Abstract
Hospitalization rates over time of childhood cancer survivors (CCS) provide insight into the burden of unfavorable health conditions on CCS and health care resources. The objective of our study was to examine trends in hospitalizations of CCS and risk factors in comparison with the general population. We performed a medical record linkage study of a cohort of 1564 ≥five-year CCS with national registers. We obtained a random sample of the general population matched on year of birth, gender and calendar year per CCS retrieved. We quantified and compared hospitalization rates of CCS and reference persons from 1995 until 2005, and we analyzed risk factors for hospitalization within the CCS cohort with multivariable Poisson models. We retrieved hospitalization information from 1382 CCS and 25583 reference persons. The overall relative hospitalization rate (RHR) was 2.2 (95%CI:1.9–2.5) for CCS compared to reference persons. CCS with central nervous system and solid tumors had highest RHRs. Hospitalization rates in CCS were increased compared to reference persons up to at least 30 years after primary diagnosis, with highest rates 5–10 and 20–30 years after primary cancer. RHRs were highest for hospitalizations due to neoplasms (10.7; 95%CI:7.1–16.3) and endocrine/nutritional/metabolic disorders (7.3; 95%CI:4.6–11.7). Female gender (P<0.001), radiotherapy to head and/or neck (P<0.001) or thorax and/or abdomen (P = 0.03) and surgery (P = 0.01) were associated with higher hospitalization rates in CCS. In conclusion, CCS have increased hospitalization rates compared to the general population, up to at least 30 years after primary cancer treatment. These findings imply a high and long-term burden of unfavorable health conditions after childhood cancer on survivors and health care resources.
Introduction
More than 75% of children with cancer become long-term survivors [1,2]. However, childhood cancer survivors (CCS) are at increased risk of unfavorable health conditions associated with their previous cancer treatment [3–8]. Among CCS aged 45 years, the cumulative prevalence estimates for serious/disabling or life-threatening chronic health conditions is 80% [5,7,9]. It is likely that late effects of cancer treatment in steadily growing numbers of CCS do not only burden the survivors themselves but also the health care system [10].
Hospitalization rates over time provide insight into the burden of unfavorable health conditions on individuals and on health care resources [11–15]. Thus far, previous studies focused on hospital admissions as a measure of burden of disease in CCS but these studies only determined first hospitalization or average hospitalization rates [16–27]. Although these studies provide important insight into the long-term morbidity of CCS, no study has analyzed hospitalization rates in CCS longitudinally by taking into account all hospitalizations within one individual. Such trends are likely to be a better measure for the total burden of unfavorable health conditions [15,28]. Insight into trends and risk factors for all hospitalizations can help to further focus and prioritize long-term follow-up care for CCS at risk of unfavorable health conditions requiring hospitalizations.
Our objective was to determine trends in hospitalization rates of CCS and associated risk factors in comparison with a random reference sample from the general population. To obtain these data, we performed medical record linkage between a study cohort of CCS and Dutch administrative registers.
Materials and Methods
Study population and linkage procedure
The Emma Children’s Hospital/Academic Medical Center (EKZ/AMC) childhood cancer survivor cohort is a single-center cohort study of CCS who survived at least five years since primary cancer diagnosis. Details of this study have been described previously [7,29]. Eligibility criteria were a primary childhood cancer diagnosis between 1966 and 1999 before age 18, survival at least five years since diagnosis and being alive at Jan 1st 1995 (N = 1564). From our database, we retrieved information on patient characteristics, cancer diagnosis, all cancer treatment before the date of five-year survival, recurrences and subsequent cancers. Written informed consent was obtained from all childhood cancer patients treated in the EKZ/AMC. The Institutional Review Board of the EKZ/AMC in Amsterdam reviewed and approved the data collection for our cohort register and the study was deemed as evaluation of patient care and was therefore exempt from the need for ethical approval. In addition, after medical record linkage of the cohort to national registers the data did not include directly identifiable variables anymore and therefore data were analyzed anonymously.
Because an individual identification number is lacking in the Hospital Discharge Register (Dutch acronym: LMR), we used a two-step record linkage approach to obtain hospitalization information from CCS. We described and validated the two-step record linkage of our cohort separately [30]. Using this approach we linked 1477 of 1564 eligible CCS to the Municipal Personal Records Database (Dutch acronym: GBA), and subsequently 1382 CCS to LMR.
We additionally obtained a random reference sample of the Dutch general population (20 persons at maximum from GBA with corresponding year of birth and gender per CCS retrieved in GBA), to whom we assigned the starting date of follow-up of the corresponding CCS, i.e. five years after the date of primary cancer diagnosis of the CCS. After record linkage to LMR, the study population consisted of 1382 CCS and of 26583 matched reference persons (both 94% of individuals retrieved from GBA) [30].
Outcome definition
We assessed the total number of hospitalizations and the total time at risk in CCS and reference persons from 1995 until 2005. We retrieved the primary diagnosis of the hospitalizations coded according to International Classification of Disease version 9 –clinical modification (ICD9-CM) [31]. The LMR contains electronic information on all hospitalizations (day case admissions and clinical hospitalizations) of almost every hospital in the Netherlands from 1995 onwards (coverage >99% until 2004 and 96.7% in 2005) [32]. Uncomplicated (day case) hospitalizations for delivery are not included in the register.
Accrual of time at risk for hospitalization began at the date of five-year survival (i.e. five years after the date of primary childhood cancer diagnosis) or January 1, 1995, whichever came later. Accrual of time at risk ended at January 1, 2006. We only counted time at risk when a person was a unique individual in GBA based on gender, date of birth and postal code[30]. Time during hospitalization was also excluded from the time at risk. When multiple unique periods were available, we summed up the time of the unique periods to define the total time at risk for hospitalization.
We hypothesized that CCS having a primary cancer recurrence or ongoing primary cancer therapy beyond the date of five-year survivorship would have increased exposure to cancer treatment and would simultaneously inflate the hospitalization rate. Because our interest was in hospitalizations beyond primary cancer survival, we censored these CCS and their corresponding reference persons at the date of five-year survival or at the incidence date of first primary cancer recurrence if this happened after the date of five-year survival. Full censoring applied to 90 CCS and partial censoring applied to 35 CCS.
