Abstract
The goal of early autism screening is earlier treatment. We pilot-tested a 12-week, low-intensity treatment with seven symptomatic infants ages 7–15 months. Parents mastered the intervention and maintained skills after treatment ended. Four comparison groups were matched from a study of infant siblings. The treated group of infants was significantly more symptomatic than most of the comparison groups at 9 months of age but was significantly less symptomatic than the two most affected groups between 18 and 36 months. At 36 months, the treated group had much lower rates of both ASD and DQs under 70 than a similarly symptomatic group who did not enroll in the treatment study. It appears feasible to identify and enroll symptomatic infants in parent-implemented intervention before 12 months, and the pilot study outcomes are promising, but testing the treatment’s efficacy awaits a randomized trial.
Keywords: ASD, Infants, Early intervention, Parents, Early Start Denver Model
Introduction
One of the most exciting areas of current autism science involves the search for infant behavioral markers of incipient autism. A number of prospective studies of infant siblings of children with autism spectrum disorder (ASD) have been carried out to help identify behavioral markers that are sensitive and specific to ASD in infancy. Some differences associated with risk status have been identified in infants as young as 5–6 months by examining group differences between infants with a sibling with autism and those with typically developing siblings (Ference and Curtin 2013; Lloyd-Fox et al. 2013). However, these studies have not yet demonstrated that such symptoms are associated with the development of ASD. Other studies have followed high-risk and low-risk groups from infancy to diagnosis at age 3 and then examined the longitudinal trajectories to find earliest evidence of differences associated with diagnosis. Using this design, several groups have demonstrated that the development of infants later diagnosed with autism begins to diverge from a typical trajectory between 6 and 12 months of age (Landa et al. 2012; Ozonoff et al. 2010), with no group differences evident, as a group, at 6 months, but differences already marked and statistically significant by 12 months. Differences in rate of development have been documented across multiple domains, including motor, social, communication, and cognitive. In the approximately 25 %of infants with older siblings with ASD who do not develop ASD themselves, but display other atypicalities in development (Messinger et al. 2013), the inflection point at which their development begins to diverge from typical infants is similar, during the 6–12 month period (Ozonoff et al. 2014). Infant sibling studies have also identified behavioral markers associated with later ASD diagnosis as early as 10–12 months of age (Zwaigenbaum et al. 2005; Ozonoff et al. 2008; Landa et al. 2012; Sacrey et al. 2013; Wan et al. 2013). Collectively, these studies suggest that it will be especially fruitful to identify predictive markers in the 6–9 month period, before the marked developmental delays and autism behavior patterns already detectable at 12 months take hold. While many infants who will later develop autism do not show symptoms in the 6–9 month period (Zwaigenbaum et al. 2005), case studies have shown that a significant subgroup does (Bryson et al. 2007). The symptoms detectable in the 6–12 month period involve six specific risk indices: (1) unusual visual examination and fixations; (2) unusual repetitive patterns of object exploration; (3) lack of intentional communicative acts; (4) lack of age-appropriate phonemic development; (5) lack of coordinated gaze, affect, and voice in reciprocal social-communicative interactions; and (6) decreased eye contact, social interest, and engagement. For this subgroup of early onset children, Bryson et al. (2007) report that the course of onset appears more rapid, and the degree of delay and atypicality more severe, than those infants whose onset occurs later. Thus, infants with symptoms before 12 months may be a particularly high-risk group.
The primary purpose of early detection of ASD is to prevent or mitigate the full onset of autism and its associated severe disabilities through early referral to effective treatment. Early detection science requires that early treatment science develop in parallel so that tested treatments are ready for identified infants. Well-structured, long-term early intervention is currently the most effective intervention for decreasing the level of disability associated with ASD (Lovaas 1987; McEachin et al. 1993; Dawson et al. 2010; Rogers and Vismara 2014). This evidence, however, involves children who are mostly 2 years and older. For younger infants, there are only two pilot intervention studies in the literature. The first is a case series focused on increasing parental responsivity in a sample of parent-infant sibling dyads for infants selected by sibling status rather than by symptoms (Green et al. 2013). The second (Steiner et al. 2013) reports a single subject design using Pivotal Response Training for three infants under the age of 1 year, resulting in an increase in specific social-communication behaviors. We currently lack methodologically rigorous, efficacious intervention studies for ASD-symptomatic infants.
In contrast, such high quality studies have been carried out with infants with other kinds of developmental delays, and these studies report several practices that appear to improve outcomes and can provide a starting point for designing effective interventions for infants with autism symptoms. Wallace and Rogers (2010) identified five central ingredients in efficacious interventions for infants. One practice involves parent coaching, including parent use of the interventions daily at home and therapist modeling of the intervention to the parent. Sanz and Menendez (1996) and Sanz-Aparicio and Balaña (2003) experimentally demonstrated the superiority of such methods over the use of written materials with parents of infants with Down Syndrome. There is robust evidence that parents can effectively deliver interventions for children with autism and effect desired child changes (Koegel et al. 1978; Harris et al. 1981; Short 1984; Laski et al. 1988; Koegel et al. 1996; Schreibman and Koegel 1997; Charlop-Christy and Carpenter 2000; Diggle et al. 2002).
A second practice identified by Wallace and Rogers (2010) involves the frequency and length of the intervention. The majority of the effective studies involved weekly sessions in the clinic or at home across the entire 6–11 month age range. In contrast, many ineffective interventions in the literature were short-term, consisting of few or widely-spaced contacts.
Third, most of the effective interventions involved individualized activities designed to meet the developmental needs of each child. Parental use of specific developmental activities was a major component of a number of efficacious infant interventions (Sanz and Menendez 1996; Sanz-Aparicio and Balaña 2002, 2003; Sloper et al. 1986, with Down Syndrome; Ross 1984, with very premature infants). Many were based upon a manualized curriculum that allowed for individualization of the parent activities and adjustments based on child progress (Resnick et al. 1988; Sanz-Aparicio and Balaña 2002).
A fourth practice involved beginning the interventions as early as possible. Outcomes from these early delivered interventions were strong and long-lasting (Brooks-Gunn et al. 1992). Sanz-Aparicio and Balaña (2002) experimentally demonstrated the benefit of earlier intervention for infants with Down syndrome involving greater gains in motor, verbal, social adaptation, and social relationships.
Fifth, several studies demonstrated the positive effects of increasing parental sensitivity and responsivity to infant cues (Barrera et al. 1990; Seifer et al. 1991). Such parenting practices also have positive impact on the development of typical infants and toddlers. The impacts are particularly seen in child language and social development (Tomasello 1992; Tamis-LeMonda and Bornstein 1994; Chapman 2000; Pan et al. 2005; Simpson et al. 2007). These five intervention practices, and the efficacious practices of the Early Start Denver Model (ESDM; Dawson et al. 2010; Rogers et al. 2012a, b) provided the basis for designing an intervention approach for infants who were at high risk for ASD.
Methods
The study’s overall goals were to develop, pilot test, and examine the feasibility of a manualized, parent-delivered intervention for infants age 6–15 months of age who were highly symptomatic for ASD, many of whom were also at familial risk for autism. The intervention aimed to reduce or alter six target symptoms and developmental patterns of early ASD. Feasibility of identifying such infants and enrolling them in treatment was an important second question.
Hypotheses
Infants with high numbers of autism symptoms and developmental delays under 15 months of age can be identified and enrolled in a treatment program.
Parents will learn and deliver the intervention at high levels of fidelity during the treatment phase and maintain it after treatment ends.
Parents will report high levels of satisfaction with the study intervention and positive working alliances with their therapist.
The group of infants who receive the study intervention will demonstrate fewer symptoms of ASD at 24 and 36 months compared to two matched comparison groups of infants: (1) a group of infants with similar behavioral profiles at 9 months who later developed ASD, and (2) a group of infants who met all treatment study eligibility criteria and were referred to the study intervention but declined to enroll.
The group of infants who receive the study intervention will demonstrate faster developmental progress and less developmental delays, reflected in higher developmental quotients, at 24 and 36 months compared to both of the above described comparison groups.
