using a fate mapping model where the expression of the Cre recombinase was inducible in Kit-expressing cells (Kit-MeriCreMer mice) 70. Importantly, this study represents an independent confirmation that most adult resident macrophages originate from embryonic and fetal progenitors and persist in adults independently of adult HSCs, as Kit+ progenitors do not seem to contribute to resident tissue macrophages after E16.5 70. The authors also conclude that most resident macrophages, except microglia, are descendants of fetal HSCs and not of EMP, based on the observation that labeling of Kit+Sca1+ EMP at day E7.5 results in the labeling of microglia only, while labeling of Kit+ progenitors at E8.5 or E9.5 results in contrast in the labeling of all adult resident macrophages and HSC-derived cells. However, EMP do not express Sca1 (Table 1 and 35), while EMP and fetal HSC both express Kit and co-exist in the fetal liver until E16.5 18, 35. Thus, Kit expression and Kit-based fate mapping may not allow to discriminate between EMP and fetal HSC.
Recent work in chicken suggests that in contrast to mice, yolk sac-derived macrophages are not retained after hatching and, surprisingly, that tissue macrophage in adult birds originate from a macrophage-restricted, self-renewing progenitor cell in bone marrow that does not give rise to any other lineage 73. These findings, if confirmed, would indicate that myelopoiesis in birds is markedly different from its counterparts in mice, zebrafish and human, because a macrophage-restricted, self-renewing progenitor cell in the bone marrow or its equivalent was not identified in these species.
Maintenance of macrophages by local proliferation or recruitment
As indicated above, a proportion of alveolar macrophages, kidney macrophages and heart macrophages appear to be replaced during the normal ageing process 12, 18, 75. A partial replacement of resident macrophages is also observed following gamma irradiation, bone marrow transplantation or adoptive transfer experiments 3, 4, 77 and in macrophage depletion studies, such as intravenous injections of clodronate liposomes. Liver Kupffer cells are also reported to be replaced by bone marrow derived progenitors following, for example, the death of Kupffer cells in severe experimental Listeria infection 29. However, resident macrophages such as Langerhans cells, Kupffer cells, microglia, alveolar macrophages, peritoneal macrophages and splenic macrophages all have the potential to proliferate 15, 22, 23, 27, 78, 79, 80, 81, 82. In some cases, resident macrophages can immediately replenish themselves following a severe depletion 15, 23, 82. A growing body of evidence now clearly shows that fully differentiated resident macrophages can re-enter cell cycle and maintain their numbers by local proliferation 23, 24, 25, 26, 27. Macrophage proliferation increases dramatically during inflammatory conditions, such as skin inflammation, where up to 30% of Langerhans cells express the proliferation marker Ki67 22. During an acute inflammatory response, tissue-resident peritoneal macrophages that survive undergo a transient and intense proliferative burst in situ to repopulate the tissue 23. In response to helminthes infection, resident macrophages in the pleura and peritoneal cavities expand by local proliferation 81. In glucan-induced granuloma formation in monocytopenic mice, Kupffer cells proliferate actively and contribute to granuloma formation 78. After conditional ablation of microglia in adult mice, the microglial compartment is reconstituted within one week of depletion by CNS-resident cells, independent of bone-marrow-derived precursors 82. However, it is yet unclear whether the tissue-resident macrophage populations contain a local ‘progenitor compartment’, or whether all macrophages have the capacity to undergo mitosis. The mechanisms that may underlie this ability of resident macrophages to self-maintain and to proliferate are still unclear. An important role of the Maf family of transcription factors is likely, as combined deficiency for the transcription factors MafB and c-Maf enables the KLF4- and c-Myc–dependent proliferation of differentiated macrophages in culture in response to Csf1 83. In the case of epidermal Langerhans cell, proliferation in vivo was shown to be driven by an (unknown) keratinocyte-derived signal 22. In the case of nematode infection, macrophage proliferation was impaired in the absence of IL-4 81, 84. The cellular and molecular mechanisms that control macrophage maintenance and expansion in adult mice, and the consequences of ageing on these processes, are an area of active investigation, as their elucidation will shed light on the pathophysiology of inflammation.