Statistical analysis
We determined average hospitalization rates during the complete study period of CCS compared to their matched reference persons. We calculated relative hospitalization rates (RHR) and absolute excess risks (AER) of hospitalization per 1000 person-years at risk for the total group. We did the same for CCS categories as well as for specific ICD9-CM hospitalization diagnosis groups. In subsequent analyses, we allowed for changes in hospitalization rates over time in both groups, by including follow-up time since primary cancer diagnosis or attained age as covariate in the regression model. We modeled both covariates via natural cubic splines (using 7 knots) to allow for non-linear trends over time. Parameter estimates of the individual spline components are hard to interpret. Therefore, we used graphs to describe trends. We also estimated hospitalization trends over follow-up time for CCS categories. In all analyses we used a Poisson regression model in which we corrected for recurrent hospitalizations via generalized estimating equations (GEE). We assumed an exchangeable correlation structure.
Finally, we examined risk factors associated with hospitalization rates within the cohort of CCS. In addition to follow-up time since primary cancer diagnosis, we included gender, calendar year of primary cancer diagnosis, age at primary cancer diagnosis and cancer treatments given before the date of five-year survival (cancer surgery; anthracyclines; alkylating agents; other chemotherapy; radiotherapy to the head and/or neck; radiotherapy to the thorax and/or abdomen; radiotherapy to extremities).
We again used a Poisson model, again correcting for recurrent hospitalizations via GEE. We explored effect modification between all included variables and follow-up time, between non-treatment variables (i.e. gender, calendar year of primary cancer diagnosis and age at primary cancer diagnosis) and main cancer treatment modalities (i.e. surgery, chemotherapy, radiotherapy), and between the main cancer treatments themselves. We included them in the final model when statistically significant. We modeled the effects of follow-up time, calendar year of primary cancer diagnosis and age at primary cancer diagnosis via natural cubic splines (using 4 or 5 knots) to allow for a non-linear relationship with the hospitalization rate. Besides providing P-values for the overall effects of the variables, we also present results graphically.
We performed analyses using statistical software R version (2.15.0) using survreg for average RHR and over time, and ggplot2 for the graphs. P values <0.05 were considered statistically significant.
Results
After medical record linkage, included CCS and reference persons contributed a total of 10,622 and 194,094 years of time at risk respectively (Table 1 and S1 Fig). Median attained ages were 25.7 and 25.9 years. Median duration of follow-up time since (corresponding) date of primary cancer diagnosis was 18.6 years.
Table 1. Characteristics of CCS and reference persons contributing to unique follow-up time.
| CCS (n = 1382) | Reference persons (n = 26583) | |||
|---|---|---|---|---|
| n | % | N | % | |
| Gender | ||||
| Male | 738 | 53.4 | 14347 | 54.0 |
| Female | 644 | 46.6 | 12236 | 46.0 |
| Year of birth | ||||
| 1954–1969 | 205 | 14.8 | 4066 | 15.3 |
| 1970–1985 | 819 | 59.3 | 15462 | 58.2 |
| 1986–1999 | 358 | 25.9 | 7055 | 26.5 |
| Calendar year of primary cancer diagnosisa | ||||
| 1966–1974 | 117 | 8.5 | 2309 | 8.7 |
| 1975–1984 | 464 | 33.6 | 8932 | 33.6 |
| 1985–1994 | 529 | 38.3 | 10037 | 37.8 |
| 1995–1999 | 272 | 19.7 | 5305 | 20.0 |
| Age at primary cancer diagnosisa | ||||
| Median (range) | 6.1 | 0–17.8 | 6.0 | 0–18.4 |
| 0–4 yr | 607 | 43.9 | 11518 | 43.3 |
| 5–9 yr | 364 | 26.3 | 7197 | 27.1 |
| 10–14 yr | 318 | 23.0 | 6118 | 23.0 |
| 15–18 yr | 93 | 6.7 | 1750 | 6.6 |
| Primary cancer diagnosis | ||||
| Leukemia/lymphoma | 624 | 45.2 | ||
| CNS tumor | 98 | 7.1 | ||
| Sarcoma | 269 | 19.5 | ||
| Other solid tumors | 356 | 25.8 | ||
| Other and unspecified tumors | 35 | 2.5 | ||
| Recurrences of primary cancer | ||||
| None | 1161 | 84.0 | ||
| Any recurrence | 221 | 16.0 | ||
| Second tumors | ||||
| None | 1310 | 94.8 | ||
| Any second tumor | 74 | 5.4 | ||
| Cancer treatment groupsb,c | ||||
| No chemotherapy/radiotherapy (± surgery) | 112 | 8.1 | ||
| Chemotherapy (± surgery) | 726 | 52.5 | ||
| Radiotherapy (± surgery) | 83 | 6.0 | ||
| Chemotherapy and radiotherapy (± surgery) | 460 | 33.3 | ||
| Specific cancer treatments before five-year survivalc,d | ||||
| Anthracyclines | 586 | 42.4 | ||
| Alkylating agents | 700 | 50.7 | ||
| Other chemotherapy | 364 | 26.3 | ||
| Radiotherapy to head and/or neck region | 374 | 27.1 | ||
| Radiotherapy to thoracic and/or abdominal region | 302 | 21.9 | ||
| Radiotherapy to extremitiese | 92 | 6.7 | ||
| Vital status at the end of follow-up according to GBA | ||||
| Living | 1334 | 96.5 | 26491 | 99.7 |
| Deceased | 48 | 3.5 | 92 | 0.3 |
| Attained age at the end of follow-up | ||||
| Median | 25.3 | 25.3 | ||
| Range | 5.9–51.3 | 6.1–52.0 | ||
| Follow-up time since (correspondinga) date of primary cancer diagnosis | ||||
| Median | 18.6 | 18.6 | ||
| Range | 5.0–39.8 | 5.7–39.8 | ||
| Years at risk for hospitalization (1995–2005) | ||||
| Sum | 10,622 | 194,094 | ||
| Median | 8.8 | 8.1 | ||
| Range | 0.1–11.0 | 0.0–11.0 | ||
Abbreviations: CCS: childhood cancer survivors; n: number; GBA: Dutch acronym for Municipal Personal Records Database
a Corresponding date of primary cancer diagnosis of a CCS was assigned to matching reference persons in order to analyze data per survival year (starting at the 5th) and to adjust for calendar period and age.
b Cancer treatment groups were mutually exclusive, i.e. persons could contribute to one cell only. Treatment categories were irrespective of surgical treatment.
c We took all cancer treatment that was given before the date of five-year survival into account.
d Totals add up to more than 1382 because of overlapping categories
e Including 8 CCS with radiotherapy localization defined as “other”.
