SUMMARY
The ability to shift between multiple decision-making strategies during natural behavior allows animals to strike a balance between flexibility and efficiency. We investigated odor-guided navigation by mice to understand how decision making strategies are balanced during a complex natural behavior. Mice navigated to odor sources in an open arena using naturally fluctuating airborne odor cues as their positions were recorded precisely in real time. When mice had limited prior experience of source locations, their search behavior was consistent with a gradient ascent algorithm that utilized directional cues in the plume to navigate to the odor source. Gradient climbing was effective because the arena size allowed animals to conduct their search mainly within the odor plume, with frequent odor contacts. With increased experience, mice shifted their strategy from this flexible, sensory-driven search behavior to a more efficient and stereotyped foraging approach that varied little in response to odor plumes. This study demonstrates that mice use prior knowledge to adaptively balance flexibility and efficiency during complex behavior guided by dynamic natural stimuli.
INTRODUCTION
Psychological studies conducted during the past century have established that multiple strategies control decision-making with a primary distinction being made between strategies that support flexible behavior and those that enforce more efficient, stereotyped stimulus-response relationships that take advantage of learned contingencies during well-trained behaviors [1–3]. Previous work has also shown that sensory cues used to perform complex tasks such as path integration [4] and odor trail-tracking [5–7] can be adaptively selected [8]. We sought to define the strategies and sensory cues used by mice to perform a complex, ethologically relevant behavior in response to natural sensory stimuli.
Since mice are macrosmatic animals that use the sense of smell to locate food [9] and avoid predators at a distance, we designed an olfaction-based search task to probe their decision making strategies in the context of natural stimuli. Previous behavioral studies have generally focused on trail tracking, where an animal uses odorants on the ground to navigate [10,11]. Here, we leverage the advantages of controlled laboratory conditions to demonstrate that mice can make use of airborne odor cues to locate a source. The complexity of airborne odor plumes is well-documented [12], creating a rich and dynamic stimulus for odor-based navigation. Work using simplified odor cues has suggested that rodents may be able to measure odor gradients with just one or two sniffs [13,14]. Gradient climbing can be an effective strategy to locate the source of a smoothly changing odor but it generally fails in the presence of turbulence [15].
In the present study we combine computational fluid dynamics with precise motion tracking and behavioral analysis to investigate the strategies and algorithms that mice use to navigate based upon airborne odor plumes. Our computational analysis and experimental measures show that in our experiments the odor fluctuates considerably as a result of turbulent transport. Surprisingly, we find that the simple readout of the gradient is enough to locate the odor source with performances comparable to the experimental data. We demonstrate that this is due to mice having frequent encounters with the plume. Such encounters become increasingly sparse with increasing distance from the source [12,16–19], implying that diverse search strategies and/or modalities must be used at varying distances from an odor source [14,20–23]. Additionally, we find that mice use different search strategies when they have varying amounts of prior information of resource location. With limited prior knowledge they follow the fluctuating odor plumes to the odor sources. As mice learn resource locations they adaptively use this prior knowledge to inform their search strategies, shifting from a sensory-guided strategy to a more stereotyped and efficient foraging pattern.
RESULTS
Mice navigate to odor sources using airborne cues
Mice were trained that water was available from a spout located adjacent to the source of an airborne odor (randomly selected from three possible sources for each trial; Figure 1A, locations 1, 2, and 3; see methods for training procedure). Once a mouse arrived at the odor source location and persisted there for at least 1.5 s (see Methods), it received water for the remainder of the odor emission period (20 seconds of odor emission per trial; Figure 1B). After receiving water, the mouse remained in the arena and was free to explore until the next trial started. Performance was measured as the time that a mouse spent at the odor source (from 0–20 seconds). To ensure that the search was odor-based, all behavior took place under dim far red light (660 nm) that eliminated visual cues for the mice [24]. W e monitored mice using a dual camera system that provided the animal's three-dimensional location at 16 Hz.
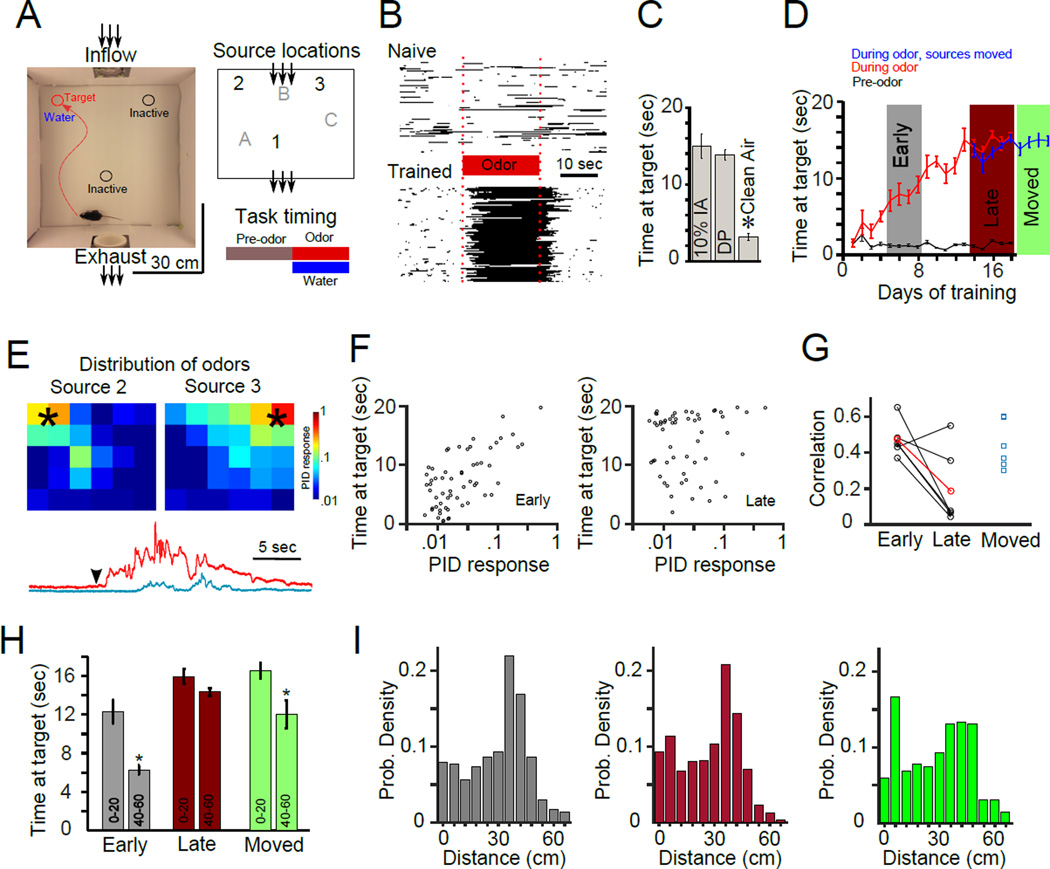
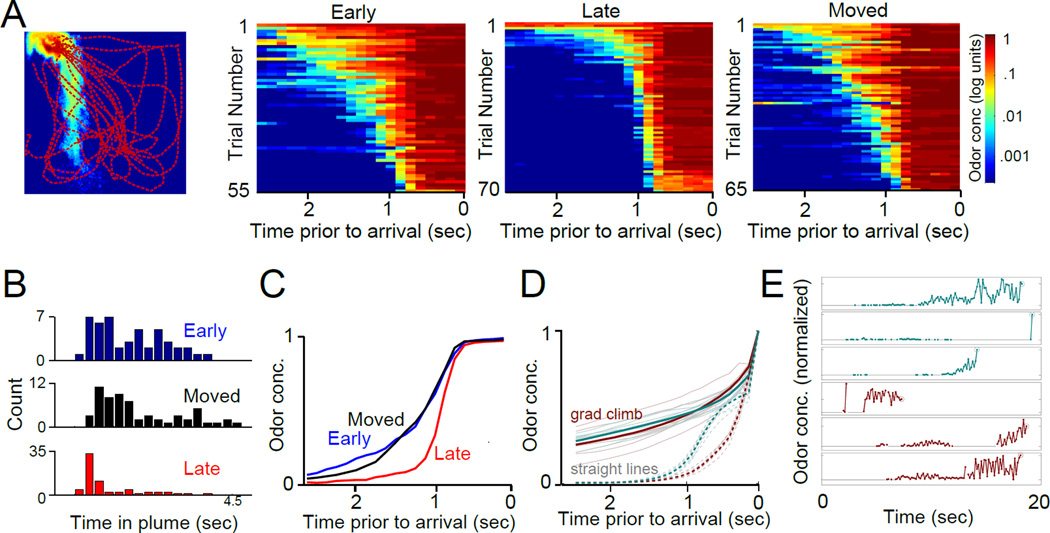
Figure 1. An odor-guided search task for mice.