Conclusions and perspectives on macrophage functions
A revised model for macrophage development and functions is therefore emerging, which distinguishes the resident macrophages that develop during embryogenesis from the passengers myeloid cells that originate and renew from bone marrow HSC. This distinction between passenger and resident macrophages is in line with earlier kinetic studies of the splenic-myeloid compartment in adult mice, which proposed a dual origin for myeloid cells, with half of myeloid cells renewing from blood and the other half being produced within the spleen 85. It also explains the observations that Langerhans cells do not exchange between parabiotic mice 13 and that fetal microglia persists in adult mice 14.
This distinction is relevant to disease pathogenesis, as it is quite clear that the functions of HSC-derived monocytes can be very different from those of tissue-resident macrophages within the same inflammatory environment. The two cell types have distinct expansion mechanisms and distinct functions in the brain during experimental auto-immune encephalitis (EAE) 86. Infiltrating monocytes are recruited via extravasation from blood vessels and produce inflammatory mediators important for disease progression, but do not persist after the resolution of inflammation, while, in contrast, activated resident microglia proliferate locally, persist and return to quiescence following remission 86. Following helminthe parasite infection, pleural resident macrophages adopt anti-inflammatory phenotypes, while passenger macrophages exhibit pro-inflammatory responses 81. However, in a model of chronic neurodegeneration, proliferating resident microglial cells contribute to neuronal damage during the development of the disease 87. Thus, it might be inaccurate to describe resident macrophages as always being ‘anti-inflammatory’ as opposed to passenger macrophages being ‘pro-inflammatory’. The respective roles of resident and passenger macrophages remain to be studied in detail, and most experimental models available, at present, do not easily elucidate the roles of resident and recruited macrophages in adult mouse tissues. Future work should help characterize the roles of (EMP-derived) resident macrophages, separate them from the function of HSC-derived passenger macrophages and characterize the underlying molecular mechanisms involved.
It is also important to recognize that the distinct subsets of resident macrophages associated with different tissues, for example microglia, Langerhans cells, Kupffer cells, peritoneal macrophages, red pulp macrophages or alveolar macrophages have different phenotypes, transcriptional profiles and chromatin landscape 88, 89, require specific growth factors and rely on distinct transcription factors for their differentiation or maintenance. For example, colony stimulating factor 1 (CSF1/M-CSF) is essential for Kupffer cells 90, TGFβ1 and IL-34 for microglia and Langerhans cells 88, 91, 92, 93, colony stimulating factor 2 (CSF2/GM-CSF) for the differentiation or maintenance of alveolar macrophages in mice and humans 94, 95, 96, 97, 98, 99, and retinoic acid and GATA6 for large peritoneal macrophages 88, 100, 101. SPIC is important for the development of red pulp macrophages 102, while LXR-deficient mice are defective in the generation of maginal zone and metallophilic macrophages 103.
Tissue-specific environment are important for the maintainance of macrophages identities 88, 89. Several studies also suggest that individual resident macrophage subsets follow a differentiation program during embryogenesis and early post-natal life that result in the establishment of stable phenotypic and functional subsets 22, 48. Future works investigating the the respective contribution and interplay of lineage-specific ‘hard-wired’ differentiation programs and of the local tissue micro-environment for the differentiation, maintenance and activation of tissue-specific resident macrophages will contribute to the understanding of their functions.