After applying the censoring for cancer treatment for primary cancer (recurrences) beyond five year survival, we identified 1736 hospitalizations in 1292 CCS, with an average rate of 172 hospitalizations per 1000 person-years. The hospitalization rate in matched reference persons was 79 per 1000 person-years. See S1 Methods for more details on the results of censoring.
Table 2 shows average RHR and AER of CCS compared to reference persons. The overall RHR and AER were 2.2 (95%CI:1.9–2.5) and 93.3 per 1000 person-years at risk, respectively. RHRs and AERs were increased in all CCS cancer diagnosis and treatment categories, with the highest RHRs for CCS originally diagnosed with primary central nervous system (CNS) tumors (RHR:3.4;95%CI:2.7–4.4) and other solid tumors RHR:2.6;95%CI:2.0–3.5). CCS not treated with chemotherapy or radiotherapy had a RHR of 2.5 (95%CI:1.5–4.1). Radiotherapy (with or without surgery, without chemotherapy) was associated with the highest RHR (3.4;95%CI:2.2–5.0) compared to reference persons.
Table 2. Average hospitalization rates, relative hospitalization rates and absolute excess rates in CCS and matched reference persons.
| CCSa | Matched reference personsb | ||||||
|---|---|---|---|---|---|---|---|
| Hospitalizations | Hospitalization rate per 1000 py at risk | Hospitalizations | Hospitalization rate per 1000 py at risk | RHR | 95%CI | AER per 1000 py at risk | |
| All individuals | 1736 | 172.4 | 13765 | 79.1 | 2.2 | 1.9–2.5 | 93.3 |
| Gender | |||||||
| Male | 693 | 129.5 | 4876 | 53.1 | 2.4 | 2.0–3.0 | 76.4 |
| Female | 1043 | 221.0 | 8889 | 108.2 | 2.0 | 1.7–2.4 | 112.8 |
| Primary cancer diagnosis | |||||||
| Leukemia/lymphoma | 530 | 120.0 | 5536 | 72.8 | 1.6 | 1.4–2.0 | 46.7 |
| CNS | 184 | 264.3 | 939 | 77.2 | 3.4 | 2.7–4.4 | 187.1 |
| Sarcomas | 369 | 185.9 | 3224 | 90.8 | 2.0 | 1.5–2.8 | 95.1 |
| Other solid tumors | 563 | 210.1 | 3631 | 79.3 | 2.6 | 2.0–3.5 | 130.8 |
| Other and unspecified cancers | 90 | 329.8 | 435 | 98.0 | 3.4 | 2.0–5.5 | 231.8 |
| Recurrences of primary cancer before five-year survival | |||||||
| None | 1436 | 159.8 | 11995 | 78.6 | 2.0 | 1.8–2.4 | 81.2 |
| Any | 300 | 277.2 | 1770 | 82.9 | 3.3 | 2.5–4.5 | 194.3 |
| Cancer treatmentc, d | |||||||
| No chemotherapy or radiotherapy | 180 | 219.5 | 1255 | 88.6 | 2.5 | 1.5–4.1 | 130.9 |
| Chemotherapy | 614 | 119.0 | 6020 | 67.8 | 1.8 | 1.4–2.2 | 51.2 |
| Radiotherapy | 240 | 344.6 | 1188 | 102.0 | 3.4 | 2.2–5.0 | 242.7 |
| Chemotherapy and radiotherapy | 701 | 206.9 | 5298 | 89.4 | 2.3 | 2.0–2.7 | 117.6 |
a Time at risk in CCS was censored at the date of five-year survival in case of ongoing primary cancer recurrence treatment or at the incidence date of first primary cancer recurrence after the date of five-year survival.
b Up to 20 reference persons were sampled per survivor and categorized into cancer diagnosis and treatment groups according to the corresponding CCS
c Cancer treatment groups were mutually exclusive, i.e. persons could contribute to one cell only. Treatment categories were irrespective of surgical treatment.
d We took all cancer treatment that was given before the date of five-year survival into account.
Abbreviations: CCS: childhood cancer survivors; py: person years; RHR: relative hospitalization rate; CI: confidence interval; AER: absolute excess rate.
Table 3 shows that CCS had significantly higher hospitalization rates in comparison to reference persons for 11 of 20 diagnosis groups, especially for neoplasms (RHR:10.7;95%CI:7.1–16.3,AER:24.2), endocrine/nutritional/metabolic disorders (RHR:7.3;95%CI:4.6–11.7,AER:6.3), diseases of the eye (RHR:4.4;95%CI:2.7–7.3,AER:2.9) and diseases of the circulatory system (RHR:3.5;95%CI:2.4–5.1,AER:4.3).
Table 3. Average hospitalization rates, relative hospitalization rates and absolute excess rates for ICD9-CM hospitalization diagnosis groups in CCS and reference persons.