(A) Left: Diagram of the arena with a mouse present. Direction of incoming and outgoing air is shown. Right, top: Schematic of odor source locations within the arena. Water dispensers are co-localized with odor sources. Right, bottom: Temporal structure of each trial. A pre-odor period (30 seconds) precedes odor release from one of the three sources (20 seconds). During odor release, water is available at the odor source. (B) Raster plots of 100 trials from one mouse naïve to the task (top, first day in the arena) and 100 trials from one trained mouse (14 days of training). Dark color indicates the presence of the mouse at the odor source location, with odor (and water) being present only during the time indicated by the red bar. (C) Performance of well-trained mice in response to a variety of odorant concentrations as well as the solvent alone (diethyl phthalate). Mice were capable of navigating not only to the source of a strong odor (isoamyl acetate at 10% v/v in the solvent diethyl phthalate; average time at the target (Ttarget) 14.91 ± 1.53 seconds, n = 6 mice), but also to the solvent alone (1 ml of diethyl phthalate; average Ttarget 13.77 ± 0.68 seconds, n = 6 mice). Importantly, mice were unable to locate the source without an odor cue, which we tested by replacing the odor source tube with an empty tube in the olfactometer (“Clean Air” in Figure 1C, average Ttarget 3.11 ± 0.42 seconds, n = 6 mice). Performance on trials during which an empty tube was used in place of an odor was significantly impaired (n = 6 mice across 14 sessions, p<0.001, Mann-Whitney U-test). See also Figures S1 and S2. (D) Performance of mice as a function of training session. For subsequent analysis, mice were separated into three groups - an early training group (“Early”, sessions 5–8), a late training group (“Late”, sessions 14–18) and a group that was trained to the performance level of the late group and then introduced to new odor source locations (blue line, “Moved” condition). As a control, the black line indicates the amount of time spent at the possible odor source between trials (when odor was not being emitted). (E) Top panels: Distribution of odors in the arena determined through direct measurements of odorant concentration with a PID (average of maximal odor amplitude across 5 repetitions). Bottom traces: single trial examples of fluctuations in odor concentration as detected by a PID adjacent to a source (red) and 30 cm downwind of a source (blue). Odor onset is indicated by the arrowhead. (F) Scatter plots of task performance (time at odor source when starting from a given location) as a function of average odor concentration detected by the PID at that location. Data are taken from the same mouse early (left, correlation coefficient = 0.66) and late (right, correlation coefficient = 0.11) in training. (G) Line plot showing correlation coefficients between mouse performance and odor concentration for all mice with black lines representing individual mice and the red line the group mean. In conditions with limited training on source locations (Early and Moved) performance was significantly better correlated with odor concentration (0.47 ± 0.03 for pooled early and moved conditions with 12 of 12 animals showing a significant correlation, 0.23 ± 0.09 for late training with 2 of 6 animals showing a significant correlation, p=0.032). (H) Performance of mice at the indicated distances from the odor source. Trials were divided into a group close to the source (0–20 cm) and a group far from the source (40–60 cm). Mice with limited exposure to the source locations (“Early” and “Moved”) performed significantly worse when navigating far from the source while mice with more experience with source locations (“Late”) did not (Ttarget for all conditions: Early: 12.31 ± 1.26 sec for close and 6.25 ± 0.48 sec for far; Moved: 16.48 ± 0.79 close and 12 ± 1.45 far; Late 15.85 ± 0.74 close and 14.28 ± 0.4 far, n = 6 mice for all conditions, significant decreases (p<0.05) are marked with an asterisk, Mann-Whitney U-test). (I) Distribution of initial distances of mice from the active source for each trial, across all 3 sources for each training condition. The distribution for the “Moved” training condition (far right) shows a peak relatively close to source locations necessitating careful selection of starting points for further analysis.
After a few days of training mice learned to locate odor sources and receive water (Figure 1B). Mice navigated to the source of both an odor dissolved in a solvent (10% Isoamyl acetate dissolved in Diethyl Phthalate) as well as the solvent alone (Diethyl Phthalate, which results in a far weaker signal). Importantly, when all odor cues were eliminated (by using an empty odor tube), mice were unable to perform the task (Figure 1C and Figure S1B) despite water being available at the correct source location. Performance reached a plateau after 12 sessions of training (Figure 1D and Figure S1). Both individual and average performances improved gradually (Figure S1), with no evidence for abrupt changes observed in other systems [25]. Application of additional “background” sources of the same odor scattered across the arena blocked the ability of mice to navigate to the correct odor sources, further confirming that mice relied on odor cues (Figure S2).
Mice shift strategies with increased experience of source locations
To examine how mice employed sensory cues to perform this behavior, we evaluated the distribution of odors, using direct measurement of odorants with a photo-ionization detector (PID; Figure 1E). Under the airflow conditions in the arena, odors were dispersed into fluctuating plumes by turbulent flow (Figure 1E, bottom). The odor signal was more intense close to the source and decreased with distance, with some anisotropy caused by the wind direction (Figure 1E). Location 1 was the source generating the smallest odor plume (due to being located near the exhaust) and was consistently the most challenging to track as mice learned the task (Figure S1D). Due to the lack of a plume from this source, we have used this data minimally. The two upwind source locations are intentionally asymmetrical, so as to generate different odor plumes (Figure 1E), as discussed in more detail later. For the upwind sources, the time to travel from any point in the arena to the odor source was correlated with the concentration of odor at that point for mice early in training (“Early” condition in Figure 1F and 1G). After several additional days of experience with the given odor source configuration, mice showed improved performance navigating from locations where odor was not detected by the PID (“Late” condition in Figure 1F and 1G) and to the downwind Location 1 generating the smallest plume (Figure S1D). To test whether this was an effect specifically of extended exposure to the given source configuration or rather due to general familiarity with the task we trained a second set of mice on a different configuration of odor sources (sources A, B, and C in the schematic of Figure 1A) and then moved the sources to the same locations as those tested for the first set of mice (Locations 1, 2, and 3). We found that these mice behaved as if they were in early training and navigated less effectively from areas where odor cues were weaker (“Moved” condition in Figure 1G). These results suggest that extended experience with a set of fixed resource locations resulted in mice navigating largely independently of dynamic odor cues. Indeed, while all mice with limited exposure to source locations showed performance that was significantly correlated with the distribution of odor, the performance of 5 of 6 mice with extended exposure to the source locations was significantly less correlated with odor concentration (Figure 1G, p = 0.0182, Mann-Whitney U-test). This transition in strategy to a seemingly less odor-guided behavior enabled mice to quickly navigate to odor sources even when starting from locations distant from the source (Figure 1H). However, even late in training, mice relied upon encounters with the odor near the source, as shown by a decrease in performance when odor cues were masked (Figure S2). We found that the distribution of starting locations was different across the sets of animals (Figure 1I), which skewed performance of the Moved condition to be similar to the Late condition (Figure 1D). Below we compare the two conditions after controlling for the initial locations.
To quantitatively compare the strategies used during the task, we next analyzed the trajectories that mice took to the odor source. In order to eliminate the bias given by the initial condition (Figure 1I), for this analysis we chose the trajectories that resulted when mice happened to start a trial in a small region in the center of the arena (“Start” in Figure 2A) and then tracked to one of the two upwind odor sources (sources 2 and 3). We classified the resulting paths to the odor source as direct (first approaching the correct source; Figure 2A, trajectory “D”) and indirect (first approaching the incorrect source; Figure 2A, trajectory “I”). We found marked differences that correlated with the animals' familiarity with the odor source locations. With limited experience of the source locations, m ice followed the airborne plume directly to the source (“Early” and “Moved” conditions, Figure 2B,C). In contrast, after extensive training with the source locations mice mostly ignored the plume and rapidly sampled the possible source locations. In this condition, mice used airborne plumes exclusively right next to port locations, to stop at the correct port. This resulted in significantly less direct approaches to the source location, closer to 50% (“Late” condition in Figure 2B,C). This was an adaptive strategy because despite the increased indirect approaches, mice travelled faster (Figure 2D,E) and arrived at the odor source more quickly (Figure 2F) since navigating using a priori information of source location freed mice from the need to sample and follow the plume [26]. Indeed, the movement of the mice during putative plume-based navigation exhibited significantly more pauses and lower velocity compared to trajectories from mice that were extensively trained on source locations (Figure 2D,E), consistent with a need to sample the odor as well as an increased cognitive load [27] due to plume following (see Movie S1). Mice late in training typically paused only when they reached possible odor sources (dip in median velocity in Figure 2D; see also Movie S1, Late Condition). As assayed by the animals’ velocity during the search, this change in behavior occurred gradually over several training sessions, without a sudden shift from one strategy to another (Figure 2G).