Participants
Recruitment
The infant start treatment group (IS) consisted of seven infants who were either (1) identified through their participation in a prospective study of younger siblings of children with ASD (n = 4; Infant Sibling Project) or (2) were referred from the community by parents or other clinicians due to early symptoms (n = 3). At the beginning of treatment, the infants ranged from 6–15 months of age.
Eligibility Criteria
There were seven inclusion criteria for enrollment: (1) Scores on the Autism Observation Scale for Infants (AOSI) of 7 or higher at initial assessment and at re-assessment 2 weeks later; (2) Presence of two or more target symptoms defined by ratings of 2 or higher on related AOSI items at initial assessment and at re-assessment in the clinic 2 weeks later; (3) Scores on the Infant-Toddler Checklist in the risk range (ITC; Wetherby and Prizant 2002); (4) Concerns based on expert clinical judgment involving direct, independent observations of Dr. Rogers and Dr. Ozonoff; (5) English as one language spoken in the home; (6) Hearing and vision screen within the normal range; (7) Residence within 1 h of the MIND Institute; and (8) Infant age of 15 months or younger at time of identification.
Exclusion criteria involved the presence of a genetic disorder related to ASD, like Fragile × Syndrome, significant abnormalities in the pre-, peri- and postnatal period, significant chronic illness, gestational age younger than 36 weeks, vision and hearing impairments, and severe motor impairments. See Table 1 for a description of the gender, ethnicity, age, referral source, and family socioeconomic status for each of the IS infants.
Table 1.
Descriptions of the ethnicity and household income of the five groups
| Infant start N =7 N (%) |
Declined referral N =4 N (%) |
Autism outcome N =7 N (%) |
Low risk N =7 N (%) |
High risk N =7 N (%) |
|
|---|---|---|---|---|---|
| Gender | |||||
| Male | 5 (71.4) | 2 (50) | 4 (57.1) | 5 (71.4) | 5 (71.4) |
| Female | 2 (28.6) | 2 (50) | 3 (42.9) | 2 (28.6) | 2 (28.6) |
| Ethnicity | |||||
| Hispanic | 2 (28.6) | 0 (0) | 1 (14.3) | 1 (14.3) | 3 (42.9) |
| Non-Hispanic | 6 (71.4) | 4 (100) | 6 (85.7) | 6 (85.7) | 4 (57.1) |
| Household income | |||||
| Under $25k | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 1 (14.3) |
| $25k–$49k | 1 (14.3) | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) |
| $50k–$74k | 1 (14.3) | 2 (50) | 1 (14.3) | 2 (28.6) | 1 (14.3) |
| $75k–$99k | 4 (57.1) | 0 (0) | 0 (0) | 2 (28.6) | 1 (14.3) |
| $100k–$124k | 1 (14.3) | 0 (0) | 0 (0) | 2 (28.6) | 3 (42.9) |
| $125k and above | 0 (0) | 0 (0) | 2 (28.6) | 1 (14.3) | 1 (14.3) |
Comparison groups for the treatment group were constructed by sampling from the entire Infant Sibling project cohort (n = 126). We constructed three different comparison groups that were matched to the treatment group: (1) high-risk (HR) children who were younger siblings of a child diagnosed with ASD but who did not themselves develop ASD; (2) low-risk (LR) children who were younger siblings of a child with no developmental disorders; and (3) autism outcome (AO) children who were younger siblings diagnosed with ASD by their 36-month visit. Each child in these three comparison groups was directly matched to one of the seven treatment group infants based on AOSI total score, Mullen Scales of Early Learning (MSEL; Mullen 1995) early learning composite at 9 months, and gender. The procedure involved algorithms that repeatedly selected the top five matches for each individual treatment group infant from the total group of infants for each comparison group. This function was run 100 times, with every potential match receiving a score after every iteration. The seven participants with the highest total score in each comparison group were selected as the matched cases.
A fourth comparison group was also constructed. The declined referral (DR) group consisted of four children who were identified as potentially eligible for the infant start treatment due to elevated AOSI scores and clinician concerns, but whose family chose not to enroll in the study. See Table 2 for a description of the five groups.
Table 2.
Means and standard deviations of child outcome measures
| Variable | Age | IS Mean (SD) |
DR Mean (SD) |
AO Mean (SD) |
HR Mean (SD) |
LR Mean (SD) |
|---|---|---|---|---|---|---|
| AOSI markers | 9 | 7.71 (3.73) | 5.75 (2.87) | 4.14 (1.46) | 4.71 (1.89) | 5.00 (1.92) |
| 12 | 6.00 (2.94) | 6.75 (2.87) | 4.71 (3.09) | 4.86 (2.48) | 3.00 (2.10) | |
| 15 | 6.43 (2.94) | 4.25 (3.30) | 6.57 (3.55) | 4.00 (0.89) | 2.86 (1.46) | |
| AOSI total | 9 | 12.57 (6.83) | 7.75 (4.35) | 5.71 (1.70) | 6.14 (2.73) | 7.43 (3.10) |
| 12 | 10.86 (6.64) | 11.00 (6.33) | 7.86 (5.58) | 6.71 (3.40) | 4.00 (2.37) | |
| 15 | 11.00 (6.06) | 7.75 (6.60) | 10.86 (6.59) | 5.00 (1.10) | 3.57 (2.15) | |
| ADOS severity | 18 | 4.43 (2.44) | 6.00 (2.71) | 5.42 (2.92) | 1.00 (0.00) | 1.00 (0.00) |
| 24 | 3.34 (3.41) | 6.25 (3.86) | 6.71 (0.95) | 1.50 (1.23) | 1.00 (0.00) | |
| 36 | 3.34 (2.30) | 5.25 (3.40) | 7.71 (1.60) | 1.71 (0.76) | 1.43 (0.54) | |
| VRDQ | 9 | 102.14 (25.60) | 97.63 (19.81) | 115.17 (18.03) | 110.97 (20.79) | 94.53 (9.26) |
| 12 | 106.87 (22.03) | 93.98 (26.59) | 103.40 (9.52) | 110.67 (10.96) | 111.37 (15.31) | |
| 15 | 95.29 (9.60) | 91.47 (9.57) | 93.27 (8.52) | 97.34 (10.73) | 103.00 (8.11) | |
| 18 | 95.31 (14.05) | 77.19 (23.69) | 84.20 (8.22) | 93.84 (11.16) | 97.83 (16.19) | |
| 24 | 96.07 (16.44) | 78.65 (9.28) | 78.38 (10.78) | 96.93 (13.17) | 112.45 (21.31) | |
| 36 | 102.06 (29.44) | 60.39 (21.76) | 78.76 (27.17) | 115.22 (17.19) | 126.56 (21.32) | |
| LDQ | 9 | 58.97 (5.62) | 79.53 (17.38) | 68.73 (10.13) | 80.45 (11.64) | 80.50 (13.78) |
| 12 | 64.42 (15.13) | 67.59 (26.03) | 68.01 (19.62) | 88.60 (18.96) | 92.38 (16.00) | |
| 15 | 59.00 (10.85) | 67.59 (20.82) | 66.43 (13.17) | 89.78 (16.48) | 93.19 (6.83) | |
| 18 | 74.02 (29.86) | 55.0 (17.35) | 65.14 (16.30) | 90.22 (20.53) | 89.02 (39.39) | |
| 24 | 92.42 (29.46) | 45.62 (20.25) | 59.00 (12.54) | 95.45 (22.67) | 103.93 (10.20) | |
| 36 | 90.75 (26.89) | 57.41 (19.77) | 71.41 (19.63) | 95.06 (4.96) | 107.61 (9.46) | |
| Total intervention hours | 1,048.93 (1,100.26) | 1,383.50 (1,473.34) | 901.00 (580.01) | 16.63 (40.72) | 0 (0.00) |
IS infant start group, DR declined referral group, AO autism outcome group, HR high risk group, LR low risk group, VRDQ visual reception developmental quotient; LDQ language developmental quotient
Enrollment Procedure
All IS-referred infants were first screened via telephone interview with a parent to determine inclusion criteria. The Infant Toddler Checklist (ITC; Wetherby and Prizant 2002) was conducted to verify the presence of autism symptoms. For infants who received scores in the ITC defined risk range (ITC social composite score 12th percentile), an assessment visit was scheduled, and the ITC was re-administered.