| CCSa | Reference persons | ||||||
|---|---|---|---|---|---|---|---|
| Hospitalizations | Hospitalization rate per 1000 py at risk | Hospitalizations | Hospitalization rate per 1000 py at risk | RHR | 95%CI | AER per 1000 py at risk | |
| ICD group | |||||||
| Infectious and parasitic diseases | 14 | 1.4 | 101 | 0.6 | 2.4 | 1.1–5.1 | 0.8 |
| Neoplasms | 269 | 26.7 | 433 | 2.5 | 10.7 | 7.1–16.3 | 24.2 |
| Diseases of blood, blood forming organs and disorders involving immune mechanism | 17 | 1.7 | 135 | 0.8 | 2.2 | 0.7–6.5 | 0.9 |
| Endocrine, nutritional and metabolic diseases | 74 | 7.4 | 174 | 1.0 | 7.3 | 4.6–11.7 | 6.3 |
| Mental and behavioral disorders | 14 | 1.4 | 161 | 0.9 | 1.5 | 0.8–2.8 | 0.5 |
| Diseases of the nervous system | 39 | 3.9 | 428 | 2.5 | 1.6 | 0.7–3.3 | 1.4 |
| Diseases of the eye and adnexa | 38 | 3.8 | 149 | 0.9 | 4.4 | 2.7–7.3 | 2.9 |
| Diseases of the ear and mastoid process | 28 | 2.8 | 332 | 1.9 | 1.5 | 0.7–2.9 | 0.9 |
| Diseases of the circulatory system | 61 | 6.1 | 300 | 1.7 | 3.5 | 2.4–5.1 | 4.3 |
| Diseases of the respiratory system | 89 | 8.8 | 1055 | 6.1 | 1.5 | 0.9–2.3 | 2.8 |
| Diseases of the digestive system | 125 | 12.4 | 1112 | 6.4 | 1.9 | 1.3–3.0 | 6.0 |
| Diseases of the skin and subcutaneous tissue | 35 | 3.5 | 335 | 1.9 | 1.8 | 0.9–3.7 | 1.6 |
| Diseases of the musculoskeletal system and connective tissue | 95 | 9.4 | 1680 | 9.7 | 1.0 | 0.7–1.3 | -0.2 |
| Diseases of the genitourinary system | 143 | 14.2 | 911 | 5.2 | 2.7 | 1.7–4.2 | 9.0 |
| Pregnancy, childbirth and the puerperium | 188 | 18.7 | 3231 | 18.6 | 1.0 | 0.8–1.3 | 0.1 |
| Conditions originating in the perinatal period | <10b | - | <10b | - | - | - | - |
| Congenital malformations, deformations and chromosomal abnormalities | 34 | 3.4 | 222 | 1.3 | 2.6 | 1.7–4.2 | 2.1 |
| Symptoms, signs and abnormal clinical findings not elsewhere specified | 173 | 17.2 | 789 | 4.5 | 3.8 | 2.4–5.9 | 12.6 |
| Injury, poisoning and other consequences of external causes | 88 | 8.7 | 1046 | 6.0 | 1.5 | 1.1–1.9 | 2.7 |
| Factors influencing health status and contact with health services | 212 | 21.1 | 1167 | 6.7 | 3.1 | 2.5–3.9 | 14.3 |
Abbreviations: CCS: childhood cancer survivors; py: person years; RHR: relative hospitalization rate; CI: confidence interval; AER: absolute excess rate
a Time at risk in CCS was censored at the date of five-year survival in case of ongoing primary cancer recurrence treatment or at the incidence date of first primary cancer recurrence after the date of five-year survival
b Less than 10 units (not shown as per Statistics Netherlands patient confidentiality regulations).
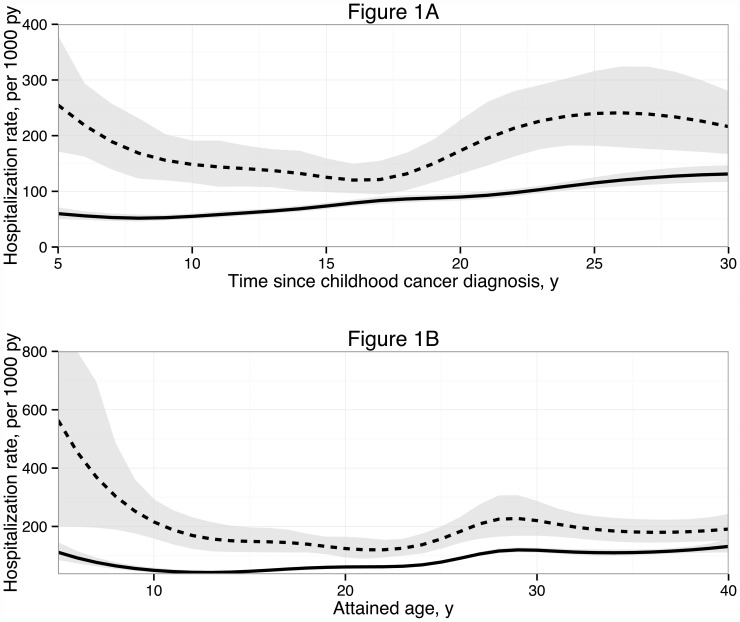
Fig 1 shows the hospitalization rates over follow-up time since (corresponding) date of primary cancer diagnosis (Fig 1a) and attained age (Fig 1b) for CCS and reference persons. Hospitalization rates in CCS were the highest between 5–10 and 20–30 years since primary cancer diagnosis, and before the attained age of 10 and between the attained ages of 25–40 years.
Fig 1. Hospitalization rate of CCS and reference persons over follow-up time since (corresponding) date of primary childhood cancer diagnosis (Fig 1A) and over attained age (Fig 1B).
Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis (Fig 1A) and over attained age (Fig 1B). Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. See S1 Table for further information on the differential follow-up time per calendar year of primary cancer diagnosis.
We found generally similar time trends in most other CCS categories, such as in males and females, in groups based on diagnosis, in groups based on recurrence status, and in treatment groups (S2A–S2M Fig).
We identified risk factors for hospitalization within CCS, based on our final multivariate model (Table 4). In this model we included the following significant effect modifiers: follow-up time for gender (P = 0.04), radiotherapy for gender (P = 0.02), follow-up time for surgery (P = 0.06) and calendar year of primary cancer for radiotherapy (P = 0.04). Overall, hospitalization rate was not constant over follow-up time since primary cancer diagnosis (P<0.001). Hospitalization rates were higher in females (P<0.001, non-monotone trend over follow-up time, see S2N Fig), after treatment with surgery (P = 0.01, non-monotone trend over follow-up time, see S2O Fig), after treatment with radiotherapy to thorax and/or abdomen (P = 0.04) and after treatment with radiotherapy to the head and/or neck (P<0.001). Calendar year of primary cancer diagnosis did not significantly influence hospitalization rate (P = 0.08).
Table 4. Multivariable model of risk factors for hospitalizations within CCSa.
| RHR (95% CI) | P-value | Figure of RHR | |
|---|---|---|---|
| Characteristic | |||
| Follow-up time since primary cancer diagnosis | - | <0.001 | |
| Gender (female versus male) | Increased risk, non-monotone trend over follow-up time | <0.001 | See S2N Fig |
| Surgery | Increased risk, non-monotone trend over follow-up time (non-significant) | 0.01 | See S2O Fig |
| Calendar year of primary cancer diagnosis | - | 0.08 | |
| Radiotherapy to thorax and/or abdomenb | 1.2 (0.9–1.6) | 0.04 | |
| Radiotherapy to head and/or neckb | 1.7 (1.2–2.4) | <0.001 | |
| Radiotherapy to extremitiesb,c | 1.2 (0.8–1.7) | 0.08 | |
| Age at primary cancer diagnosis | - | 0.17 | |
| Anthracyclines | 0.9 (0.7–1.3) | 0.72 | |
| Alkylating agents | 0.8 (0.6–1.1) | 0.12 | |
| Other chemotherapy | 0.9 (0.6–1.3) | 0.52 |
Abbreviations: CCS: childhood cancer survivors; RHR: relative hospitalization rate
a The final model includes the following effect modifiers: follow-up time for gender (P = 0.04), gender for radiotherapy (P = 0.02), follow-up time for surgery (P = 0.06), and calendar year of primary cancer diagnosis for radiotherapy (P = 0.04). P-values in the table correspond to test for overall effect of the respective variables.
b RHR of radiotherapy groups given for the reference calendar year of primary cancer diagnosis (1986) because calendar year is an effect modifier for radiotherapy in the model. No figure provided because the overall effect of calendar year of primary cancer diagnosis was not significant. For the specific RT groups, p-values are based on tests that include the interaction of radiotherapy (any location) with calendar year.
c Including 8 CCS with radiotherapy localization defined as “other”.
