Abstract
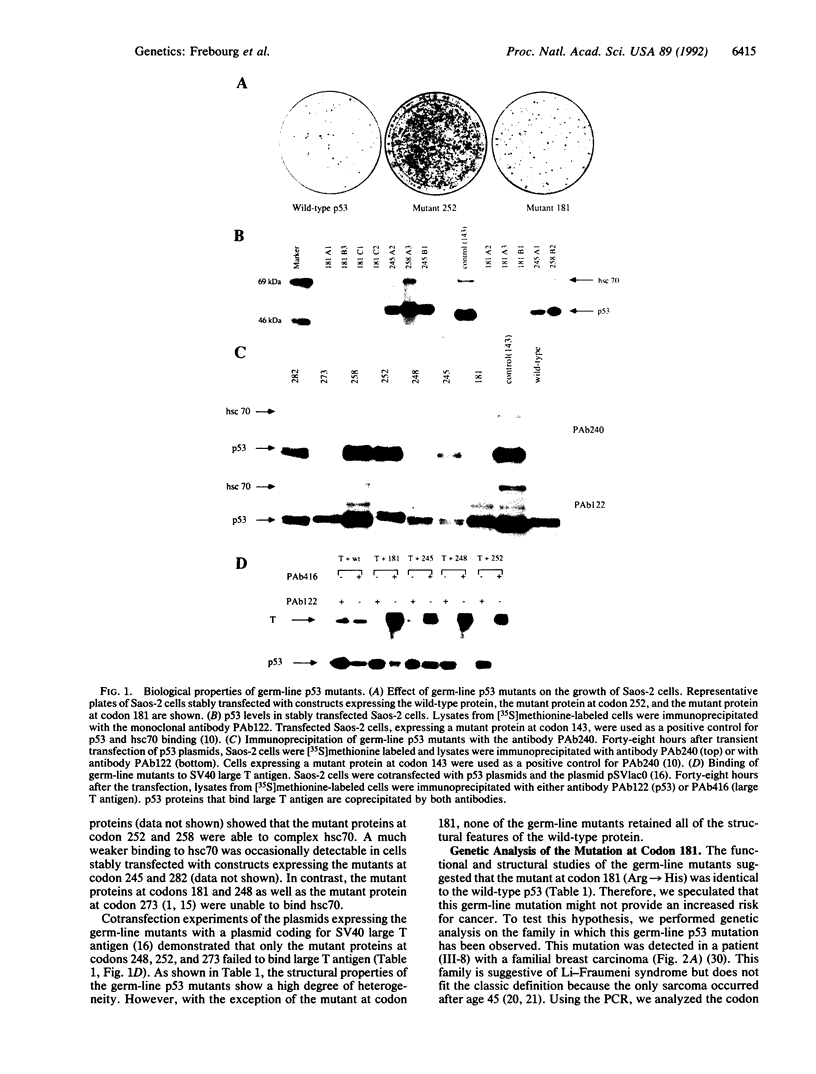
Germ-line mutations in the p53 tumor suppressor gene have been observed in patients with Li-Fraumeni syndrome, brain tumors, second malignancies, and breast cancers. It is unclear whether all of these mutations have inactivated p53 and thereby provide an increased risk for cancer. Therefore, it is necessary to establish the biological significance of these germ-line mutations by the functional and structural analysis of the resulting mutant p53 proteins. We analyzed the ability of seven germ-line mutant proteins observed in patients with Li-Fraumeni syndrome, second primary neoplasms, or familial breast cancer to block the growth of malignant cells and compared the structural properties of the mutant proteins to that of the wild-type protein. Six of seven missense mutations disrupted the growth inhibitory properties and structure of the wild-type protein. One germ-line mutation retained the features of the wild-type p53. Genetic analysis of the breast cancer family in which this mutation was observed indicated that this germ-line mutation was not associated with the development of cancer. These results demonstrate that germ-line p53 mutations observed in patients with Li-Fraumeni syndrome and with second malignancies have inactivated the p53 tumor suppressor gene. The inability of the germ-line p53 mutants to block the growth of malignant cells can explain why patients with these germ-line mutations have an increased risk for cancer. The observation of a functionally silent germ-line mutation indicates that, before associating a germ-line tumor suppressor gene mutation with cancer risk, it is prudent to consider its functional significance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Brown M., Figge J., Hansen U., Wright C., Jeang K. T., Khoury G., Livingston D. M., Roberts T. M. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987 Jun 5;49(5):603–612. doi: 10.1016/0092-8674(87)90536-8. [DOI] [PubMed] [Google Scholar]
- Børresen A. L., Andersen T. I., Garber J., Barbier-Piraux N., Thorlacius S., Eyfjörd J., Ottestad L., Smith-Sørensen B., Hovig E., Malkin D. Screening for germ line TP53 mutations in breast cancer patients. Cancer Res. 1992 Jun 1;52(11):3234–3236. [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Casey G., Lo-Hsueh M., Lopez M. E., Vogelstein B., Stanbridge E. J. Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene. 1991 Oct;6(10):1791–1797. [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Carter B. S., Ewing C. M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991 Sep 1;51(17):4716–4720. [PubMed] [Google Scholar]
- Johnson P., Gray D., Mowat M., Benchimol S. Expression of wild-type p53 is not compatible with continued growth of p53-negative tumor cells. Mol Cell Biol. 1991 Jan;11(1):1–11. doi: 10.1128/mcb.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Law J. C., Strong L. C., Chidambaram A., Ferrell R. E. A germ line mutation in exon 5 of the p53 gene in an extended cancer family. Cancer Res. 1991 Dec 1;51(23 Pt 1):6385–6387. [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst. 1969 Dec;43(6):1365–1373. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Malkin D., Jolly K. W., Barbier N., Look A. T., Friend S. H., Gebhardt M. C., Andersen T. I., Børresen A. L., Li F. P., Garber J. Germline mutations of the p53 tumor-suppressor gene in children and young adults with second malignant neoplasms. N Engl J Med. 1992 May 14;326(20):1309–1315. doi: 10.1056/NEJM199205143262002. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Amin M., Sauve G. J., Appella E., Ullrich S. J., Romano J. W. Wild type human p53 is antiproliferative in SV40-transformed hamster cells. Oncogene. 1990 Jul;5(7):973–980. [PubMed] [Google Scholar]
- Mercer W. E., Shields M. T., Amin M., Sauve G. J., Appella E., Romano J. W., Ullrich S. J. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger A. K., Sheffield V. C., Duyk G., Daneshvar L., Edwards M. S., Cogen P. H. Identification of a germ-line mutation in the p53 gene in a patient with an intracranial ependymoma. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7825–7829. doi: 10.1073/pnas.88.17.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies C., Houldsworth J., Chaganti R. S. Extraction of DNA from paraffin blocks for Southern blot analysis. Am J Surg Pathol. 1991 Feb;15(2):169–174. doi: 10.1097/00000478-199102000-00010. [DOI] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky D., Tokino T., Helzlsouer K., Zehnbauer B., Rausch G., Shelton B., Prestigiacomo L., Vogelstein B., Davidson N. Inherited p53 gene mutations in breast cancer. Cancer Res. 1992 May 15;52(10):2984–2986. [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Yamaguchi T., Dayton S. H., Beauchamp R. L., Herrera G. E., Ishizaki K., Yamamuro T., Meyers P. A., Little J. B., Sasaki M. S. Prevalence and spectrum of germline mutations of the p53 gene among patients with sarcoma. N Engl J Med. 1992 May 14;326(20):1301–1308. doi: 10.1056/NEJM199205143262001. [DOI] [PubMed] [Google Scholar]