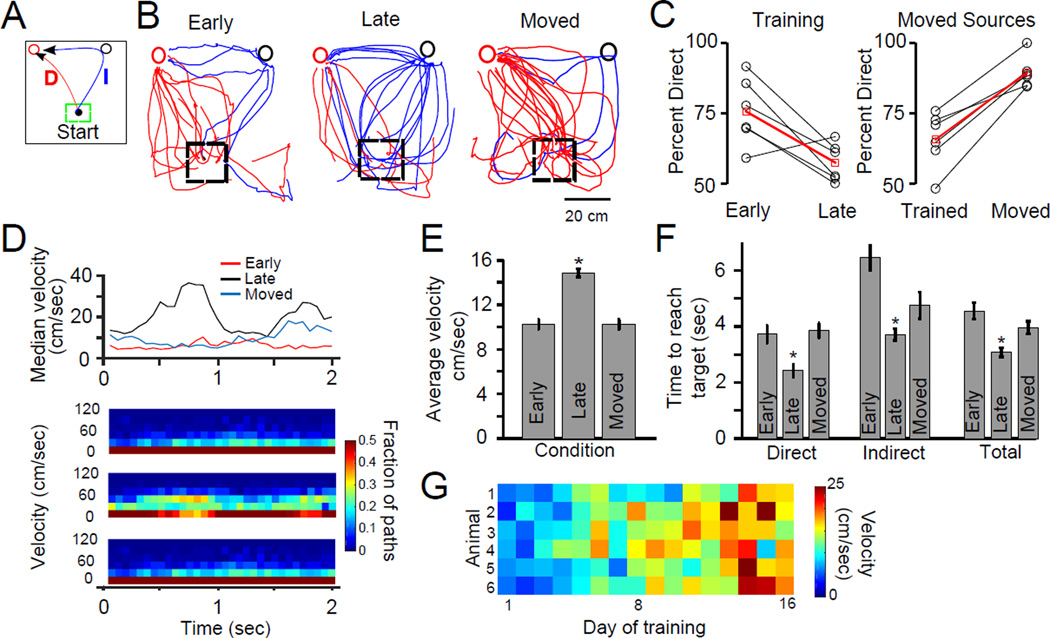
Figure 2. Mice use airborne plumes to directly navigate to odor sources when source locations are not well known.
For this figure we use trials where mice tracked Location 2 and 3 only. (A) Diagram illustrating possible trajectories. Only trials in which the mouse passed through the center location (indicated by dashed box) are included for analysis. From this location, the mouse could either proceed directly to the odor source location (red circle, path “D”), or it could check the inactive location first and then proceed to the odor source (black circle, path “I”). (B) Paths of mice as they navigate to the odor source (for “Early” and “Late” conditions all trajectories are from the same 2 mice). Mice with less familiarity with source locations (“Early and “Moved”) show frequent direct approaches to the odor source (red circle) without first checking the inactive source, while those with more experience randomly select which location to approach first. Individual trajectories are colored. (C) Left panel, line plot: Summary of the percent direct approaches to the odor source for mice early and late in training. Black lines represent individual mice and the population mean is the red line. Mice had a significantly higher percentage of direct approaches in early training (early: 81 ± 3%, late 63 ± 4%, p = 0.013). Right panel: The same analysis for mice that were well trained in the task on one configuration of odor sources (Trained, 69 ± 2%) and then challenged with a novel configuration (Moved, 91 ± 3%, p = 0.002). (D) Velocity profile of mice during the first 2 seconds of the search. Top panel: median values of velocity at each time point. Bottom panel: the same data expanded and shown as a series of histograms (colormap) for each 1/16 second time point. Mice well-trained on source locations show rapid movement at the start of the search while those less familiar with source locations move much more slowly. See also Figures S3 and S4. (E) The mean velocity late in training is significantly greater than that in the early and moved conditions (Early: average velocity = 10.24 ± 0.43 cm/sec, Moved average velocity: 10.23 ± 0.41, Late: average velocity = 14.85 ± 0.36, p< 0.001). (F) Time taken by mice to reach the odor source under the 3 different training conditions divided into direct approaches to the odor source (path “D” in panel a), indirect approaches (path “I” in panel a) and all trajectories (Total). When navigating to well-known sources mice performed significantly better (Statistics for total performance: Early: 4.56 ± 0.3 sec, Moved: 3.96 ± 0.23, Late: 3.08 ± 0.16; Late condition significantly less time to source compared to Early and Moved under all approach conditions, significance indicated by asterisk (p<0.05)). Mann-Whitney U-test was used to establish significance for all comparisons, data are shown as ± standard error of the mean. (G) Velocity for each mouse for the first 2 seconds of a search a veraged over each day of training. Only search trajectories that began at least 20 cm from a source were included. Velocity increased with training for all mice but did not show an abrupt state-shift. See also Figure S3 and Movie S1.
Decreased odor sampling in the latter period was also supported by the less frequent head rearing (a pause with the nose placed upward to sample the air) by mice that were well-trained on source locations (Figure S3). An increase in stereotyped search trajectories with training also occurred during free foraging between trials when no odor was present (Figure S3), which suggests a shift in overall foraging strategy by the mice towards rapid and organized searches near the known possible source locations. Indeed, when odor sources were moved, mice required multiple days of training to completely eliminate a preference to visit areas previously associated with reward (Figure S4). Our results demonstrate that mice initially followed plumes directly to odor sources and that with experience this cognitively demanding strategy, requiring moment-to-moment responses to time-varying stimuli, was abandoned in favor of rapid foraging informed by knowledge of possible source locations. While limiting the use of such demanding strategies is advantageous because these strategies consume more cognitive capacity [27], in this case the rapid, stereotyped search behavior was also the most efficient strategy for reaching the odor source and maximizing reward.