Discussion
We showed that CCS have increased hospitalization rates compared to the general population up to at least 30 years after primary cancer diagnosis. The average hospitalization rate in our CCS cohort was increased 2.2-fold compared to the general population. Especially survivors originally diagnosed with CNS and other solid tumors have an increased hospitalization rate compared to the general population. Within the CCS cohort, we identified that hospitalization rates over follow-up time were higher after treatment with surgery or radiotherapy and in females. These findings imply an increased and long-term burden of unfavorable health conditions after childhood cancer on survivors and health care resources. Since we only studied health conditions that lead to hospitalization, the true burden of unfavorable health conditions after childhood cancer is likely to be even higher.
It is concerning that, after an initial decline in hospitalization rate, we observed a late enhanced increase between the 20th and 30th follow-up year since primary cancer diagnosis compared to the general population. Although we are limited by a hospital register that does not contain electronic data before 1995, likely explanations for this trend could be the deterioration of existing health conditions in CCS as well as new, late onset health conditions.
We found increased hospitalization rates for several hospitalization diagnosis groups including neoplasms, endocrine disorders, circulatory diseases and diseases of the eye in CCS compared to the general population. These numbers imply that several well-known adverse events in CCS translate into an increased risk of hospitalization. The increased risk of hospitalization for neoplasms will be mainly due to secondary neoplasms since we applied censoring at late primary cancer recurrences and treatment. The increased risk of secondary neoplasms in CCS is well known and primarily related to radiotherapy [33,34]. The high risk of endocrine disorders and circulatory diseases in CCS has also been described and is mainly related to local radiotherapy as well as several chemotherapeutic agents [21–23,35–42]. The risk of diseases of the eye is known, but less often described in CCS. Likely explanations for this increased hospitalization rate are diseases such as cataract after radiotherapy and other problems after orbital tumors, retinoblastoma and glucocorticoids, in combination with a low background risk of (hospitalization for) eye disease in the general population [43,44]. Finally, the increased rates in the diagnosis groups “factors influencing health status and contact with health services” and “symptoms, signs and abnormal findings not elsewhere specified” could be explained by clinical signs and symptoms in CCS unusual for the age range and low-threshold clinical evaluations because of anxiety for cancer recurrence. Future studies should explore the underlying diagnoses of the hospitalization diagnosis groups.
The main treatment-related risk factors for hospitalization within CCS were surgery and radiotherapy, especially head and/or neck irradiation. These findings confirm previous study findings that irradiated survivors are the most vulnerable CCS risk group [45]. CCS treated with surgery only have generally been treated with extensive surgery. We confirmed previous findings within our cohort that this treatment modality is associated with an increased burden of health conditions [7]. In our analyses within the CCS cohort we found that female survivors are at increased risk of hospitalization compared to male survivors. Female gender has been previously linked to unfavorable health conditions in CCS. However, we found that males had a higher relative risk but a lower excess risk of hospitalization than females when comparing hospitalizations of CCS to the general population for both sexes [46]. Thus, the increased risk in hospitalization among females CCS in our analyses might be partly due to increased risks of hospitalization in females in the general population in this age range.
Other studies that used hospital discharge registers and determined hospitalization rates in CCS in general found increased risks of hospitalization [16,19,20]. Reported risks were an odds ratio (OR) for any hospitalization of 4.4 [16], a standardized hospitalization ratio (SHR) of any hospitalization of 2.8 [19] and a hospital admission rate ratio of 1.7 per year [20]. These studies were not able to determine hospitalization rates in CCS longitudinally by taking into account all hospitalizations within one individual and did not include multivariate detailed treatment-related risk factor analyses. Two other studies have used questionnaires to determine average hospitalization rates in CCS [17,18]. These studies compared hospitalizations rates to available reference rates obtained from existing surveys. Both studies also found an increased risk of hospitalization in CCS (OR 1.6 and relative rate 1.9 respectively). They found an increased risk of hospitalization for CNS tumors [18] female survivors and after radiotherapy [17].
The most important strength of our study is that we determined hospitalization rates over follow-up time by taking into account repeated hospitalizations within one individual. Using this approach, we showed that hospitalization rates differ over time and show a clinically relevant late enhanced increase between the 20th and 30th follow-up year since primary cancer diagnosis. We were also able to link hospitalizations to complete information on an individual’s previous cancer treatment. Our analysis accounted for recurrent hospitalizations, as well as for the confounding influences of age, gender, calendar period and late recurrences of primary cancer. Without applying the censoring at late primary cancer recurrences in our analyses, the average RHR would have increased substantially to 2.8, primarily due to hospitalizations in the first 5 to 10 years since cancer diagnosis (data not shown). Finally, hospitalizations in our study were prospectively registered in a national administrative register in the same way for CCS and reference persons. We therefore had an appropriate reference group, no risk of selection bias due to (non-)response and low risk of differential misclassification of the outcome.
A limitation of our study is that we could not directly link CCS to the hospital discharge register with one unique person identifier. However, by using unique time at risk based on linkage parameters, we analyzed hospitalization rates over follow-up time in a valid manner [30]. Another limitation is the differential follow-up for survivors treated in different time periods in our study. The data of CCS diagnosed in the 1990’s will have contributed mostly to the 5–15 years follow-up time, while the data of CCS diagnosed in the 1970’s contributed mostly to the ≥20 years follow-up time (S1 Table). However, when we added calendar year of primary cancer diagnosis to the model, this effect was not significant and the effect of follow-up time did not change much (S2P Fig). Because of the differential follow-up within our study design, we could not further explore whether the effect of follow-up time differed by calendar year of diagnosis. Nevertheless, we accounted for our longitudinal design with left-truncation by including the entry-time in our statistical analyses [47]. Finally, for this study we rely on correct hospitalization registration by Dutch hospitals, although this applies to both CCS and reference persons and has been found to be acceptable by others [48] and by ourselves [30].