REFERENCES
- 1.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282(5736):324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 4.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nature immunology. 2007;8(6):578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. The Journal of experimental medicine. 2009;206(3):595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultze JL, Freeman T, Hume DA, Latz E. A transcriptional perspective on human macrophage biology. Seminars in immunology. 2015;27(1):44–50. doi: 10.1016/j.smim.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 13.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature immunology. 2002;3(12):1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. Journal of leukocyte biology. 1989;45(2):87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- 21.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain research Developmental brain research. 1999;117(2):145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 22.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. The Journal of experimental medicine. 2009;206(13):3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. European journal of immunology. 2011;41(8):2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 24.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 25.Ghigo C, Mondor I, Jorquera A, Nowak J, Wienert S, Zahner SP, et al. Multicolor fate mapping of Langerhans cell homeostasis. The Journal of experimental medicine. 2013;210(9):1657–1664. doi: 10.1084/jem.20130403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanitakis J, Morelon E, Petruzzo P, Badet L, Dubernard JM. Self-renewal capacity of human epidermal Langerhans cells: observations made on a composite tissue allograft. Experimental dermatology. 2011;20(2):145–146. doi: 10.1111/j.1600-0625.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 27.Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6(4):718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- 28.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature immunology. 2014;15(10):929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42(1):145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Naito M, Takahashi K, Nishikawa S. Development, differentiation, and maturation of macrophages in the fetal mouse liver. Journal of leukocyte biology. 1990;48(1):27–37. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Takahashi H, Naito M, Sato T, Kojima M. Ultrastructural and functional development of macrophages in the dermal tissue of rat fetuses. Cell and tissue research. 1983;232(3):539–552. doi: 10.1007/BF00216427. [DOI] [PubMed] [Google Scholar]
- 32.Deimann W, Fahimi HD. Peroxidase cytochemistry and ultrastructure of resident macrophages in fetal rat liver. A developmental study. Developmental biology. 1978;66(1):43–56. doi: 10.1016/0012-1606(78)90272-5. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102(1):134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106(9):3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 35.McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, et al. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell reports. 2015;11(12):1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 37.Palis J. Primitive and definitive erythropoiesis in mammals. Frontiers in physiology. 2014;53 doi: 10.3389/fphys.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Developmental cell. 2006;11(4):519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 40.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 41.Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111(1):132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 42.England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117(9):2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell stem cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111(7):3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rampon C, Huber P. Multilineage hematopoietic progenitor activity generated autonomously in the mouse yolk sac: analysis using angiogenesis-defective embryos. The International journal of developmental biology. 2003;47(4):273–280. [PubMed] [Google Scholar]
- 47.Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139(19):3521–3530. doi: 10.1242/dev.079210. [DOI] [PubMed] [Google Scholar]
- 48.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature neuroscience. 2013;16(3):273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 49.Sumner R, Crawford A, Mucenski M, Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19(30):3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 50.Bartunek P, Kralova J, Blendinger G, Dvorak M, Zenke M. GATA-1 and c-myb crosstalk during red blood cell differentiation through GATA-1 binding sites in the cmyb promoter. Oncogene. 2003;22(13):1927–1935. doi: 10.1038/sj.onc.1206281. [DOI] [PubMed] [Google Scholar]
- 51.Davidson CJ, Tirouvanziam R, Herzenberg LA, Lipsick JS. Functional evolution of the vertebrate Myb gene family: B-Myb, but neither A-Myb nor C-Myb, complements drosophila Myb in hemocytes. Genetics. 2005;169(1):215–229. doi: 10.1534/genetics.104.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annual review of immunology. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 53.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 56.Zovein AC, Turlo KA, Ponec RM, Lynch MR, Chen KC, Hofmann JJ, et al. Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood. 2010;116(18):3435–3444. doi: 10.1182/blood-2010-04-279497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4(1):97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- 58.Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 59.Mukouyama Y, Chiba N, Mucenski ML, Satake M, Miyajima A, Hara T, et al. Hematopoietic cells in cultures of the murine embryonic aorta-gonad-mesonephros region are induced by c-Myb. Current biology : CB. 1999;9(15):833–836. doi: 10.1016/s0960-9822(99)80368-6. [DOI] [PubMed] [Google Scholar]