Modeling odor transport and searching behavior
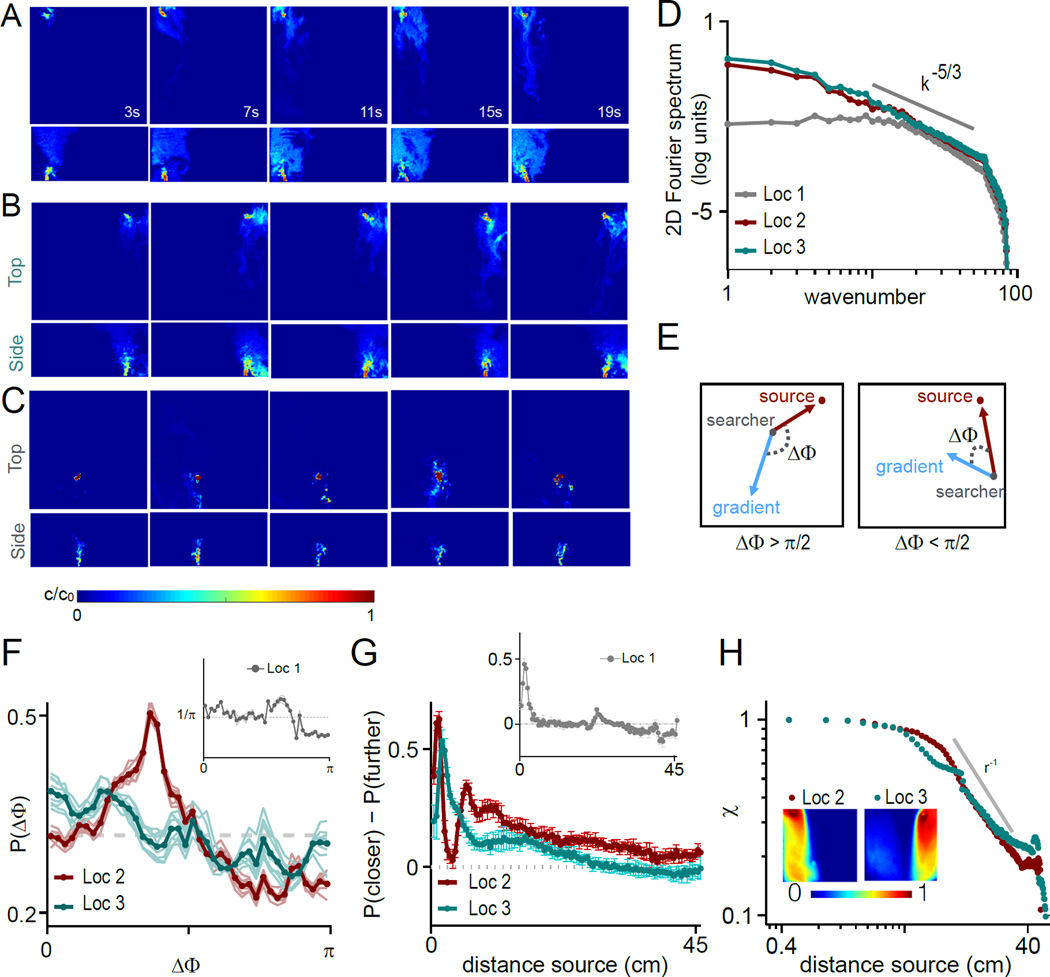
To gain insight into the searching behavior we observed in mice, we first characterized the dynamic odor plumes that mice employ to reach the source. We performed direct, 3-dimensional numerical simulations of the odor concentration in the arena, constraining the model with experimentally measured airflow velocities (see methods for details). In our simulations the odor is injected from one of the three ports represented in the sketch in Figure S5A. Figure 3A–C show a series of horizontal and vertical snapshots of the odor field, for one specific realization of the odor field emitted from Locations 1,2 and 3. Because of the stochastic nature of the air flow, the odor distribution varies for each trial. We simulated eight different realizations of the velocity field and odor concentration for each of the two upwind port locations (see Movies S2 and S3). Our simulation of odor flow correctly reproduces key aspects of experimental measures of odor plumes taken with PIDs. To compare the spatial distributions we correlated the time average of the signal obtained by PID with the time average of the simulated odor field after resampling the simulation at the points where PID measures were taken. For all 8 simulated realizations of each source, the correlation with the PID was significant (Source 2: average correlation coefficient 0.62 ± 0.019, Source 3: 0.69 ± 0.02, p<0.008, Wilcoxon signed rank test).
Figure 3. Odor concentration in the arena established through numerical simulations.
The signal fluctuates considerably, and displays the power law spectra typical of turbulent transport [16,28](Figure 3D). The plume generated from the downwind source is smaller, as seen from the small wave number cutoff of the spectrum (Figure 3D). Despite the complex nature of the signal, the gradient of the odor concentration carries information on the location of the source. To demonstrate this, we considered a putative searcher at a given location in space and time and traced two vectors from its location: the concentration gradient and the vector pointing to the source. When these two vectors overlap, the gradient points precisely toward the source. In general however this is not the case, as the concentration fluctuates widely in space and time and its gradient sometimes points toward the source and sometimes away from it. Hence when a searcher purely follows the concentration gradient, it performs a random walk. To establish whether the (possibly biased) random walk leads toward the source, we define with ΔΦ the angle between the concentration gradient and the vector pointing to the source. Two examples of a small and a large ΔΦ are sketched in Figure 3C, bottom panels: These correspond to instances where following the gradient the searcher gets a step closer to or further from the source respectively. In our simulations, we find that small angles are more probable than large angles for upwind sources (bias 0.18 ± 0.02 and 0.09± 0.02 for Location 2 and 3 respectively - Figure 3E), so that following the gradient the searcher eventually localizes the source. The concentration gradient, however, only contains information about source location at short ranges [19]. Consistently we observe that the bias toward the source decays with distance from the source (Figure 3F). The bias vanishes outside of the plume, at about 30 and 38 cm from Location 2 and 3 respectively (Figure 3F). The downwind source generates a small plume of about 5 cm, so that the distribution of ΔΦ is nearly flat (Figure 3E inset) and the bias vanishes few cm from the source (Figure 3F inset).
This analysis demonstrates that odor encounters are statistically informative. However, our plumes are intermittent, i.e. a searcher spends a considerable amount of time without encountering the odor. We quantify this property for the upwind sources by defining the intermittency factor χ as the fraction of time the signal is above zero, or above sensitivity in the simulations, at any given location [12]: χ = Ton/Toff. Here, Ton and Toff are, respectively, the time intervals over which the searcher does and does not experience an odor cue. The intermittency factor is 1 at the source and falls off with the distance r from the source (exponent close to −1 see Figure 3G); its average is close to 50% in the correct half of the arena and close to 0 in the wrong half of the arena (Figure 3G inset). As described below, we find that intermittency is the most severe constraint to source localization.
Gradient ascent is sufficient to localize the source in the absence of prior information
Since the odor gradient bears information about the location of the source, we focused on a gradient ascent algorithm. Through empirical optimization (see Supplemental Methods for details) we obtained the following algorithm:
| (1) |
where xt is the position of the searcher at time t; α is the step length i.e. the distance travelled in the time between subsequent sniffs and c(x,t) is the two dimensional horizontal snapshot of odor concentration at time t, computed at mouse nose height; the gradient ∇c is calculated at the current position of the searcher. We let 3000 searchers start at a random initial position. Each searcher continues to update its position according to eq (1) until it comes closer than a threshold ℓ to the correct source location. ℒ is the size of the region surrounding the port where the concentration is essentially undiluted and it is of the order of few cm. When the searcher does not experience an odor cue, it proceeds in straight lines in the direction of the previous step. Similar results hold when the behavior in the absence of odor is a random walk rather than a straight line, or if memory of the previous direction is only partial. Time is equally spaced in 150 time points spanning 20s, with frequency 7.5Hz comparable with experimental sniffing rates and we consider a constant speed ν = 20cm/s enforcing α = 2.5 cm. More complex behavior is left for future studies, where sniffing rates will be monitored.
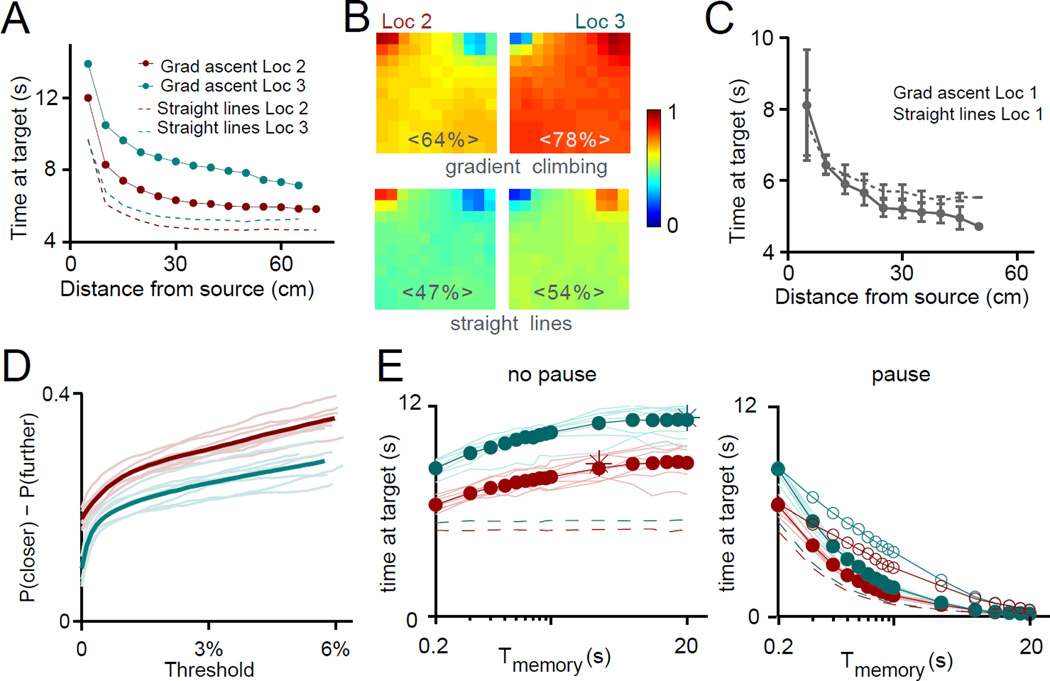
We find that the algorithm successfully localizes the upwind sources within 20 s in 57 ± 6 % and 73 ± 6 % of the trials for Location 2 and 3 respectively, with an average time at target of 6.4 ± 1.6 s and 8.5 ± 1.7 s respectively (Figure 4A). Straight lines alone eventually localize the source as well, since the arena is closed, but their performance is considerably poorer – successful localization in 45 ± 5 % and 50 ± 4 % of the trials for Location 2 and 3 respectively, with an average time at target of 4.9 ± 1.3 s and 5.5 ± 1.1 s respectively (Figure 4A). Additionally, straight lines track both sources with nearly equal probability, whereas gradient ascent tracks directly the correct location 71% of the times (Figure 4B). The algorithm is successful because following the fluctuating plume the searcher performs a biased random walk, and since the gradient points more often toward the source than away from it, the random walk is biased toward the source. Stochastic gradient ascent is a short-range strategy, since this bias vanishes further than about 30 – 38 cm from the source (Figure 3F). More sophisticated algorithms are required to navigate from larger distances, where tracking turbulent plumes becomes notoriously difficult [18]. Consistently, gradient ascent is largely ineffective when tracking the small plume generated by Location 1: it only outperforms slightly the straight-line behavior very close to the source (average time at target starting closer than 5 cm to the source is 9±6 s and 8±6 s respectively, p-value<10−20 - Figure 4C).