Conclusions
We showed that CCS have increased hospitalization rates compared to the general population up to many years after reaching adulthood, especially survivors originally diagnosed with CNS and other solid tumors. CCS treated with surgery or radiotherapy are at highest risk for hospitalization. Further refinements in the trends of hospitalization over time and evaluation of disease specific hospitalization rates in relation to cancer treatments are needed. The high and long-term burden of unfavorable health conditions on CCS and on health care resources underscores the need for awareness and knowledge about these health conditions among survivors and health care professionals.
Supporting Information
1 We censored CCS (and corresponding reference persons) who developed a first primary cancer recurrence after the date of five-year survival (n = 86) and with ongoing cancer therapy for a primary cancer recurrence at the date of five-year survival (n = 39). Complete censoring applied to 90 CCS (51 of the 86 CCS who developed a primary cancer recurrence before 1995 plus the 39 CCS). Abbreviations: EKZ/AMC: Emma Children’s Hospital/Academic Medical Center; CCS: childhood cancer survivors; N: number; GBA: Municipal Personal Records Database (In Dutch: Gemeentelijke Basisadministratie).
(TIF)
(A) Hospitalization rate of male CCS and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (B) Hospitalization rate of female CCS and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (C) Hospitalization rate of CCS previously diagnosed with leukemia or lymphoma and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (D) Hospitalization rate of CCS previously diagnosed with a central nervous system tumor and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (E) Hospitalization rate of CCS previously diagnosed with a sarcoma and matched reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (F) Hospitalization rate of CCS previously diagnosed with other solid tumors and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (G) Hospitalization rate of CCS previously diagnosed with other and unspecified cancers and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (H) Hospitalization rate of CCS without a recurrence before five-year survival and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (I) Hospitalization rate of CCS with a recurrence before five-year survival and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (J) Hospitalization rate of CCS treated without chemotherapy and radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (K) Hospitalization rate of CCS treated with chemotherapy and without radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (L) Hospitalization rate of CCS treated with radiotherapy and without chemotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (M) Hospitalization rate of CCS treated with chemotherapy and radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (N) RHR of female CCS versus male CCS treated with (Yes) or without (No) radiotherapy over follow-up time. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. The final model includes the following effect modifiers: follow-up time for gender, radiotherapy for gender, follow-up time for surgery, and calendar year of primary cancer diagnosis for radiotherapy. Other variables included are radiotherapy to thorax and/or abdomen; radiotherapy to head and/or neck; radiotherapy to extremities (including 8 CCS with radiotherapy localization defined as “other”); anthracyclines; alkylating agents; other chemotherapy. Grey areas represent 95% confidence intervals. Abbreviations: RHR: relative hospitalization rate; CCS: childhood cancer survivors; py: person years; y: years. (O) RHR of CCS treated with versus without surgery over follow-up time. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. The final model includes the following effect modifiers: follow-up time for gender, radiotherapy for gender, follow-up time for surgery, and calendar year of primary cancer diagnosis for radiotherapy. Other variables included are radiotherapy to thorax and/or abdomen; radiotherapy to head and/or neck; radiotherapy to extremities (including 8 CCS with radiotherapy localization defined as “other”); anthracyclines; alkylating agents; other chemotherapy. Grey areas represent 95% confidence intervals. After 30 years of follow-up numbers were too small to give appropriate estimates. Abbreviations: RHR: relative hospitalization rate; CCS: childhood cancer survivors; py: person years; y: years. (P) Hospitalization rate of CCS and reference persons over follow-up time and adjusting for calendar year of primary cancer diagnosis. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis and adjusting for calendar year (the plot presents four calendar year periods: 1995, 1985, 1980 and 1975 from left to right). Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: py: person years; y: years.
(ZIP)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank all staff members involved in the Childhood Cancer Register of the Emma Children’s Hospital / Academic Medical Center and staff of Statistics Netherlands (CBS) involved in this project. We are indebted to the patients for giving their permission to participate in the study.
Data Availability
Third-party data are owned by Statistics Netherlands. Due to legal restrictions, interested researchers may obtain the same data the authors received if the conditions laid down in the Statistics Netherlands Act are fulfilled. This study can be replicated by a researcher who is employed by an authorised institution, follows the access procedures and covers the costs for the microdata services. The anonymized data used in the authors' analysis is available to a researcher who is employed by an authorised institution, follows the access procedures and covers the costs for the microdata services, as the authors did when obtaining the original dataset. For further information please visit: http://www.cbs.nl/en-GB/menu/informatie/beleid/zelf-onderzoeken/default.htm.