- 60.Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(40):17304–17308. doi: 10.1073/pnas.1004640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18(5):699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 62.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 63.Tober J, McGrath KE, Palis J. Primitive erythropoiesis and megakaryopoiesis in the yolk sac are independent of c-myb. Blood. 2008;111(5):2636–2639. doi: 10.1182/blood-2007-11-124685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokomizo T, Ogawa M, Osato M, Kanno T, Yoshida H, Fujimoto T, et al. Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes to cells : devoted to molecular & cellular mechanisms. 2001;6(1):13–23. doi: 10.1046/j.1365-2443.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 65.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(15):6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythromyeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. The Journal of experimental medicine. 2012 doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of experimental medicine. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43(2):382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 71.Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 72.Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108(13):4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- 73.Garceau V, Balic A, Garcia-Morales C, Sauter KA, McGrew MJ, Smith J, et al. The development and maintenance of the mononuclear phagocyte system of the chick is controlled by signals from the macrophage colony-stimulating factor receptor. BMC biology. 2015;13:12. doi: 10.1186/s12915-015-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buza-Vidas N, Woll P, Hultquist A, Duarte S, Lutteropp M, Bouriez-Jones T, et al. FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood. 2011;118(6):1544–1548. doi: 10.1182/blood-2010-10-316232. [DOI] [PubMed] [Google Scholar]
- 75.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. The Journal of experimental medicine. 2014;211(11):2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 77.Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. Journal of neuropathology and experimental neurology. 1992;51(3):246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Yamada M, Naito M, Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. Journal of leukocyte biology. 1990;47(3):195–205. [PubMed] [Google Scholar]
- 79.Golde DW, Byers LA, Finley TN. Proliferative capacity of human alveolar macrophage. Nature. 1974;247(440):373–375. doi: 10.1038/247373a0. [DOI] [PubMed] [Google Scholar]
- 80.Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Laboratory investigation; a journal of technical methods and pathology. 1982;46(2):165–170. [PubMed] [Google Scholar]
- 81.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity. 2015;43(1):92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326(5954):867–871. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 84.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. The Journal of experimental medicine. 2013;210(11):2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. The Journal of experimental medicine. 1984;160(5):1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14(9):1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(6):2481–2493. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto T, Kaizu C, Kawasaki T, Hasegawa G, Umezu H, Ohashi R, et al. Macrophage colony-stimulating factor is indispensable for repopulation and differentiation of Kupffer cells but not for splenic red pulp macrophages in osteopetrotic (op/op) mice after macrophage depletion. Cell and tissue research. 2008;332(2):245–256. doi: 10.1007/s00441-008-0586-8. [DOI] [PubMed] [Google Scholar]
- 91.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nature immunology. 2012;13(8):753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. The Journal of experimental medicine. 1996;184(6):2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lieschke GJ, Stanley E, Grail D, Hodgson G, Sinickas V, Gall JA, et al. Mice lacking both macrophage- and granulocyte-macrophage colony-stimulating factor have macrophages and coexistent osteopetrosis and severe lung disease. Blood. 1994;84(1):27–35. [PubMed] [Google Scholar]
- 95.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15(4):557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 96.Happle C, Lachmann N, Skuljec J, Wetzke M, Ackermann M, Brennig S, et al. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Science translational medicine. 2014;6(250):250ra113. doi: 10.1126/scitranslmed.3009750. [DOI] [PubMed] [Google Scholar]
- 97.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(6):2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akagawa KS, Kamoshita K, Tokunaga T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J Immunol. 1988;141(10):3383–3390. [PubMed] [Google Scholar]
- 99.Chen BD, Mueller M, Chou TH. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J Immunol. 1988;141(1):139–144. [PubMed] [Google Scholar]
- 100.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344(6184):645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.N AG, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, et al. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nature immunology. 2013;14(8):831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]