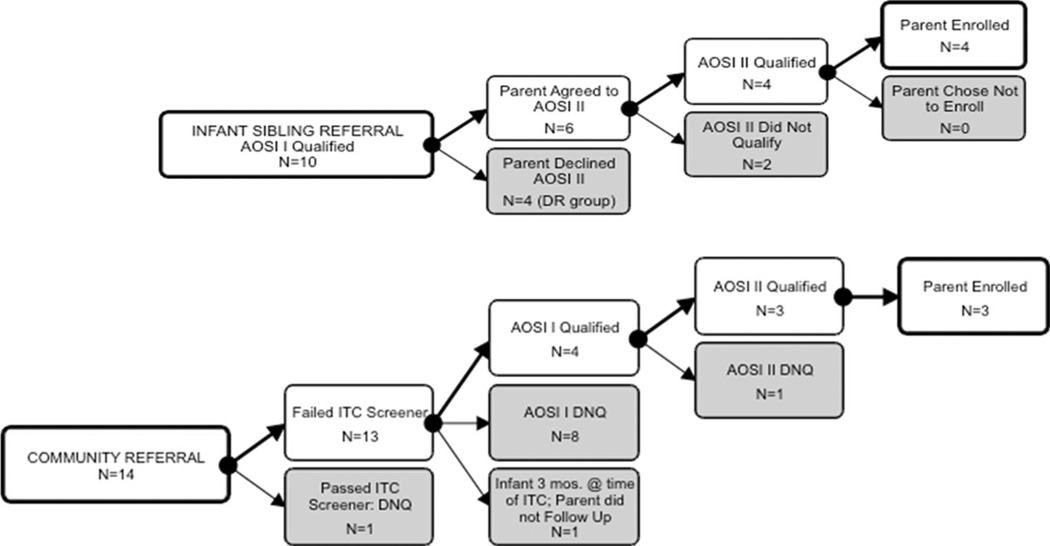
As can be seen in Fig. 1, ten children were recruited from the Infant Sibling Study; six consented to further screening, of whom four met qualifications and were enrolled. Four families did not respond to the referral and did not enroll their children (these are the children who became the DR group). Fourteen children were referred by families in the community. Three of these referrals met enrollment criteria and participated.
Fig. 1.
Flow chart of subject identification, screening, and enrollment
Measures
Parent Measures
Infant Start Parent Fidelity Measure (Rogers et al. 2012c)
This measure was a therapist rating of parent use of treatment techniques collected during each treatment and follow-up session. Parents were rated across a 3-point Likert scale on the targeted skills described in Table 3. Scores across 19 individual items were averaged to create a total score.
Table 3.
The treatment goal and approach for each of six target symptoms
| Symptom | Theme | Goal | Procedure |
|---|---|---|---|
| (1) Visual fixations on objects | Joining into toy play |
Facilitate: attention shifting from object to parent; parallel play; and sharing of emotion regarding the object |
Follow infant interest to an object and develop a turn-taking social game (trading turns with the object or using double objects) |
| (2) Abnormal repetitive behaviors | Encouraging flexible and varied actions and play |
Increase number and maturity of schemas child uses |
(for repetitive object behaviors); Follow infant interest while developing age- appropriate sensory motor schemas for object play (for repetitive body movements) Shape motor movements into communicative gestures using graduated, or least to most, prompting hierarchy |
| (3) Lack of intentional communicative acts and (4) lack of coordination of gaze, affect, and voice in reciprocal, turn-taking interactions |
Increasing engagement and interaction |
Elicit communicative gestures, vocalizations, and integrated communicative behaviors for varied pragmatic intents |
Offer and follow the child into preferred activities and dyadic and triadic joint activities; then increase and shape these three behaviors via prompting, shaping, fading, and differential reinforcement |
| (5) Lack of age-appropriate phonemic development |
Developing the foundations of speech |
Increase frequency of child vocalizations and shape specific consonant and vowel |
Use imitation and other interaction strategies and differential reinforcement, shaping, and prompting |
| (6) Decreasing gaze, social interest and engagement |
Maximizing social attention |
Maximize gaze and increase infant pleasure and engagement in social interaction |
Position self and child for maximal face- to face orientation and provide object and social games that follow infant preferences, delivered to maximize infant attention and pause for infant turns |
Parent Satisfaction Rating (Charlop-Christy and Carpenter 2000)
This is a measure of social validity, or acceptability, of the experimental treatment, to parents. Parents of children were asked to fill out this questionnaire at the end of the 12-week intervention program during the final intervention session to rate the ease of implementation in the home and their opinions concerning treatment utility.
Working Alliance Scale for Interventions with Children (Davis et al. 2006)
This measure was created as an adaptation of an existing working alliance scale. This psychometrically strong measure was administered at the end of the 12-week program to describe the response of the families to the experimental intervention, and thus constitutes another measure of social validity.
Infant Enrollment Measures
Infant Toddler Checklist (ITC; Wetherby and Prizant 2002)
The ITC is a parent questionnaire developed to determine risk for communication disorders which also has an algorithm validated to identify possible ASD. Screening cutoffs and standard scores are available at monthly intervals from 6 to 24 months based on a normative sample of over 2,188 children.
Autism Observation Scale for Infants (AOSI; Bryson et al. 2008)
The AOSI is an assessment of autism symptoms in infants. It was administered at two time points, spaced 2 weeks apart, as part of the inclusion criteria for the study. The measure was also given at 6, 9, 12, and 15 months of age. Two variables were used from this measure: the total score (number of symptoms and severity of each) and the number of markers (number of symptoms shown regardless of severity).
Infant Treatment Curriculum Measures
The Carolina Curriculum for Infants and Toddlers with Special Needs, 2nd Edition (Johnson-Martin et al. 1991)
This tool provides curriculum items that assess all aspects of early development arranged hierarchically across the 0–36 month period. It was administered to children at the start of their intervention. It was used to construct individualized treatment objectives. This curriculum has strong psychometric data, including data on reliability, validity, and program efficacy.
ESDM Curriculum Checklist (Rogers and Dawson 2010)
This tool provides a very detailed list of items for ASD-specific social and preverbal communication development arranged hierarchically across the 8–48 month period. It was administered to children at the start of intervention and was used to construct individualized treatment objectives.
Infant Outcome Measures
Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000)
This is a structured 40-min observational assessment that provides a number of opportunities for interaction (e.g., play, turn-taking games, looking at books, etc.) and measures social and communicative behaviors used in the diagnosis of autism. Each item is scored from 0 (typical for age or not autistic in quality) to 3 (unquestionably abnormal and autistic in quality). The ADOS was administered at the 18, 24 and 36-month visits. To account for the use of modules 1 and 2, severity scores were calculated according to Gotham et al. (2009).
Mullen Scales of Early Learning (MSEL; Mullen 1995)
This is a standardized, normed developmental assessment. It was administered at 6, 9, 12, 15, 18, 24, and 36 months. Two developmental quotient scores were generated: visual reception developmental quotient (VRDQ), constructed by dividing developmental age by chronological age, and language developmental quotient (LDQ), constructed by averaging the two language subscale developmental age scores together and dividing by chronological age.
Total Intervention Hours (CPEA Network, Unpublished)
Parents reported enrollment in treatment programs for developmental delays or concerns from ages 9 to 36 months. Types of treatment included: applied behavior analysis, other in-home programs, speech therapy, occupational therapy, and physical therapy. Participation in generalized socialization classes (e.g., Gymboree®) and typical preschool were excluded. The start and end dates of each type of intervention were recorded as well as average hours received per week. Weeks in treatment were calculated and then multiplied by average hours a week for an estimate of total hours enrolled in treatment.
Clinical Best Estimate (CBE) Outcome classification
At the end of the 36-month visit, examiners classified each child into one of two CBE categories, ASD or no ASD. Children classified with ASD met DSM-IV-TR criteria for Autistic Disorder or Pervasive Developmental Disorder-Not Otherwise Specified (PDDNOS) and had an ADOS score over the ASD cutoff (APA 2000). All other participants were classified with Typical Development.