Funding Statement
This work was supported by Stichting Kinderen Kankervrij (KiKa), Project 84, https://www.kika.nl/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45: 992–1005. 10.1016/j.ejca.2008.11.042 [DOI] [PubMed] [Google Scholar]
- 2.Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, Lacour B, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364: 2097–105. [DOI] [PubMed] [Google Scholar]
- 3.Mulrooney DA, Neglia JP, Hudson MM. Caring for adult survivors of childhood cancer. Curr Treat Options Oncol. 2008;9: 51–66. 10.1007/s11864-008-0054-4 [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Hematol Oncol Clin North Am. 2010;24: 129–49. 10.1016/j.hoc.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355: 1572–82. [DOI] [PubMed] [Google Scholar]
- 6.Wallace WH, Green DM. Late effects of childhood cancer. London: Arnold; 2004. [Google Scholar]
- 7.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297: 2705–15. [DOI] [PubMed] [Google Scholar]
- 8.Survivors of childhood and adolescent cancer A multidisciplinary approach. 2nd ed Berlin: Springer; 2005. [Google Scholar]
- 9.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309: 2371–81. 10.1001/jama.2013.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry HL, Parkes SE, Powell JE, Mann JR. Caring for survivors of childhood cancers: the size of the problem. Eur J Cancer. 2006;42: 501–8. [DOI] [PubMed] [Google Scholar]
- 11.Kilian R, Matschinger H, Angermeyer MC. The impact of chronic illness on subjective quality of life: A comparison between general population and hospital inpatients with somatic and psychiatric diseases. Clin Psychol Psychother. 2001;8: 206–13. [Google Scholar]
- 12.Reynolds MR, Morais E, Zimetbaum P. Impact of hospitalization on health-related quality of life in atrial fibrillation patients in Canada and the United States: results from an observational registry. Am Heart J. 2010;160: 752–8. 10.1016/j.ahj.2010.06.034 [DOI] [PubMed] [Google Scholar]
- 13.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4: 361–71. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JW, Krauss NA. Spending and service use among people with the fifteen most costly medical conditions, 1997. Health Aff (Millwood). 2003;22: 129–38. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe C, Thompson SG, Cowie MR, Sharples LD. The use of hospital admission data as a measure of outcome in clinical studies of heart failure. Eur Heart J. 2003;24: 105–12. [DOI] [PubMed] [Google Scholar]
- 16.Bradley NM, Lorenzi MF, Abanto Z, Sheps S, Broemeling AM, Spinelli JJ, et al. Hospitalisations 1998–2000 in a British Columbia population-based cohort of young cancer survivors: report of the Childhood/Adolescent/Young Adult Cancer Survivors (CAYACS) Research Program. Eur J Cancer. 2010;46: 2441–8. 10.1016/j.ejca.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Kurt BA, Nolan VG, Ness KK, Neglia JP, Tersak JM, Hudson MM, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59: 126–32. 10.1002/pbc.24017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebholz CE, Reulen RC, Toogood AA, Frobisher C, Lancashire ER, Winter DL, et al. Health care use of long-term survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2011;29: 4181–8. 10.1200/JCO.2011.36.5619 [DOI] [PubMed] [Google Scholar]
- 19.Brewster DH, Clark D, Hopkins L, Bauer J, Wild SH, Edgar AB, et al. Subsequent hospitalisation experience of 5-year survivors of childhood, adolescent, and young adult cancer in Scotland: a population based, retrospective cohort study. Br J Cancer. 2013;110: 1342–50. 10.1038/bjc.2013.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff AC, Fluchel MN, Wright J, Ying J, Sweeney C, Bodson J, et al. Risk of hospitalization for survivors of childhood and adolescent cancer. Cancer Epidemiol Biomarkers Prev. 2014;23: 1280–9. 10.1158/1055-9965.EPI-13-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Fine Licht S, Winther JF, Gudmundsdottir T, Holmqvist AS, Bonnesen TG, Asdahl PH, et al. Hospital contacts for endocrine disorders in Adult Life after Childhood Cancer in Scandinavia (ALiCCS): a population-based cohort study. The Lancet. 2014;383:1981–9. [DOI] [PubMed] [Google Scholar]
- 22.Holmqvist AS, Olsen JH, Andersen KK, Licht Sde F, Hjorth L, Garwicz S, et al. Adult life after childhood cancer in Scandinavia: diabetes mellitus following treatment for cancer in childhood. Eur J Cancer. 2014;50: 1169–75. 10.1016/j.ejca.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Olsen M, Schmidt M, Lash TL, Sørensen K, Pedersen L, Sørensen HT. Cardiovascular disease risk in childhood cancer survivors. Am J Epidemiol. 2014;180: 120–3. 10.1093/aje/kwu144 [DOI] [PubMed] [Google Scholar]
- 24.Gunn ME, Lähdesmäki T, Malila N, Arola M, Matomäki J, Lähteenmäki PM. Late morbidity in long-term survivors of childhood brain tumors: a nationwide registry-based study in Finland. Neuro Oncol. 2015;17: 747–56. 10.1093/neuonc/nou321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzi MF, Xie L, Rogers PC, Pritchard S, Goddard K, McBride ML. Hospital-related morbidity among childhood cancer survivors in British Columbia, Canada: Report of the childhood, adolescent, young adult cancer survivors (CAYACS) program. Int J Cancer. 2011;128: 1624–31. 10.1002/ijc.25751 [DOI] [PubMed] [Google Scholar]
- 26.Lund LW, Winther JF, Dalton SO, Cederkvist L, Jeppesen P, Deltour I, et al. Hospital contact for mental disorders in survivors of childhood cancer and their siblings in Denmark: a population-based cohort study. The Lancet Oncology. 2013;14: 971–80. 10.1016/S1470-2045(13)70351-6 [DOI] [PubMed] [Google Scholar]
- 27.Ross L, Johansen C, Dalton SO, Mellemkjaer L, Thomassen LH, Mortensen PB, et al. Psychiatric Hospitalizations among Survivors of Cancer in Childhood or Adolescence. N Engl J Med. 2003;349: 650–7. [DOI] [PubMed] [Google Scholar]
- 28.Dijkgraaf MGW, Luijben AHP, Postma MJ, Borleffs JCC, Schrijvers AJP, Jager JC. Lifetime hospitalization profiles for symptomatic, HIV-infected persons. Health Policy. 1996;35: 13–32. [DOI] [PubMed] [Google Scholar]
- 29.Sieswerda E, Mulder RL, van Dijk IW, van Dalen EC, Knijnenburg SL, van der Pal HJ, et al. The EKZ/AMC childhood cancer survivor cohort: methodology, clinical characteristics, and data availability. J Cancer Surviv. 2013;7: 439–54. 10.1007/s11764-013-0283-9 [DOI] [PubMed] [Google Scholar]
- 30.Sieswerda E, Font-Gonzalez A, Dijkgraaf MGW, Geskus RB, Heinen RC, van der Pal HJ, et al. Studying Hospitalizations and Mortality in the Netherlands: Feasible and Valid Using Two-Step Medical Record Linkage with Nationwide Registers. PLoS One. 2015;10: e0132444 10.1371/journal.pone.0132444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stichting Medische Registratie. Classification of Diseases 1980, based on the International Classification of Disease 9th revision, Clinical Modification (ICD9-CM) [article in Dutch]. 1980.