Figure 4. Performance of a simulated searcher using a gradient descent algorithm.
(A) Performance of the gradient ascent algorithm as a function of initial distance of the searcher from the source. The algorithm performs comparably to mice in the arena and best toward Location 3 that generates the largest plume. Gradient ascent toward the upstream sources always outperforms the straight line trajectories (dashed lines): for Location 2 and 3 respectively, average success rate for gradient ascent 57 ± 6 % and 73 ± 6 % vs 45 ± 5% and 50 ± 4% for straight trajectories (p<10−31); average time at target gradient ascent 6.4 ± 1.6 s and 8.5 ± 1.7 s vs 4.9 ± 1.3 s and 5.5 ± 1.1 s of the straight trajectories (p<10−34). (B) Fraction of direct localization as a function of initial position of the searcher. Performance is better for Location 3 (78.5 ± 1.1 %, top right) than for Location 2 (64.0 ± 1.5 %, left). This asymmetry is almost absent for the straight line behavior, for which fraction of direct localization is closer to 50% (46.5 ± 0.1 % and 53.6 ± 0.1 % for Location 2 and 3 respectively – bottom two panels). (C) Same as (A) for Location 1: due to the small plume generated by Location 1, the gradient ascent algorithm is inefficient. It only outperforms the straight line trajectories when the searcher starts within the first few cm from the source. (D) Bias, defined as P(closer) − P(further) as a function of a threshold measured as a fraction of the concentration at the source, below which gradients are discarded. Large gradients (higher thresholds in the plot) are more reliably biased toward the source than small gradients. (E) Performance of the algorithm increases with longer memory up to ~ 6 s, beyond which performance saturates. For infinite memory, adding a small perturbation is slightly advantageous, as it avoids getting trapped into local minima (star). For zero memory, adding a small perturbation is unnecessary, as turbulent fluctuations play the role of noise (data not shown). Right: performance when the mouse only updates its position once every time unit of Tmemory required to compute the average. Filled dots: the searcher always waits Tmemory before updating its position. Empty dots: the searcher only pauses when it gets the signal. In both cases, performance is monotonically decreasing with Tmemory: when accounting for the fact that the mouse has to pause to compute the average, averaging is never advantageous. See also Figure S6.
We found that simple ways to enhance reliability of the signal fail to improve performance. For example, although larger gradients are more reliable than small gradients (see Figure 4D), as already observed [12,29], simply avoiding response to sub-threshold gradients decreases performance, no matter how small the threshold is. Similarly, although long time averages are useful to smooth the signal (Figure 4E left), to compute static time averages mice would have to stop moving and average sequentially across sniffs. We find that it is never beneficial to pause and average the signal to decrease noise, no matter how long the averaging window (Figure 4E right). It is possible that the animals may be using more complex averaging algorithms while in motion: access to sniffing behavior will be the first step for future work that will generate answers to these questions.
Finally we compared performance toward the two upstream odor sources. Location 2 is slightly further from the midline, so that the resulting signal is slightly less fluctuating and more reliable than that coming from Location 3 (Figure 4D). However, Location 3 is easier to localize, with a mean time to reach the source about 1s faster than the time to reach Location 2 (Figure 4A). The reason for this seeming discrepancy is that because Location 3 is closer to the central air stream, odor is transported faster across the arena and the corresponding plume is slightly larger. Average first encounter time along the searchers’ trajectory is about 1s sooner than for odor coming from Location 2, which points to intermittency rather than reliability as a more severe constraint on performance.
Correlating mouse behavior to search algorithms
We next compared our search algorithms based on numerical simulations of the air flow and odor transport, to the trajectories taken by mice to reach the odor sources. Gradient ascent performs poorly when tracking the small plume generated by Location 1 (success rate of 47 ± 5% for gradient ascent vs 52 ± 4% for straight trajectories; time at target of 5.6 ± 1.4 s for gradient ascent vs 5.9 ± 1.1 s for straight trajectories - Figure 4C). Consistently, mouse performance toward Location 1 is poorer than toward the upwind sources early in training when the odor matters (Figure S1D; 9.86 ± 0.76 s time at target for upwind sources, 6.33 ± 0.68 s for downwind source, p=0.0027). The difference disappears later in training when the structure of the odor plume becomes irrelevant as the animals rely on prior information on source location (16.43 ± 0.67 s for upwind sources, 15.25 ± 0.67 s for downwind source, p=0.16). Given the small plume for source 1, for the next analyses (Figures 5 and 6) we focused on data for Locations 2 and 3 only. We found that mice that were less familiar with source locations travelled longer within the regions of the arena that corresponded to frequent contact with odor plumes than mice that had more experience of the source locations (Figure 5A–C). This behavior is consistent with a more prevalent use of odor-guided strategies in the absence of prior information on source location. Indeed, performing the gradient ascent algorithm, the simulated searchers experience fluctuating odor cues (Figure 5D - right), whose average is smoothly increasing as they approach the source (Figure 5D - left); while the increase is abrupt for the straight-line trajectories that ignore the odor cues (Figure 5D - left). This observation can be used to quantify the amount of time animals stay in the plume, and thus potentially extract information from the airborne odor signal.
Figure 5. Comparison of mouse search trajectories t o simulated plume distributions.
(A) Left panel: In order to calculate probable odor concentrations along each trajectory, measured mouse trajectories were merged with a dynamic simulation of odor flow from sources 2 and 3. Odor concentrations are displayed as the log of the signal. Right 3 panels: Estimated concentration (log units) of odor along the paths used to approach the odor source when the source is less well-known (“Early” and “Moved”) and late in training with well-known sources (“Late”). The last 2.5 seconds before arrival at the source are shown. Trials are sorted within each condition according to the amount of time spent in the plume. (B) The curves shown are the average for each condition shown in panel g. (C) Histograms of the time spent in the odor plume for each condition. Mice spent significantly longer within the plume when navigating in epochs where source locations were not well-known (Early: 2.6 ± 0.03 sec; Moved: 2.52 ± 0.19 sec; Late 1.6 ± 0.15 sec; Late significantly less time in plume than Early and Moved, p< 0.0001, Mann-Whitney U-test; ± standard error of the mean). (D) Left panel: Average odor concentration along the simulated searcher trajectories. Solid lines: searchers performing the gradient climb algorithm; dotted lines: searchers performing straight line trajectories that ignore the odor. (E) Odor traces normalized by odor at target, experienced by few representative searchers along their trajectory for Location 2 (bottom three) and Location 3 (top three). Grey lines represent blanks. Considering all searchers, the average intermittency defined as the fraction of time the searcher receives a non-vanishing signal is 66%.