Infant Start Therapist Fidelity Measure (Rogers et al. 2012c)
This measure was a rating of therapist use of specified techniques and behaviors to be used in the work with parents. It was rated following each treatment and follow-up session using a 3-point Likert scale with 16 items. Therapists’ self-rated scores were averaged to create an overall score for each session; the mean for 42 sessions with complete data = 2.76, SD .20. Other trained therapists rated 27 sessions either during observation or via video review; the mean was 2.73, SD .21, showing excellent agreement between self-rating and ratings by others overall.
Procedures
Overview of Family Procedures
Treatment began immediately after enrollment and continued for 12 clinic treatment sessions scheduled 1 week apart, followed by an assessment. A 6-week maintenance period then followed involving 1-h clinic visits with the therapist at post-treatment weeks 2, 4, and 6. Maintenance sessions included: discussion of child and parent progress; discussion of challenges and problems; and observation of play interactions. Children that were reported by their parents to show delayed or poor progress on any of the six targeted topics were seen for additional 1-h bimonthly booster sessions until improvements in related learning objectives occurred for two consecutive sessions. Three families attended these booster sessions after the completion of the maintenance phase, with the number of sessions ranging from 0 to 5, depending on the needs and wishes of the families. Finally, children received follow-up assessments at 15, 18, 24, and 36 months of age. None of the enrolled families discontinued participation in the study. At any assessment in which the child demonstrated clinical problems on standardized measures, families were provided with intervention referrals for public intervention services. Three families sought autism specific intervention services at some point during their enrollment, and two additional families sought speech therapy.
Treatment Procedures
The treatment consisted of 12 consecutive weekly 1-h clinic sessions. Sessions were conducted by the first, second, and third authors of this paper who developed the parent curriculum (Rogers et al. 2012a) from ESDM techniques (Rogers and Dawson 2010), adapted the coaching methods (Hanft et al. 2003), and developed the parent and therapist fidelity of implementation measures. All were highly experienced, credentialed professionals with many years of experience working with families and young children with ASD.
The sessions were organized as follows. Session 1 was devoted to developing 5–6 measurable child learning objectives from curriculum tools for parents to practice with their child throughout the intervention phase, based on parental goals and the target autism symptoms. Across sessions 2–12, parents were sequentially coached on parenting techniques to address developmental needs related to one of the six target symptoms, with one area focused on for two consecutive weeks. These were taught in random order to the families. The six symptoms and related topics and techniques are outlined in Table 3.
In addition to the six target symptom interventions, therapists also provided parents with specific interventions for other delays, which were individualized for each child to address weaknesses identified during the curriculum assessment, embedded into everyday routines.
Sessions included six sequenced 5–10 min activities: (1) A greeting and parent progress sharing; (2) Warm-up period of parent–child play, after which both parent and therapist reflected on the activity related to intervention goals and elicited child behaviors. If necessary, additional coaching and practice occurred at this point to strengthen parent’s practice of this particular topic; (3) Therapist introduced a new topic through verbal description and written materials from the manual, with discussion fitting the new topic into parent’s goals; (4) Parent practiced new technique while the therapist provided coaching, followed by reflection; (5) Parent practiced and was coached on the topic skill across one or two other play and caregiving activities (e.g., books, feeding, dressing or changing, toy play, and social play) until the parent demonstrated the technique at a fidelity of implementation rate of 80 % or higher; (6) Session concluded with discussion and visualization of generalization of a new skill in various activities and settings at home and in the community, and time for discussion of any other topics the parent introduced during the session. The parent left with self-instructional manual materials on the target technique to review. Each session was videotaped for clinical supervision and fidelity coding of parent and therapist.
Fidelity of Treatment Implementation by Parent and by Therapist
Fidelity of treatment implementation measures were developed and used to assess ongoing parent and therapist fidelity. The Infant Start 19-item parent fidelity tool (Rogers et al. 2012c) uses a Likert-based, 3-point rating system (1 = seldom present, 2 = sometimes, 3 = consistently present) associated with the six target symptoms and related parenting interventions (described above) that were the topics of the weekly treatment sessions. Parent fidelity was coded by therapists during the first “warm-up” parent–child play activity of each session, before any coaching or teaching had been carried out. Therapists coded this after establishing initial inter-rater reliability of 80 % or better of total item scores. Mean score was the variable used for weekly analysis and could range from 1 to 3, with a higher score reflecting interactions closer to the intervention strategies taught to parents.
Therapist fidelity of implementation measures similarly consisted of a 3-point (1 = not present; 2 = sometimes present; 3 = clearly present) 17-item Likert-based rating system. Items included: presence of five phases of the session (initiation, observation, action, reflection, evaluation); the six coaching characteristics (collaborative, reflective, nonjudgmental, conversational and reciprocal, performance-based and contextually-linked); and five general parent learning goals. Fidelity was self-assessed by the therapists immediately after the sessions.
Results
Analytic Approach
We first present our study enrollment data. We then present the parent fidelity data from the Infant Start treatment as a single-case design, followed by descriptions of therapist fidelity of implementation and measures of parent satisfaction. Then we present the group analyses starting from 9 months of age, the first point at which all seven infants in the IS group had assessment data, up to 36 months of age.
In terms of the outcome measures, for the analysis of autism symptom severity, we ran group comparisons separately at pre- and post-treatment because we used two different autism symptom measures due to age limitations for each measure. At 9 months of age, groups were compared on AOSI scores with a univariate analysis of variance. At 18, 24, and 36 months, groups were compared within a linear mixed effects model (LME). In the LME model of ADOS severity scores, age (18, 24, and 36 months) and group were included as fixed effects as well as a group by age interaction. Total intervention hours were included in the model as a covariate. Developmental scores were calculated from the MSEL across all time points, so variables from those measures were analyzed with a linear mixed effects model (LME) approach with maximum likelihood estimation. In the LME model, age (9–36 months) and group were included in the model as fixed effects as well as a group by age interaction. Total intervention hours were added to the model as a covariate. Significant effects were followed up with post hoc comparisons with a Bonferroni adjustment for multiple comparisons. For all significant simple comparisons, Cohen’s d calculated from estimated marginal means is also reported.
Parent Fidelity
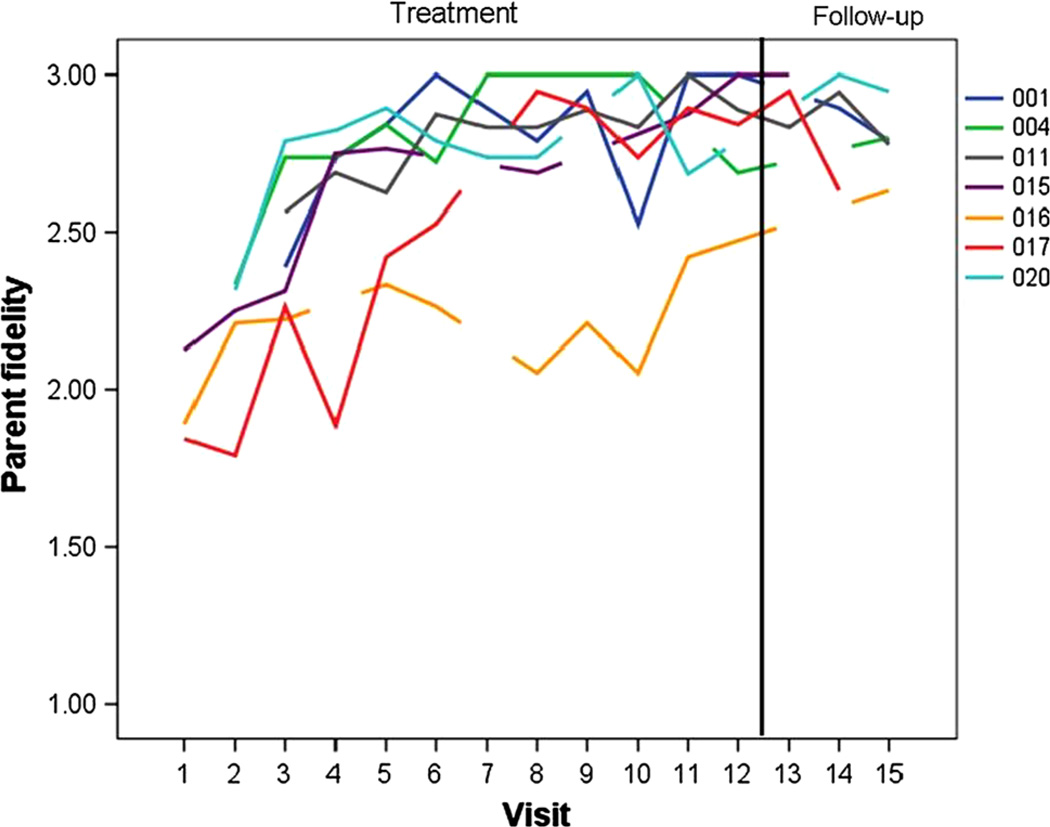
Fidelity scores for each parent are presented in Fig. 2. All parents demonstrated improvement across the 12 weeks of treatment and maintained skills across three follow-up visits. Potential range of scores is 1–3. A paired sample t test of average scores across the first three treatment sessions compared to average scores across the three post-treatment follow-up visits revealed a significant increase of more than 1.5 SD in scores (t(6) = 6.13, p = .001; start of treatment, M = 2.33, SD = .24; follow-up, M = 2.84, SD = .12).