- 32.de Bruin A, Kardaun JWPF, Gast A, de Bruin E, van Sijl M, Verweij G. Record linkage of hospital discharge register with population register: experiences at Statistics Netherlands. Statistical Journal of the United Nations Economic Commission for Europe. 2004;21(1): 23–32. [Google Scholar]
- 33.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27: 2356–62. 10.1200/JCO.2008.21.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305: 2311–9. 10.1001/jama.2011.747 [DOI] [PubMed] [Google Scholar]
- 35.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97: 663–73. [DOI] [PubMed] [Google Scholar]
- 36.Gurney JG, Ness KK, Sibley SD, O'Leary M, Dengel DR, Lee JM, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107: 1303–12. [DOI] [PubMed] [Google Scholar]
- 37.Mulder RL, Kremer LC, van Santen HM, Ket JL, van Trotsenburg AS, Koning CC, et al. Prevalence and risk factors of radiation-induced growth hormone deficiency in childhood cancer survivors: a systematic review. Cancer Treat Rev. 2009;35: 616–32. 10.1016/j.ctrv.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 38.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339: b4606 10.1136/bmj.b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poutanen T, Tikanoja T, Riikonen P, Silvast A, Perkkio M. Long-term prospective follow-up study of cardiac function after cardiotoxic therapy for malignancy in children. J Clin Oncol. 2003;21: 2349–56. [DOI] [PubMed] [Google Scholar]
- 40.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42: 3191–8. [DOI] [PubMed] [Google Scholar]
- 41.van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170: 1247–55. 10.1001/archinternmed.2010.233 [DOI] [PubMed] [Google Scholar]
- 42.Gudmundsdottir T, Winther JF, de Fine Licht S, Bonnesen TG, Asdahl PH, Tryggvadottir L, et al. Cardiovascular disease in adult life after childhood cancer in scandinavia (ALiCCS): A population-based cohort study of 32,308 one-year survivors. Int J Cancer. 2015;137: 1176–1186. 10.1002/ijc.29468 [DOI] [PubMed] [Google Scholar]
- 43.Whelan KF, Stratton K, Kawashima T, Waterbor JW, Castleberry RP, Stovall M, et al. Ocular late effects in childhood and adolescent cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2010;54: 103–9. 10.1002/pbc.22277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punyko JA, Mertens AC, Gurney JG, Yasui Y, Donaldson SS, Rodeberg DA, et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2005;44: 643–53. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res. 2010;174: 840–50. 10.1667/RR1903.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25: 4477–89. [DOI] [PubMed] [Google Scholar]
- 47.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165: 444–52. [DOI] [PubMed] [Google Scholar]
- 48.Paas GR, Veenhuizen KC. Research on the validity of the LMR. Utrecht: Prismant; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1 We censored CCS (and corresponding reference persons) who developed a first primary cancer recurrence after the date of five-year survival (n = 86) and with ongoing cancer therapy for a primary cancer recurrence at the date of five-year survival (n = 39). Complete censoring applied to 90 CCS (51 of the 86 CCS who developed a primary cancer recurrence before 1995 plus the 39 CCS). Abbreviations: EKZ/AMC: Emma Children’s Hospital/Academic Medical Center; CCS: childhood cancer survivors; N: number; GBA: Municipal Personal Records Database (In Dutch: Gemeentelijke Basisadministratie).
(TIF)
(A) Hospitalization rate of male CCS and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (B) Hospitalization rate of female CCS and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (C) Hospitalization rate of CCS previously diagnosed with leukemia or lymphoma and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (D) Hospitalization rate of CCS previously diagnosed with a central nervous system tumor and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (E) Hospitalization rate of CCS previously diagnosed with a sarcoma and matched reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (F) Hospitalization rate of CCS previously diagnosed with other solid tumors and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (G) Hospitalization rate of CCS previously diagnosed with other and unspecified cancers and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (H) Hospitalization rate of CCS without a recurrence before five-year survival and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (I) Hospitalization rate of CCS with a recurrence before five-year survival and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (J) Hospitalization rate of CCS treated without chemotherapy and radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (K) Hospitalization rate of CCS treated with chemotherapy and without radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (L) Hospitalization rate of CCS treated with radiotherapy and without chemotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (M) Hospitalization rate of CCS treated with chemotherapy and radiotherapy (with or without surgery) and reference persons over follow-up time. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis. Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: CCS: childhood cancer survivors; py: person years; y: years. Please note that numbers of hospitalizations differed between categories and that we adjusted the y-axes accordingly in the figures. (N) RHR of female CCS versus male CCS treated with (Yes) or without (No) radiotherapy over follow-up time. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. The final model includes the following effect modifiers: follow-up time for gender, radiotherapy for gender, follow-up time for surgery, and calendar year of primary cancer diagnosis for radiotherapy. Other variables included are radiotherapy to thorax and/or abdomen; radiotherapy to head and/or neck; radiotherapy to extremities (including 8 CCS with radiotherapy localization defined as “other”); anthracyclines; alkylating agents; other chemotherapy. Grey areas represent 95% confidence intervals. Abbreviations: RHR: relative hospitalization rate; CCS: childhood cancer survivors; py: person years; y: years. (O) RHR of CCS treated with versus without surgery over follow-up time. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. The final model includes the following effect modifiers: follow-up time for gender, radiotherapy for gender, follow-up time for surgery, and calendar year of primary cancer diagnosis for radiotherapy. Other variables included are radiotherapy to thorax and/or abdomen; radiotherapy to head and/or neck; radiotherapy to extremities (including 8 CCS with radiotherapy localization defined as “other”); anthracyclines; alkylating agents; other chemotherapy. Grey areas represent 95% confidence intervals. After 30 years of follow-up numbers were too small to give appropriate estimates. Abbreviations: RHR: relative hospitalization rate; CCS: childhood cancer survivors; py: person years; y: years. (P) Hospitalization rate of CCS and reference persons over follow-up time and adjusting for calendar year of primary cancer diagnosis. Hospitalization rates per 1000 person years of CCS (dotted line) and reference persons (continuous line) over follow-up time since (corresponding) date of primary childhood cancer diagnosis and adjusting for calendar year (the plot presents four calendar year periods: 1995, 1985, 1980 and 1975 from left to right). Grey areas represent 95% confidence intervals. Estimates were made with a Poisson regression model corrected for recurrent hospitalizations. Abbreviations: py: person years; y: years.
(ZIP)
(DOCX)
(DOCX)
Data Availability Statement
Third-party data are owned by Statistics Netherlands. Due to legal restrictions, interested researchers may obtain the same data the authors received if the conditions laid down in the Statistics Netherlands Act are fulfilled. This study can be replicated by a researcher who is employed by an authorised institution, follows the access procedures and covers the costs for the microdata services. The anonymized data used in the authors' analysis is available to a researcher who is employed by an authorised institution, follows the access procedures and covers the costs for the microdata services, as the authors did when obtaining the original dataset. For further information please visit: http://www.cbs.nl/en-GB/menu/informatie/beleid/zelf-onderzoeken/default.htm.