Abstract
The frequent co-occurrence of antisocial behavior and other disinhibited phenotypes reflects a highly heritable externalizing spectrum. We examined the molecular genetic basis of this spectrum by testing polygenic associations with psychopathology symptoms, impulsive traits, and cognitive functions in two samples of primarily military veterans (n =537, n =194). We also investigated whether polygenic risk for externalizing moderated the effects of trauma on these phenotypes. As hypothesized, polygenic risk positively predicted externalizing psychopathology and negatively predicted performance on inhibitory control tasks. Gene-by-environment effects were also evident, with trauma exposure predicting greater impulsivity and less working memory capacity, but only at high levels of genetic liability. As expected, polygenic risk was not associated with internalizing psychopathology or episodic memory performance. This is the first independent replication of the polygenic score as a measure of genetic predispositions for externalizing and provides preliminary evidence that executive dysfunction is a heritable vulnerability for externalizing psychopathology.
Keywords: genes, veterans, inhibitory control, working memory, internalizing, impulsivity
Research has consistently demonstrated that a common dimension, termed the externalizing spectrum, underlies the co-occurrence of antisocial behavior, substance and alcohol use disorders, (lack of) constraint, novelty seeking, and childhood disruptive disorders (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002). Extensive research implicates a propensity for behavioral disinhibition, or the tendency to act recklessly and impulsively, as the defining feature of this spectrum (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Kendler et al., 2003). For example, disinhibition manifesting as poor impulse control and a failure to inhibit socially undesirable behaviors are well-established risk factors for early conduct problems and engagement in adult antisocial behavior (e.g., Babinski, Hartsough, & Lambert, 1999; Luengo, Carrillo-De-La-Pena, Otero, & Romero, 1994), both of which are core components of the externalizing spectrum. Although not all antisocial behavior is characterized by this type of disinhibited profile (c.f., psychopathic fearless-dominance and callous-unemotional traits; Blonigen, Hicks, Krueger, Patrick, & Iacono, 2005; Frick & White, 2008), externalizing is a consistently strong predictor of chronic and severe antisocial trajectories and related problem behaviors (Krueger et al., 2002). Thus, it represents a potent etiological pathway for a range of harmful behaviors stemming from problems with disinhibition, including criminal behavior, reactive aggression, risk-taking, and drug problems.
A number of studies indicate that the externalizing spectrum is highly heritable and represents a predominately genetic vulnerability that manifests as distinctive phenotypes (e.g., as drug abuse or antisocial behavior; Kruger et al., 2002). This evidence comes from a number of twin studies, which estimate that genetic effects account for 70–80% of the comorbidity among the externalizing spectrum (Krueger & Markon, 2006; Wolf et al., 2010). Efforts to isolate specific genetic mechanisms that predispose to externalizing have yielded several promising candidates (e.g., Caspi et al., 2002; Dick, 2007; Kendler et al., 2012; Logue et al., 2013, Sadeh, Javdani, & Verona, 2013). Replication failures in genetic association studies have been common (Duncan & Keller, 2011; Vassos, Collier, & Fazel, 2014), however, and it is becoming increasingly apparent that it is highly unlikely a single common variant will explain genetic liability for externalizing given its phenotypic complexity. Polygenic modeling of genetic susceptibility is more theoretically consistent with the etiology of complex phenotypes, like the broad externalizing spectrum, because this approach measures the additive effects of many thousands of common genetic variants across the genome
Recently, Salvatore and colleagues (2015) conducted the first polygenic association study of externalizing psychopathology using a polygenic risk score derived in a sample of 1,249 adults with a history of alcohol dependence. The authors found that the polygenic score accounted for approximately 6% of the variance in externalizing psychopathology and 2–7% of the variance in other disinhibited phenotypes (e.g., impulsivity, sensation-seeking) among adolescents and young adults. Gene-by-environment effects were also evident such that polygenic influences on externalizing were stronger in cases of low parental monitoring and high peer substance use. These data provide insight into how additive genetic risk for externalizing unfolds across development and how it interacts with environmental conditions to increase risk for externalizing. However, given the novelty of these data, the replicability, generalizability, and specificity of the observed polygenic effects for externalizing still needs to be established. In particular, the ability of the risk score to predict externalizing psychopathology in an independent sample of adults has yet to be tested. Further, based on behavioral genetics research showing that there are shared genetic effects between externalizing and internalizing (e.g., Wolf et al., 2010), it is necessary to examine the specificity of the risk score for explaining externalizing psychopathology in particular, which was not examined in the original study.