Fig. 2.
Individual parent fidelity- of- treatment implementation scores from baseline through maintenance
Therapist Fidelity
A total of 69 treatment sessions (73 %) were rated for therapist fidelity. Therapist fidelity average score was a mean of 2.74 (SD = .21) on a Likert-based rating system, with scores ranging from 1 to 3 on 16 items.
Parent Satisfaction Rating
Six of the seven parents in the IS group completed the Parent Satisfaction Rating Scale at the exit of intervention. Scores on the individual items were all within the neutral to positive range (3–5). The overall mean of satisfaction across items was 4.25 (SD = .50).
Working Alliance Scale
Six of the seven parents in the IS group completed the Working Alliance Scale at the exit of intervention. All parents rated items at the highest end of the scale (range of individual item scores 6–7). The group average total score was 6.94 (SD = .11).
Autism Symptoms
At 9 months, there was a significant effect of group (F(4, 27) = 3.10, p = .03) in the model for AOSI total scores. The IS group had significantly more symptoms than all other comparison groups except the DR group (AO: d = 1.81, p = .004; HR: d = 1.70, p = .007; LR: d = 1.36, p = .03). There was a trend towards a significant effect of group on number of AOSI markers (F(4, 27) = 2.19, p = .10). No other comparisons between groups reached significance at 9 months.
In the model of ADOS scores from 18 to 36 months, there was a significant main effect of group (F(4, 55.80) = 13.19, p < .001). Main effects of age, treatment hours and the interaction between age and group did not reach significance. The IS group had significantly lower ADOS severity scores than the AO (p < .01) group and a trend towards lower scores than the DR (d = −1.98, p = .06) group. The scores of the IS group were significantly higher than the HR (d = 1.79, p < .05) group and a trend towards higher scores than the LR (d = 1.77, p = .05) group. Children in the IS group had fewer autism symptoms than children with a diagnosis or those who declined the referral to treatment, but were still exhibiting more symptoms than children with typical development in either risk group.
Developmental Scores
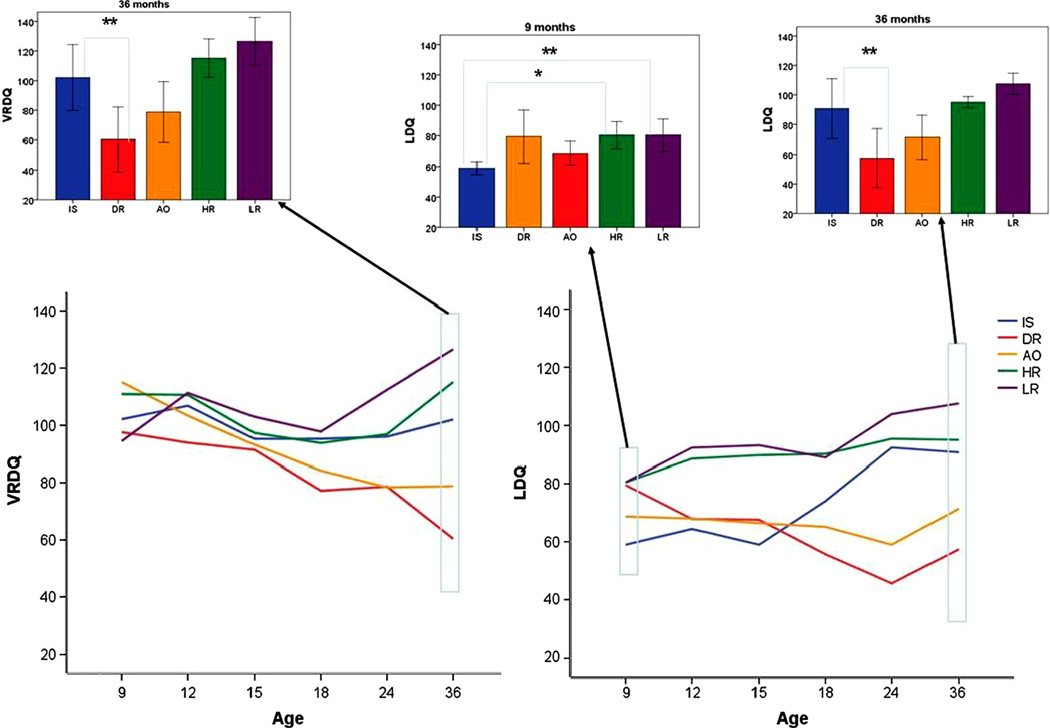
In terms of visual receptive abilities of the 5 groups, there were significant main effects of group (F(4, 131.89) = 7.46, p < .001), age (F(5, 44.75) = 3.88, p < .01), and age by group interaction (F(20, 47.30) = 1.84, p < .05). The effect of total treatment hours did not reach significance (F(1, 101.96) = 3.02, p = .09). Simple comparisons revealed no differences between the IS group and any other group at the 9, 12, and 15-month time points. At 18 months, the IS group had significantly higher scores than the DR group (d = 2.01, p = < .05). At 36 months, the IS group had higher scores than the DR group (d = 2.41, p = .01).
In terms of language development, there were significant main effects of group (F(4, 114.64) = 14.30, p < .001), a significant effect of age (F(5, 50.41) = 2.52, p < .05) and a significant age by group interaction (F(20, 52.63) = 2.49, p < .012). The effect of total treatment hours did not reach significance (F(1, 116.89) = 2.29, p = .l3). At 9 months, the IS group had significantly lower LDQ scores than the HR and LR groups (HR: d = −1.70, p < .05; LR: d = −1.98, p = .01). At 12 months, the IS group only had lower scores than the LR group (d = −.78, p < .05). At 15 months, the IS group had lower scores than the HR and LR groups (HR d = −2.23, p < .01; LR d = −3.02, p < .001). However, by 18 months, the IS group did not significantly differ from any other group. At 24 months, the IS group had higher scores than the AO (d = 1.99, p < .01) and DR (d = 2.99, p = .001) groups. At 36 months, the IS group continued to have higher scores than the DR group (d = 2.42, p = .01), but the difference between the IS and AO groups no longer reached significance (d = .90, p = .52), although it continued to show a moderate effect size. There were no significant differences between the IS and HR or LR group from 18 to 24 months of age. See Fig. 3 for representations of these group differences.
Fig. 3.
Visual reception DQ and Language DQ across the treatment and follow-up period. Insert bar graphs present significant pairwise comparisons at pre-treatment (9 months) and post-treatment (36 months) *p ≤.05, **p ≤ .01
To determine whether differences in rates of Expressive and Receptive Language development in the groups might affect the results, we also repeated the analysis using models built separately from Receptive and Expressive Language scores. The results from these did not differ from the models run with the scores combined, as described above.