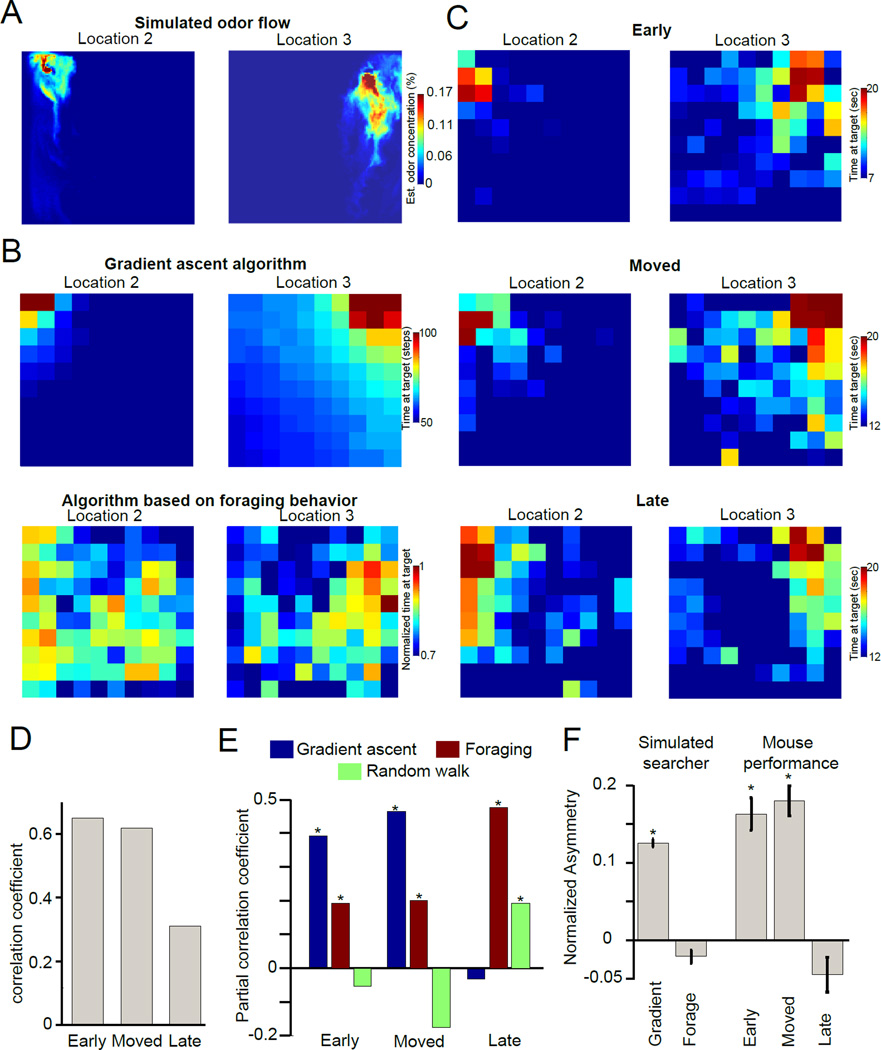
Figure 6. A switch in search behavior drive n by prior information.
(A) Horizontal snapshots of odor concentration at mouse nose height (3 cm, estimated from mouse behavior monitored in 3 dimensions) of one realization of simulated flow from the indicated odor source location (12 seconds after odor release; see Figure 4 and Movie S2). (B) Average time spent at odor source by simulated searchers starting at different locations using a modified gradient ascent algorithm (top panels; see text and Supplementary Methods for description of the algorithm) and a non plume guided strategy based on repetitive foraging behavior (bottom panels). (C) Average time spent at the odor source location when mice begin at various locations in the arena. Data is shown for different training sessions. Top panels show early training, middle panels show well-trained mice that have been trained on a different constellation of source locations and are new to these odor source locations, and the bottom panels are mice that have been well-trained with these odor source locations. (D) Correlation coefficients between mouse performance and the gradient ascent algorithm. All conditions show significant correlations (Early: 0.65, Moved 0.62, Late 0.31) due to all measures having some correlation with distance from the source. (E) Partial correlation coefficients for the time to source in each training condition correlated with the gradient ascent algorithm (panel b), the non plume-guided sequential foraging strategy, and random walk. Correlation coefficients significantly greater than zero are indicated with asterisks (p<0.00001). (F) Asymmetry of odor search performance defined as the difference between the time at odor source 2 and the time at odor source 3, with all values divided by the maximum possible time at the source (gradient ascent asymmetry: 0.13 ± 0.005; sequential foraging asymmetry: −0.02 ± 0.009; early condition asymmetry: 0.16 ± 0.02; moved condition asymmetry: 0.18 ± 0.019; late condition asymmetry: −0.04 ± 0.022; statistics across grid values (n = 100), conditions with values significantly greater than foraging behavior are indicated (Mann-Whitney U-test). Error bars indicate standard error of the mean.
Our results suggest that mice use both sensory cues and prior information of source location to solve the task and that the prior becomes stronger with more sessions of training. We next compared two limiting cases, where behavior is entirely dominated by either the sensory cues or prior experience. The gradient ascent algorithm discussed above is the first limiting case, in which odor cues are used exclusively to track the plume to its source, with no prior information about source location (Figure 6A,B). The second model ignored the odor plume and only stopped the search when a high concentration of odor was encountered at a possible source location (Figure 6B, bottom). To generate the spontaneous search behavior of the mouse in the second model we employed the foraging behavior of the mice between trials (when odor was not present). As mice forage more often near the sources, this behavior can be considered as representative of their prior information on source location. In fact, as the training proceeds, mice develop a stereotyped foraging behavior that involves sequentially sampling each source location (Figure S3), somewhat similar to the enhanced foraging that is observed with practice during an auditory search task [30]. We then compared the performance of these models to actual mouse behavior, measured as the time at the target odor source from a grid of starting locations (Figure 6C). All measures were somewhat correlated, since each has a trivial dependence on distance from the source (Figure 6D; Early: 0.65, Moved 0.62, Late 0.31). We thus used partial correlation analysis and found that only under conditions with limited exposure to source locations was mouse performance significantly correlated with the gradient ascent algorithm (Figure 6E; Early 0.39, p <0.00001; Moved 0.47, p <0.00001; Late −0.03, p= 0.64). To further confirm these findings we took advantage of the asymmetry of the odor cues in our arena (due to more effective transport of odor from location 3). We found that the performance of the gradient ascent algorithm was significantly better towards the odor source that provided a larger plume. This was also the case for mouse performance when mice had less experience with the source locations, but the asymmetry was eliminated with experience (Figure 6F; gradient ascent asymmetry: 0.13 ± 0.005; sequential foraging asymmetry: −0.02 ± 0.009; early condition asymmetry: 0.16 ± 0.02; moved condition asymmetry: 0.18 ± 0.019; late condition asymmetry: − 0.04 ± 0.022, with gradient ascent, early, and moved conditions p<0.05 when compared to foraging).
DISCUSSION
Both dynamic sensory cues from the environment as well as previously learned information inform decision-making during complex behaviors such as foraging and navigation [1,2]. The strategies adopted to perform these behaviors arise from a balance that must be struck between flexibly interpreting and following sensory cues and efficiently performing a task guided by information gained from experience [3]. In the current study, we investigated how mice weigh these two factors during odor-guided navigation using natural, fluctuating airborne odor plumes. We found that while mice were capable of following dynamic odor plumes, as they learned probable odor source locations they shifted their strategy to weight prior information more heavily. The gain in efficiency from using prior information allowed mice to more effectively exploit odor cues and find reward sources more quickly.
There is currently little information available on how terrestrial animals track airborne odors [14,31], with most information coming from studies using invertebrates [15]. Here, we used controlled laboratory conditions to demonstrate that mice can make use of airborne odor cues to locate a source. This type of odor tracking is notoriously difficult, as under natural conditions, even with little wind, airborne cues will rarely be transported by laminar flow [12]. We have shown that under conditions of frequent plume contact, as might occur within a patch of food items [9] simple gradient- based algorithms could be used to inform a biased random walk and locate odor sources using fluctuating airborne plumes. Further extensions of the task presented here, with more challenging situations and with measurement of sniffing behavior, are necessary to establish which algorithms are used under various behavioral and environmental conditions. For example, mice could sense air motion with their whiskers and preferentially move upwind upon encounter with the odor, an anemotactic behavior that has been well characterized in insects [21–23]. The cost function in our algorithm can be modified to include these effects, and the qualitative result is that performance improves. Moreover, downwind motion, which occasionally occurs when purely following the gradient, decreases (data not shown). As instances of downwind motion are rarely observed in the experiments, additional cues, including wind direction, are likely to be relevant for this task. Note also that in the wild, mice use odors to navigate within plant canopies, where interaction of vegetation with the wind considerably modifies the turbulence [32]. Further work is needed to establish how these findings using a flat arena apply to such conditions and how performance depends on the properties of the fluctuating plumes (e.g. Reynolds number).