An important next step in validating the externalizing polygenic risk score is to identify intermediate mechanisms that are positioned between genetic variation and the phenotype, such as heritable cognitive or neurobiological vulnerabilities. Isolating such mechanisms would greatly advance etiological models of externalizing by pointing to specific causal pathways that link genes to the development of psychopathology. Despite the promise this approach holds, very little research to date has examined the mechanisms by which genetic liability confers risk for externalizing, and no data are currently published in relation to the externalizing polygenic risk score. One mechanism by which genes may confer susceptibility for the development of externalizing is through executive functions, which are highly heritable cognitive processes (estimates over .75; Miyake & Friedman, 2012) that are found to be impaired in disinhibited and externalizing individuals (Finn et al., 2009; Iacono, Malone, & McGue, 2008; Nigg, 2000). Thus, dysfunction in these processes may represent an inherited vulnerability for the development of externalizing. Consistent with this hypothesis, Young and colleagues (2009) found that externalizing in early and late adolescence predicted poorer performance on tasks of response inhibition (i.e., antisaccade task, stop-signal task, Stroop task) at age 17 in twin pairs. This association was almost entirely genetic in nature (heritability estimate = .61), suggesting a shared biological vulnerability for executive dysfunction was an inherited liability for the development of externalizing psychopathology. Similarly, Finn and colleagues have shown that worse performance on tasks of working memory (e.g., auditory consonant trigram test) and short-term memory (e.g., digit span test) are associated with externalizing problems in adolescents and adults (Bogg & Finn, 2010; Finn et al., 2009). Based on these and similar findings, deficits in inhibitory control and working memory capacity are cognitive processes by which genes may confer risk for externalizing, making them promising candidates as intermediate mechanisms.
Finally, a large body of research indicates that exposure to certain environmental conditions interacts with genetic predispositions to confer risk for externalizing. One of the most widely studied and strongly linked environmental risk factors for externalizing psychopathology is exposure to traumatic events (e.g., Douglas et al., 2010; Luntz & Widom, 1994; Miller, Greif, & Smith, 2003; Wilson, Stover, & Berkowitz, 2009), making it an important environmental moderator to consider in models of polygenic risk. For example, a number of studies have found that the prevalence of externalizing psychopathology is significantly higher in samples saturated by trauma, including criminal-justice-involved persons and military veterans (Elbogen et al., 2014; Wolff et al., 2011; Wolff, & Shi, 2012; Wright, Foran, Wood, Eckford, & McGurk, 2012). The prevalence of trauma exposure is estimated to be 18 to 27 times higher among inmates than the general population (e.g., Crisanti & Frueh, 2011), underscoring the importance of environmental adversity for antisocial behavior and other externalizing outcomes. Furthermore, moderation effects are frequently observed in genetic studies such that the risk traumatic experiences confer for externalizing psychopathology is greater at higher levels of genetic liability. For example, using a behavioral genetics design, genetic risk for the latent externalizing spectrum was consistently found to be higher in the context of six different types of environmental adversity (e.g., antisocial peers, stressful life events) in a study of 1315 twin pairs age 17 (Hicks, South, DiRago, Iacono, & McGue, 2009). Given that trauma is a risk factor common to disorders on the externalizing spectrum (Greenwald, 2002; Miller, Fogler, Wolf, Kaloupek, & Keane, 2008; Mills, Teesson, Ross, & Peters, 2006; Widom, 1989), understanding if and how a genetic predisposition for externalizing is modulated by exposure to trauma is critical for developing comprehensive etiological models of these harmful behaviors.
The goals of the present study were to further validate the externalizing polygenic risk score by examining its associations with psychopathology symptoms, impulsive traits, and cognitive functions. First, we sought to replicate the findings of Salvatore et al. (2015) in a sample of trauma-exposed adults and extend them by examining associations between the polygenic risk score and different forms of psychopathology. We hypothesized that the risk score would relate to externalizing psychopathology and impulsive traits, but not internalizing psychopathology. The second aim was to investigate polygenic risk associations with tasks of executive functioning as potential intermediate mechanisms linking genes to externalizing psychopathology. Based on research showing that externalizing is associated with executive dysfunction but necessarily cognitive functioning more broadly (Finn et al., 2009; Young et al., 2009), we hypothesized that the risk score would predict poorer performance on tasks of inhibitory control and working memory capacity, but not episodic memory. The final aim was to examine the interactive effects of trauma exposure and polygenic risk. In the light of previous gene-by-environment effects (e.g., Hicks et al., 2009; Salvatore et al., 2015), we expected that a history of traumatic events would confer greater risk for externalizing psychopathology and executive dysfunction at higher levels of polygenic risk.