Finally, we examined rates of overall DQ at or below 70 at 36 months. One IS child (14 %), 2 children in the AO group (28.6 %), and 3 children (75 %) in the DE group scored in this range.
Clinical Best Estimate Outcome Classification
At the final visit, each child was assigned a diagnostic category based on standardized assessments and clinical judgment. In the IS group, only two children received a diagnosis of ASD. One was a male infant sibling who had low developmental scores, met criteria for an intellectual disability, and was diagnosed with DSM-IV-TR Autistic Disorder. The second was a female infant sibling who was diagnosed with PDDNOS and had verbal and nonverbal MSEL scores in the normal range. The other five children were not classified as having an ASD or intellectual disability.
In the DR group, three of the four children (75 %) received a diagnosis of ASD. Two met criteria for DSM-IV-TR Autistic Disorder and also presented with intellectual disability. One met criteria for PDDNOS and also presented with language delays. Although the fourth child in the DR group did not meet criteria for ASD, she presented with intellectual disability.
Discussion
The promise of early identification of ASD is built on the premise of earlier treatment, which is thought to maximize effects of treatment and amelioration of the disabling effects of ASD due to the greater plasticity of younger neural systems and prevention of secondary effects due to environmental alterations in response to ASD symptoms. Findings from many infant sibling studies (Bryson et al. 2007; Ozonoff et al. 2010) have led to much greater awareness of evolving symptoms of ASD in the second 6 months of life in the subgroup of children who have early symptoms. The symptoms described by such studies were used in the present study to identify a group of infants with at-risk symptoms before the first year of life and to develop and test a parent-implementation intervention that could reduce symptoms and foster more typical developmental patterns and rates, thus ameliorating the effects of ASD on early development.
Five questions were addressed by this study:
-
1
Could symptomatic infants younger than 15 months of age be identified and recruited for a treatment study? This is a low incidence group of infants. Our study involved 3 years of recruitment, combining the resources of a large infant sibling study and a community that is very aware of ASD and has many services for young children with ASD. From these efforts, 24 children were referred and 7 were enrolled. Four of the six children who were referred from the infant sibling project and qualified for the study were enrolled, while 3 of 14 children referred from the community qualified for the study and were enrolled. Clearly, infants referred from the infant sibling study due to their symptoms were far more likely to meet our stringent enrollment criteria—persistent, multiple ASD symptoms and independent clinician agreement—than those referred from the community. This likely reflects the impact of several variables, including the greater risk in ASD families and the greater knowledge of the referring professionals in the infant sibling study compared to the family referrals from the general community. However, we needed both types of referrals to meet our enrollment goals, and parents of infant siblings were less inclined to enroll than were the community referrals. Those who did not enroll tended to choose to wait until a later evaluation to see if symptoms continued, thus missing the cut-off age for the study. Hence, working within an infant-sibling study may require some additional efforts to motivate families to enroll as early as autism symptoms raise concerns for the staff.
Interestingly, no families who enrolled dropped out of the study. This is quite a different picture than one sees in the population screening studies, in which there is a very large drop-out of infants screened at high risk for autism from follow-up assessments and treatment (Dietz et al. 2006). This lack of attrition may reflect the greater motivation of families who actively seek out studies and clinical services for their children due to family-recognized risk status. The low rate of referrals, however, indicates that in order to advance to the next level of treatment science, a randomized group design (Smith et al. 2007) conducted at multiple sites will be needed in order to gather reasonably sized groups in this age range.
-
2
Can parents learn and deliver the intervention with high fidelity of implementation and maintain this after short-term intervention ends? The single subject graph presented in (Fig. 2) demonstrates parent mastery of the techniques (defined as 80 % of the total possible score, or a mean score of 2.40) for all parents by the end of the 12th session. It also demonstrates their maintenance of skills after treatment ended. For 6 of the 7 parents, mastery occurred at week 7. This parallels previous publications of parent ESDM learning data (Rogers et al. 2012a, b; Vismara et al. 2009, 2012), and so replicates our previous findings that parents can learn these techniques in less than 8 contact hours. Other low intensity parent-delivered interventions also demonstrate parent fidelity of implementation (Kasari et al. 2010; Carter et al. 2011) of responsive techniques. The intervention appears to affect parent interactions in the desired directions, as measured in contexts and with experimenters that differ from the treatment sessions.
A caveat, however, involves the increased motivation that may well be present in parents who enroll in infant sibling studies and parents who call specialized centers with concerns about autism in their infants. The levels of motivation, commitment, and resources to carry out interventions at home seen in these families may not reflect that of community families identified through early screenings.
-
3
Are parents satisfied with this low intensity, short-term intervention? Parent ratings demonstrate high levels of satisfaction with the intervention, that are consistent across the 6/7 parents who provided data. Parents also report strong working alliances with their IS therapist. It follows that the intervention was well-received by the parents.
-
4
Are infants who received the intervention less impaired in terms of ASD and delays at age 3 than those who had similar amounts of symptoms at 9 months but did not receive the intervention? This question is best answered by comparing the IS group to the AO and DR groups. Compared to the AO group, IS infants had significantly more autism symptoms at 9 months of age and significantly lower autism severity scores over the 18–36 month age period. In terms of developmental scores, the IS group did not differ significantly from the AO group on visual reception scores at any age point. In terms of verbal quotients, the IS group had significantly higher scores at 24 months. At 36 months, they continued to have a higher verbal score with the difference not statistically significant but demonstrating a moderate effect size (d = .90). Thus, the IS group had less impairment in terms of ASD symptoms and developmental delays than the AO group at 36 months.
Compared to the DR group, the IS group had equivalent autism symptoms at 9 months and a trend (p = .06) towards lower ADOS severity scores from 18 to 36 months, with a large effect size (d = −1.98) and much less ASD outcome than the DR group (29 % compared to 75 %). In terms of developmental scores, the IS group had significantly higher quotients than the DR group in visual reception at 18 and 36 months. In terms of language quotients, the IS group had higher scores at 24 and 36 months than the DR group. Visual inspection of Fig. 3 reveals that the degree of developmental acceleration that the IS group experienced between 12 and 24 months stands in contrast to any of the other groups. Finally, in terms of rates of overall DQ at or below 70, one IS child (14 %), 2 children in the AO group (28.6 %), and 3 children (75 %) in the DE group scored in this range.
Without a randomized controlled trial, we do not know whether the course of these IS infants would have been more like the AO and DR groups without intervention. However, the multiple points above converge to suggest that these IS infants were in fact at high risk for autism and the intervention may have contributed to the differences in their outcomes compared to the other two groups.
What might this improvement in the IS infants mean about early ASD? In several ways, their improvements mirror the improvements that slightly older children make in the most efficacious interventions (Dawson et al. 2010; Lovaas 1987; Smith et al. 2000), so acceleration of developmental rates and decreased ASD symptoms resulting from early intervention should not be surprising. However, these infants are much younger than the children thus far studied, they are showing changes much faster than preschoolers in intensive intervention, and they are receiving far less professional intervention (though not necessarily any fewer hours per week of intervention, since the parents are integrating intervention into all their daily routines). More rapid change in younger infants should not surprise us, given the increased plasticity of infant neural development and the rapid learning capacity of infants. Additionally, the skills these infants are acquiring—language, joint attention, imitation, reciprocal communication—are skills that normally develop in the 12–24 month period. Thus, these infants are acquiring skills that are appropriate for their chronological ages, and there may be enhanced neural readiness to acquire these skills in this period, both for the affected infants and also for typically developing infants. Finally, the change in these infants adds weight to the idea that some of the problems associated with ASD may not be due to the causal biological difference, but may instead represent secondary effects of ASD, likely associated with alterations in the social-communicative environment that stem from the infants’ poor social-communication and their ongoing lack of their typical responses and initiations to their family members (see Dawson et al. 2001; Mundy and Crowson 1997, for a fuller discussion of the social reward theory of autism). All of the science and theory that has led the field to earlier screening and earlier treatment of ASD would predict this outcome: that more improvement will occur when autism is detected and treated as early as possible. Consequently, this finding may represent proof-of-principle; however, only rigorous clinical trials can actually test this hypothesis.