Importantly, we have designed our experiments and performed key controls to demonstrate that mice are indeed using airborne odor cues to locate the odor sources. We have eliminated vision by performing all experiments under dim, far red light. This range of light is invisible to mice [24]. We eliminated spatial auditory cues by co-localizing all valves used to control the release of odors 1 meter from the behavioral arena. We confirmed that mice were unable to localize the reward locations using other modalities by performing a key set of control experiments in which we used clean air from empty odor tubes as the stimulus. In these conditions none of the mice were able to navigate to the reward source, providing evidence that odor cues were an indispensable part of the behavior (Figure 1C). Finally, we showed that constant background sources of odor were capable of masking the reward source (Figure S2), further confirming that mice were using odor cues to perform the task.
An important and novel point of our work is that mice shift their strategy with increased knowledge of source locations, foregoing sensory-based navigation to perform an exhaustive search strategy. Since the arena was sm all and the source locations fixed, mice were able to systematically forage these locations quickly. Foraging to known locations was likely supported by an animal’s own odor traces [33], as the arena was cleaned prior to each animal’s session, but not between trials of the same animal (Figure S3G). Self-movement sensed through the vestibular and proprioceptive systems could also provide input to allow for dead-reckoning [34]. Finally, tactile cues sensed through the whiskers, including position of the walls and airflow may contribute to non-plume-guided navigation as well [35–37]. This stereotyped foraging strategy led mice to the source more quickly, on average, than an odor based strategy, which requires more computation and is subject to the stochastic whims of the airflow. As discussed above, such a strategy may reflect a general shift in the balance between flexibility and speed [1]. Indeed, rodents shift navigation strategies from flexible, “place” based strategies to more habitual strategies with training in a T-maze [38] or the Morris water maze [39]. Inactivation and developmental studies further suggest that these two strategies may reflect the recruitment of two different networks in the brain [39,40]. Our behavioral assay offers an excellent basis to address the time course and factors influencing this balance between strategies. In fact, in our “Moved” condition, mice continue to forage near old source locations for days (Supplementary Figure 5), offering evidence for persistent behaviors even in the face of change. In future experiments, the arena could have some predictable source locations with low value rewards and other unpredictable (on a trial-by-trial basis) sources with higher value rewards. This might help test hypotheses about how mice weigh different options and strike a balance.
Conclusion
Our results show that as mice acquire information, they adaptively shift their decision-making strategies away from flexible yet cognitively-demanding, sensory-driven searches to more efficient stereotyped foraging. This work builds the foundation for studies addressing a wide range of fundamental issues including the neural correlates of complex sensory-driven behaviors [1,2]. Additionally, we have shown that in this turbulent regime, the odor gradient provides noisy information sufficient for short-range navigation to an odor source. Taken together, our results provide the conceptual framework to combine the recently advanced understanding of odor-driven behavior [8,10,41] and neural coding [42] in rodents with the complex dynamics present in odor plumes in order to define possible algorithms [18,43,44] and neural circuit computations [45] for natural plume tracking [15] during complex decision-making.
EXPERIMENTAL PROCEDURES
Odor navigation task
The navigation arena was a fully enclosed acrylic box measuring 0.6 meters per side and 0.3 meters tall, with openings only for air inflow and exhaust. On each trial, one of 3 possible locations emitted an odor (see Figure 1A), which signaled that water was available at the water spout located adjacent to that odor source. If the mouse arrived at that odor source while odor was still being emitted (20 seconds), it received water following a 1.5 second wait at the water spout. This length of time was empirically chosen because mice did not typically pause for more than 1 second at a given odor source in the absence of an odor cue. This eliminated guessing (see Figure 1C). Following this 1.5 second initial wait, mice received a small drop of water (approx. 5 microliters) every second that they remained at the spout while odor was emitted from the source (approx. 60 microliters of water per successful trial for well-trained mice). This schedule of reward gave the mice an incentive to arrive as quickly as possible since they received more water the longer they were present at the source. For each trial the odor-emitting location was pseudo randomly assigned such that all locations were used an equal number of times every 9 trials. A minimum of 25 seconds without odor passed before the next trial began. This time was used to cycle the air in the arena and eliminate odor left from the previous trial (odor elimination was confirmed by odor measurements using a PID). During this time between trials the mouse remained in the arena and was allowed to freely explore (Figure S3). In some experiments, time between trials was determined with an exponential function such that the hazard rate for trial initiation was kept uniform from 25 seconds to 60 seconds while in other experiments time between odor off in one trial and odor on during the next was exactly 30 seconds. We did not observe a substantial difference in behavior in these two conditions, perhaps because freely moving mice did not always encounter the odor with a consistent latency from the previous trial even with the fixed 30 second interval.
Behavioral training
A total of 12 c57bl/6 mice (Charles River) were trained on the odor navigation task. Following water deprivation, a mouse was introduced to the arena and allowed to freely explore. The typical task was run during this phase, alternating at least 25 seconds of pre-odor time with 20 seconds of odor per trial. On the first 2 days of training, mice received water if they approached an odor source and remained at the water spout for 500 ms, which allowed mice to quickly establish an odor/water association. Once mice exhibited odor approach behavior, the time they had to wait to receive water was gradually increased over the next 2 days such that by the 5th day of training, mice were required to wait for 1.5 seconds to receive water. Water consumption was monitored throughout training and mice that did not receive a minimum of 1 ml of water per session were supplemented to this total following the session. Each session continued until 40 trials had been completed or until a mouse exhibited satiation (stopped performing the task). Data for a session was included in subsequent analysis only if the mouse was active and consistently performed the task. For the entire data set, two sessions (out of 204) were excluded for not meeting these criteria.
Behavioral data analysis
All analysis was performed using MATLAB (MathWorks). For all data, group comparisons were made using the non-parametric Mann-Whitney U-test with correction for multiple comparisons done using false discovery rate control (FDR). To examine correlations between algorithm performance and mouse performance in the arena (Figure 6D), partial correlation coefficients were calculated and correlations greater than zero were found using a Students t distribution. For clarity of display, all bar graphs are presented as mean ± standard error of the mean, although no assumptions of normality were made when making group comparisons.
Fluid dynamics simulations
We simulated the flow and odor transport in the arena through direct numerical simulations, in a three dimensional periodic domain 128×128×64. All airflow parameters were either known or experimentally constrained using data obtained with an anemometer. The Supplemental Methods provides detailed information regarding our implementation of the pseudospectral code used to integrate the Navier-Stokes equations and obtain odor transport statistics in the arena.
Source Localization Algorithm
We used a generic gradient ascent algorithm because the odor gradient bears information about the location of the source (Figure 4). We use a horizontal cut of the simulated odor concentration at mouse nose height and simulate the trajectories of many searchers that climb the gradient of the signal, starting from a random location in the arena. The Supplemental Methods contain details of the implementation and optimization of this algorithm leading to eq (1).
Supplementary Material
Highlights.
Mice can efficiently track odor sources using air-borne fluctuating plumes
Gradient-based algorithms are consistent with tracking behavior close to source
With knowledge of source locations, mice shift strategy to stereotyped foraging
Acknowledgments
We thank Bingni Brunton, Thomas Finger, Sam Gershman, Julian Meeks and Diego Restrepo for helpful discussion and Peter Brunjes and members of the Murthy lab for comments on the manuscript. Funding provided by NIH/NIDCD grants K99 DC013305 (DHG) and R01 DC011291 (VNM) and by Harvard University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS DHG and VNM initiated the study and designed the experiments. VK and DHG built the olfactometer and navigation arena and wrote the behavioral monitoring software. AAA and DHG performed all experiments. AS performed all airflow and odor concentration simulations and generated all figures reporting simulation results. AS developed the gradient ascent algorithm with input from DHG. DHG performed analysis of behavioral data with input from AS, VK and VNM. DHG generated all figures reporting experimental data. DHG, AS, and VNM wrote the text. All authors provided editing suggestions for the figures and text.