One surprising finding in this study was the rate of families who declined enrollment for their symptomatic infants. Four of seven infant sibling study families who qualified for the treatment study declined enrollment, compared to the community referrals who qualified, all of whom enrolled. This may reflect the fact that the infant sibling study families were not expecting to be referred to treatment, and those who declined were not concerned yet about their infants, knew that another assessment would occur in 3 months, and so chose to wait to see if the next evaluation confirmed the concerns. In contrast, the community families all had significant concerns and sought out help. In terms of effects of infant sibling status on fidelity measures, the fidelity scores in this study, both at baseline and over time, are very similar to those we have reported in previous studies of community referred toddlers in parent-implemented interventions. So far, we have not experienced clinical differences in our intervention experiences or our data reflecting parent use of technique in the infant sibling families compared to other families, though the numbers are too small to analyze this.
There is a second important contrast, involving outcomes, between the infant siblings and community referrals in this study. In terms of autism diagnosis in the third year of life, three of the seven IS infants were diagnosed with ASD at some time during the age period 12–24 months. However, one of the children’s symptoms (a community referral) improved so much that she no longer qualified for any type of diagnosis by 36 months, and a second child’s symptoms (a female sibling) were borderline (PDD-NOS) at 36 months. The third child (a male sibling) met all criteria for Autistic Disorder and also had significant developmental delays at 24 and 36 months. This was the one child who began treatment later than 12 months of age. Thus, 2 of the 7 children (28.5 %) in the IS group, both siblings, had an autism spectrum diagnosis at age 3. In contrast, none of the community-enrolled children were autism-risk siblings, and none of them were diagnosed with ASD at age 3. There are several potential implications of this difference, including greater developmental flexibility in non-siblings, early symptoms due to different causes in the two referral groups, among others. Future studies should carefully characterize community enrollees clinically to search for various types of risk factors that could be involved in these early symptoms.
As in any pilot study, there are a number of weaknesses to consider. First, the treated group is very small. With only seven infants in the treatment group, no conclusions can be drawn. The number of children located and enrolled in this study by or before their first birthday suggests that recruitment for a larger trial will be aided by using an infant sibling recruitment approach and by conducting the study across multiple sites. Second, parent fidelity ratings were based on therapist assessments. Third, the comparison groups were drawn from an existing sample of convenience and their data do not provide causal evidence that the treatment caused the improvement in the treated group. Furthermore, the baseline period was consistent for all subjects and does not demonstrate control for change due to other variables in the IS group.
There are also several strengths to be noted in this small study. First is the use of four different comparison groups all drawn from the same study and all followed longitudinally on the same measures and during the same time period. This allows us to contrast the status and growth patterns of the IS group across the entire period, from enrollment to age 3 outcomes. A second strength is the use of standardized tests and naive child raters to assess children’s development and autism symptoms. Finally, few low intensity parent-implemented toddler treatment studies have demonstrated significant changes on standard scores, relying instead on changing frequencies of one or a few discrete behaviors measured using video analyses of parent– child interaction. Change on standard scores requires that child changes being fostered by parents during everyday routines are robust enough to be elicited by strangers— the assessors—and in situations far removed from parent–child dyadic interaction—namely, a formal standardized assessment in a clinic. If these findings are replicated in a larger, controlled study, it would suggest that deep structural changes, not simple surface changes, are occurring in the infants’ learning in multiple areas and in development of more appropriate social communication capacities.
Summary and Conclusions
In this effort to alter very early autism symptoms through a parent—implemented intervention study, seven infants between the ages of 9 and 15 months were enrolled in a pilot study to examine proof-of-principle regarding infant treatment of ASD. The infants and parents were provided with 12 weeks of a low intensity parent coaching model derived from the ESDM. The infants were followed from 9 months to 36 months, and their overall developmental rates and autism symptoms were compared to four other groups of infants also at high risk for ASD due to sibling status and increased early symptoms, including one group who would be diagnosed with autism within the coming year. The treated group began as the most symptomatic and language delayed of the groups, but over the 18–36 month age period they demonstrated autism symptom scores that were significantly fewer than those children who developed ASD. The language developmental rates of the treated group accelerated more steeply than any of the other groups of infants, moving from the delayed range into the average range by 24–36 months. Because this was not a randomized study, no conclusions about the efficacy of the experimental intervention can be drawn. However, given the need for treatment approaches for this age group in response to infant autism screening and public awareness campaigns, and given the outcomes at age 3 of the treated infants in relation to four different comparison groups of infants all drawn from the same autism infant sibling study, the data from the study indicate that a controlled trial is a feasible and an important next step.
Acknowledgments
This project was funded by grants from NICHD/NIMH (R21 HD065275: Rogers R01 MH068398: Ozonoff) and support from Autism Speaks and the John and Marcia Goldman Foundation. The authors would like to acknowledge SoYeon Baik for her assistance with nearly every aspect of the project, Diane Larzelere for her assistance with manuscript preparation, and the children and families who gave of their time to participate in the study.
Contributor Information
S. J. Rogers, Email: sally.rogers@ucdmc.ucdavis.edu, MIND Institute, University of California, Davis, Sacramento, CA, USA.
L. Vismara, York University, Toronto, ON, Canada
A. L. Wagner, University of California, Davis, CA, USA
C. McCormick, MIND Institute, University of California, Davis, Sacramento, CA, USA
G. Young, MIND Institute, University of California, Davis, Sacramento, CA, USA
S. Ozonoff, MIND Institute, University of California, Davis, Sacramento, CA, USA
References
- American Psychiatric Association, editor. Diagnostic and statistical manual-IV-TR. Washington, DC: American Psychiatric Publishing Incorporated; 2000. [Google Scholar]
- Barrera ME, Doucet DA, Kitching KJ. Early home intervention and socio-emotional development of preterm infants. Infant Mental Health Journal. 1990;11(2):142–157. [Google Scholar]
- Brooks-Gunn J, Liaw FR, Klebanov PK. Effects of early intervention on cognitive function of low birth weight preterm infants. The Journal of Pediatrics. 1992;120(3):350–359. doi: 10.1016/s0022-3476(05)80896-0. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Carter AS, Messinger DS, Stone WL, Celimli S, Nahmias AS, Yoder P. A randomized controlled trial of Hanen’s ‘More Than Words’ in toddlers with early autism symptoms. Journal of Child Psychology and Psychiatry. 2011;52(7):741–752. doi: 10.1111/j.1469-7610.2011.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS. Children’s language learning: An interactionist perspective. Journal of Child Psychology and Psychiatry. 2000;41(1):33–54. [PubMed] [Google Scholar]
- Charlop-Christy MH, Carpenter MH. Modified incidental teaching sessions: A procedure for parents to increase spontaneous speech in their children with autism. Journal of Positive Behavior Interventions. 2000;2(2):98–112. [Google Scholar]
- Davis NO, Kuhn JC, Carter AS. Children’s problem behaviors and self-efficacy among mothers of toddlers with autism. The moderating role of working alliance. Poster presentation, International Meeting of Autism Researchers; Montreal. 2006. [Google Scholar]
- Dawson G, Osterling J, Rinaldi J, Carver L, McPartland J. Brief report: Recognition memory and stimulus-reward associations: Indirect support for the role of ventromedial prefrontal dysfunction in autism. Journal of Autism and Developmental Disorders. 2001;31(3):337–341. doi: 10.1023/a:1010751404865. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, et al. Randomized controlled trial of the Early Start Denver Model: A developmental behavioral intervention for toddlers with autism: Effects on IQ, adaptive behavior, and autism diagnosis. Pediatrics. 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz C, Swinkels S, van Daalen E, van Engeland H, Buitelaar JK. Screening for autistic spectrum disorder in children aged 14–15 months. II: Population screening with the Early Screening of Autistic Traits Questionnaire (ESAT). Design and general findings. Journal of Autism and Developmental Disorders. 2006;36(6):713–722. doi: 10.1007/s10803-006-0114-1. [DOI] [PubMed] [Google Scholar]
- Diggle T, McConachie HR, Randle VRL. Parent-mediated early intervention for young children with autism spectrum disorder. The Cochrane Database of Systematic Reviews. 2002;2 doi: 10.1002/14651858.CD003496. [DOI] [PubMed] [Google Scholar]
- Ference J, Curtin S. Attention to lexical stress and early vocabulary growth in 5-month-olds at risk for autism spectrum disorder. Journal of Experimental Child Psychology. 2013;116(4):891–903. doi: 10.1016/j.jecp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Wan MW, Guiraud J, Holsgrove S, McNally J, Slonims V, et al. Intervention for infants at risk of developing autism: A case series. Journal of Autism and Developmental Disorders. 2013;43(11):2502–2514. doi: 10.1007/s10803-013-1797-8. [DOI] [PubMed] [Google Scholar]
- Hanft BE, Rush DD, Shelden ML. Coaching families and colleagues in early childhood. Baltimore, MD: Brookes; 2003. [Google Scholar]
- Harris SL, Wolchik SA, Weitz S. The acquisition of language skills by autistic children: Can parents do the job? Journal of Autism and Developmental Disorders. 1981;11(4):373–384. doi: 10.1007/BF01531613. [DOI] [PubMed] [Google Scholar]
- Johnson-Martin NM, Jens KG, Attermeier SM, Hacker B. The Carolina curriculum for infants and toddlers with special needs. 2nd. Baltimore: Brookes; 1991. [Google Scholar]
- Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. Journal of Autism and Developmental Disorders. 2010;40(9):1045–1056. doi: 10.1007/s10803-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Bimbela A, Schreibman L. Collateral effects of parent training on family interactions. Journal of Autism and Developmental Disorders. 1996;26(3):347–359. doi: 10.1007/BF02172479. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Glahn TJ, Nieminen GS. Generalization of parent-training results. Journal of Applied Behavior Analysis. 1978;11(1):95–109. doi: 10.1901/jaba.1978.11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry. 2012;53(9):986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski KE, Charlop MH, Schreibman L. Training parents to use the natural language paradigm to increase their autistic children’s speech. Journal of Applied Behavior Analysis. 1988;21(4):391–400. doi: 10.1901/jaba.1988.21-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE, Charman T, Murphy D, Johnson MH. Reduced neural sensitivity to social stimuli in infants at risk for autism. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1758):20123026. doi: 10.1098/rspb.2012.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Levanthal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic. A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology. 1987;55(1):3. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- McEachin JJ, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. American Journal of Mental Retardation. 1993;97:359–372. [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, et al. Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. The mullen scales of early learning. Circle Pines, MN: American Guidance; 1995. [Google Scholar]
- Mundy P, Crowson M. Joint attention and early social communication: Implications for research on intervention with autism. Journal of Autism and Developmental Disorders. 1997;27(6):653–676. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, et al. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. PMCID: in progress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, et al. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38(4):644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BA, Rowe ML, Singer JD, Snow CE. Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development. 2005;76(4):763–782. doi: 10.1111/j.1467-8624.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Resnick MB, Armstrong S, Carter RL. Developmental intervention program for high-risk premature infants: Effects on development and parent-infant interactions. Journal of Developmental and Behavioral Pediatrics. 1988;9(2):73–78. [PubMed] [Google Scholar]
- Rogers SJ, Dawson G. The Early Start Denver Model for young children with autism: Promoting language, learning, and engagement. NY: Guilford; 2010. [Google Scholar]
- Rogers SJ, Dawson G, Vismara L. An early start for your child with autism: Using everyday activities to help kids connect, communicate and learn. NY: Guilford; 2012a. [Google Scholar]
- Rogers SJ, Estes A, Lord C, Vismara L, Winter J, Fitzpatrick A, et al. Effects of a brief Early Start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: A randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2012b;51(10):1052–1065. doi: 10.1016/j.jaac.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L. Interventions for infants and toddlers at risk for autism spectrum disorder. In: Volkmar F, Rogers SJ, Pelphry R, Paul K, editors. Handbook of autism and developmental disorders. 4th. Hoboken, NJ: Wiley; 2014. pp. 739–769. [Google Scholar]
- Rogers SJ, Vismara L, Wagner A. Infant start fidelity. University of California, Davis; 2012. Unpublished material. [Google Scholar]
- Ross GS. Home intervention for premature infants of low-income families. American Journal of Orthopsychiatry. 1984;54(2):263–270. doi: 10.1111/j.1939-0025.1984.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Sacrey LAR, Bryson SE, Zwaigenbaum L. Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: A longitudinal study from 6 to 36 months of age. Behavioural Brain Research. 2013;256:441–450. doi: 10.1016/j.bbr.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Sanz MT, Menendez J. A study of the effect of age of onset of treatment on the observed development of Down’s syndrome babies. Early Child Development and Care. 1996;118(1):93–101. [Google Scholar]
- Sanz-Aparicio MT, Balaña JM. Early language stimulation of Down’s Syndrome babies: A study on the optimum age to begin. Early Child Development and Care. 2002;172(6):651–656. [Google Scholar]
- Sanz-Aparicio MT, Balaña JM. Early learning in psychomotor training of Down’s syndrome. Early Child Development and Care. 2003;173(2–3):317–321. [Google Scholar]
- Schreibman L, Koegel RL. Fostering self-management: Parent-delivered pivotal response training for children with autistic disorder. In: Hibbs ED, Jensen PS, editors. Psychosocial treatments for child and adolescent disorders: Empirically based strategies for clinical practice. Washington, DC: American Psychological Association; 1997. [Google Scholar]
- Seifer R, Clark GN, Sameroff AJ. Positive effects of interaction coaching on infants with developmental disabilities and their mothers. American Journal on Mental Retardation. 1991;96:1–11. [PubMed] [Google Scholar]
- Short AB. Short-term treatment outcome using parents as co-therapists for their own autistic children. Journal of Child Psychology and Psychiatry. 1984;25(3):443–458. doi: 10.1111/j.1469-7610.1984.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Collins WA, Tran S, Haydon KC. Attachment and the experience and expression of emotions in romantic relationships: A developmental perspective. Journal of Personality and Social Psychology. 2007;92(2):355. doi: 10.1037/0022-3514.92.2.355. [DOI] [PubMed] [Google Scholar]
- Sloper P, Glenn SM, Cunningham CC. The effect of intensity of training on sensori-motor development in infants with Down’s syndrome. Journal of Mental Deficiency Research. 1986;30(2):149–162. doi: 10.1111/j.1365-2788.1986.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Smith T, Groen AD, Wynn JW. Randomized trial of intensive early intervention for children with pervasive developmental disorder. American Journal on Mental Retardation. 2000;105:269–285. doi: 10.1352/0895-8017(2000)105<0269:RTOIEI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Smith T, Scahill L, Dawson G, Guthrie D, Lord C, Odom S, et al. Designing research studies on psychosocial interventions in autism. Journal of Autism and Developmental Disorders. 2007;37(2):354–366. doi: 10.1007/s10803-006-0173-3. [DOI] [PubMed] [Google Scholar]
- Steiner AM, Gengoux GW, Klin A, Chawarska K. Pivotal response treatment for infants at-risk for autism spectrum disorders: A pilot study. Journal of Autism and Developmental Disorders. 2013;43(1):91–102. doi: 10.1007/s10803-012-1542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH. Specificity in mother-toddler language-play relations across the second year. Developmental Psychology. 1994;30(2):283. [Google Scholar]
- Tomasello M. The social bases of language acquisition. Social Development. 1992;1(1):67–87. [Google Scholar]
- Vismara LA, Colombi C, Rogers SJ. Can 1 hour per week of therapy lead to lasting changes in young children with autism? Autism: An International Journal. 2009;13(1):93–115. doi: 10.1177/1362361307098516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara LA, Young GS, Rogers SJ. Telehealth for expanding the reach of early autism training to parents. Autism Research and Treatment. 2012 doi: 10.1155/2012/121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KS, Rogers SJ. Intervening in infancy: Implications for autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51(12):1300–1320. doi: 10.1111/j.1469-7610.2010.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry. 2013;54(7):763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- Wetherby A, Prizant B. Communication and symbolic behavior scales developmental profile. 1st. Baltimore, MD: Paul H. Brookes; 2002. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]