REFERENCES
- 1.Dolan RJ, Dayan P. Goals and Habits in the Brain. Neuron. 2013;80:312–325. doi: 10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 3.Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4 doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whishaw IQ, Gorny B. Path integration absent in scent-tracking fimbria fornix rats: evidence for hippocampal involvement in “sense of direction” and “sense of distance” using self-movement cues. J. Neurosci. 1999;19:4662–4673. doi: 10.1523/JNEUROSCI.19-11-04662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Means LW, Alexander SR, O’Neal MF. Those cheating rats: male and female rats use odor trails in a water-escape “working memory” task. Behav. Neural Biol. 1992;58:144–151. doi: 10.1016/0163-1047(92)90387-j. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DG, Gorny B, Whishaw IQ. Rats can track odors, other rats, and themselves: implications for the study of spatial behavior. Behavioural Brain Research. 2002;131:185–192. doi: 10.1016/s0166-4328(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 7.Whishaw IQ, Gorny BP. A video demonstration of preserved piloting by scent tracking but impaired dead reckoning after fimbria-fornix lesions in the rat. J Vis Exp. 2009 doi: 10.3791/1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav. Brain Res. 1999;99:143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- 9.Howard WE, Marsh RE, Cole RE. Food detection by deer mice using olfactory rather than visual cues. Anim Behav. 1968;16:13–17. doi: 10.1016/0003-3472(68)90100-0. [DOI] [PubMed] [Google Scholar]
- 10.Khan AG, Sarangi M, Bhalla US. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun. 2012;3:703. doi: 10.1038/ncomms1712. [DOI] [PubMed] [Google Scholar]
- 11.Porter J, Craven B, Khan RM, Chang S-J, Kang I, Judkewitz B, Judkewicz B, Volpe J, Settles G, Sobel N. Mechanisms of scent-tracking in humans. Nat. Neurosci. 2007;10:27–29. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- 12.Celani A, Villermaux E, Vergassola M. Odor Landscapes in Turbulent Environments. Phys. Rev. X. 2014;4:041015. [Google Scholar]
- 13.Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- 14.Catania KC. Stereo and serial sniffing guide navigation to an odour source in a mammal. Nat Commun. 2013;4:1441. doi: 10.1038/ncomms2444. [DOI] [PubMed] [Google Scholar]
- 15.Cardé RT, Willis MA. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008;34:854–866. doi: 10.1007/s10886-008-9484-5. [DOI] [PubMed] [Google Scholar]
- 16.Falkovich G, Gawȩdzki K, Vergassola M. Particles and fields in fluid turbulence. Rev. Mod. Phys. 2001;73:913–975. [Google Scholar]
- 17.Shraiman BI, Siggia ED. Scalar turbulence. Nature. 2000;405:639–646. doi: 10.1038/35015000. [DOI] [PubMed] [Google Scholar]
- 18.Vergassola M, Villermaux E, Shraiman BI. “Infotaxis” as a strategy for searching without gradients. Nature. 2007;445:406–409. doi: 10.1038/nature05464. [DOI] [PubMed] [Google Scholar]
- 19.Balkovsky E, Shraiman BI. Olfactory search at high Reynolds number. PNAS. 2002;99:12589–12593. doi: 10.1073/pnas.192393499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy JS, Marsh D. Pheromone-regulated anemotaxis in flying moths. Science. 1974;184:999–1001. doi: 10.1126/science.184.4140.999. [DOI] [PubMed] [Google Scholar]
- 21.Dusenbery DB. Upwind searching for an odor plume is sometimes optimal. J. Chem. Ecol. 1990;16:1971–1976. doi: 10.1007/BF01020509. [DOI] [PubMed] [Google Scholar]
- 22.Sabelis MW, Schippers P. Variable wind directions and anemotactic strategies of searching for an odour plume. Oecologia. 1984;63:225–228. doi: 10.1007/BF00379881. [DOI] [PubMed] [Google Scholar]
- 23.Mafra-Neto A, Cardé RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 24.S Lipman N. Chapter 9 - Design and Management of Research Facilities for Mice. In: Smith JGFTDWQWBENL, editor. The Mouse in Biomedical Research. Second. Burlington: Academic Press; 2007. pp. 271–319. [Google Scholar]
- 25.Bazhenov M, Huerta R, Smith BH. A computational framework for understanding decision making through integration of basic learning rules. J. Neurosci. 2013;33:5686–5697. doi: 10.1523/JNEUROSCI.4145-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace DG, Whishaw IQ. NMDA lesions of Ammon’s horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur. J. Neurosci. 2003;18:513–523. doi: 10.1046/j.1460-9568.2003.02772.x. [DOI] [PubMed] [Google Scholar]
- 27.Otto AR, Gershman SJ, Markman AB, Daw ND. The curse of planning: dissecting multiple reinforcement-learning systems by taxing the central executive. Psychol Sci. 2013;24:751–761. doi: 10.1177/0956797612463080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisch U, Kolmogorov AN. Turbulence: The Legacy of A.N. Kolmogorov. Cambridge University Press; 1995. [Google Scholar]
- 29.Mydlarski L, Pumir A, Shraiman BI, Siggia ED, Warhaft Z. Structures and Multipoint Correlators for Turbulent Advection: Predictions and Experiments. Phys. Rev. Lett. 1998;81:4373–4376. [Google Scholar]
- 30.Whitton JP, Hancock KE, Polley DB. Immersive audiomotor game play enhances neural and perceptual salience of weak signals in noise. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E2606–E2615. doi: 10.1073/pnas.1322184111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharyya U, Bhalla US. Robust and rapid air borne odor tracking without casting. 2015 doi: 10.1523/ENEURO.0102-15.2015. Eneuro ENEURO.0102–0115.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnigan J. Turbulence in Plant Canopies. Annual Review of Fluid Mechanics. 2000;32:519–571. [Google Scholar]
- 33.Save E, Nerad L, Poucet B. Contribution of multiple sensory information to place field stability in hippocampal place cells. Hippocampus. 2000;10:64–76. doi: 10.1002/(SICI)1098-1063(2000)10:1<64::AID-HIPO7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DG, Hamilton DA, Whishaw IQ. Movement characteristics support a role for dead reckoning in organizing exploratory behavior. Anim Cogn. 2006;9:219–228. doi: 10.1007/s10071-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 35.Arkley K, Grant RA, Mitchinson B, Prescott TJ. Strategy Change in Vibrissal Active Sensing during Rat Locomotion. Current Biology. 2014;24:1507–1512. doi: 10.1016/j.cub.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Gener T, Perez-Mendez L, Sanchez-Vives MV. Tactile modulation of hippocampal place fields. Hippocampus. 2013;23:1453–1462. doi: 10.1002/hipo.22198. [DOI] [PubMed] [Google Scholar]
- 37.Clark BJ, Hamilton DA, Whishaw IQ. Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: modification of movement distribution, distance, location, and speed. Physiol. Behav. 2006;87:805–816. doi: 10.1016/j.physbeh.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Redhead ES. Evidence for a shift from place navigation to directional responding in one variant of the Morris water task. J Exp Psychol Anim Behav Process. 2009;35:271–278. doi: 10.1037/a0013260. [DOI] [PubMed] [Google Scholar]
- 40.Akers KG, Candelaria-Cook FT, Rice JP, Johnson TE, Hamilton DA. Delayed development of place navigation compared to directional responding in young rats. Behav. Neurosci. 2009;123:267–275. doi: 10.1037/a0014594. [DOI] [PubMed] [Google Scholar]
- 41.Kulvicius T, Tamosiunaite M, Ainge J, Dudchenko P, Wörgötter F. Odor supported place cell model and goal navigation in rodents. J Comput Neurosci. 2008;25:481–500. doi: 10.1007/s10827-008-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchida N, Poo C, Haddad R. Coding and transformations in the olfactory system. Annu. Rev. Neurosci. 2014;37:363–385. doi: 10.1146/annurev-neuro-071013-013941. [DOI] [PubMed] [Google Scholar]
- 43.Masson J-B. Olfactory searches with limited space perception. Proc. Natl. Acad. Sci. U.S.A. 2013;110:11261–11266. doi: 10.1073/pnas.1221091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grünbaum D, Willis MA. Spatial memory-based behaviors for locating sources of odor plumes. Mov Ecol. 2015;3:11. doi: 10.1186/s40462-015-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riffell JA, Shlizerman E, Sanders E, Abrell L, Medina B, Hinterwirth AJ, Kutz JN. Sensory biology. Flower discrimination by pollinators in a dynamic chemical environment. Science. 2014;344:1515–1518. doi: 10.1126/science.1251041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.