Methods
Sample and Procedures
Sample 1
The first sample consisted of 554 White, non-Hispanic (as determined through genotyping, see below) military veterans and their cohabitating intimate partners (veterans = 388, partners = 166) who enrolled in one of two VA studies with overlapping methodologies, allowing for the samples to be merged (see Logue et al., 2013). Military veterans who screened positive for posttraumatic stress disorder (PTSD) were enrolled in the first study, and trauma-exposed military veterans and their intimate partners were enrolled in the second study. Seventeen participants were excluded from analyses, because they had problems completing the protocol or withdrew before completing it. The final sample consisted of 537 participants (60% male) and ranged in age from 21 to 75 (M = 51.8, SD = 11.2). Forty-six percent of participants were either unemployed or receiving disability payments, and the remainder were employed full- or part-time (33%), retired (18%), students (3%), or did not provide employment information (<1%). There was a range of educational attainment in the sample: 22% high school diploma, GED, or below, 45% some college or vocational degree, 14% Bachelor’s degree, 18% graduate work or degree, and <1% did not provide education information.
Sample 2
The second sample consisted of 199 White, non-Hispanic (as determined through genotyping, see below) service members of Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND). Participants were consecutively enrolled in the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research & Development Traumatic Brain Injury Center of Excellence at VA Boston Healthcare System. Individuals with a history of moderate or severe traumatic brain injury were excluded (n = 5). The final sample consisted of 194 primarily male (90%) veterans ages 19 to 58 (M = 31.6, SD = 8.3). The majority of participants were employed full- or part-time (n = 133, 69%), and education levels were as follows: 35% high school diploma or GED, 55% some college or Bachelor’s degree, and 10% graduate work or degree.
Approval for the studies was obtained from all relevant Institutional Review Boards and regulatory committees. After a complete description of study procedures, written informed consent was obtained from participants.
Measures
Not all measures were available in both samples. Externalizing psychopathology and impulsive personality traits were only available in Sample 1, and cognitive task performance was only available in Sample 2. DNA genotyping and a measure of trauma exposure were available in both samples. The measures that were analyzed in each sample are described below.
Sample 1 only
(a) Adult antisocial behavior
Adult antisocial behavior was assessed with two different measures. In the veteran-only study, adult antisocial behavior was measured using the International Personality Disorder Examination (Loranger, 1999). In the intimate partner study, it was assessed using the Structured Clinical Interview for DSM-IV II (First, Spitzer, Gibbon, & Willams, 1995). To create a single adult antisocial scale across the two measures, the summary score from matching items on each measure were standardized and then combined (Miller, Wolf, Logue, & Baldwin, 2013). Inter-rater reliability for these measures was assessed in approximately 25% of the sample and was excellent (kappa = 0.89). The prevalence of antisocial personality disorder was 6%.
(b) Impulsive personality traits
A subset of participants in Sample 1 (n = 151) completed the Multidimensional Personality Questionnaire – Brief Form (MPQ-BF; Patrick, Curtin, & Tellegen, 2002), a 155-item self-report personality inventory derived from the full-length 276-item MPQ (Tellegen, 1982). Only polygenic associations with the higher-order dimension of Constraint were examined, because it is a broad measure of impulsivity (reversed). The Constraint dimension is composed of the primary trait scales of thrill-seeking or fearlessness (Harm Avoidance scale reversed), poor impulse control (Control scale reversed) and nonconformity to social norms (Traditionalism scale reversed).
(c) Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, 1994)
Lifetime Axis I disorders were assessed with the SCID-IV. Dimensional scores for each diagnosis were created by summing scores across symptoms within a module. Inter-rater reliability was calculated for approximately 25% of the sample and kappas ranged from .69 to .97 for individual diagnoses. The reliability kappas evidenced moderate agreement for fear disorders (ranging from agoraphobia = .69 to specific phobia = .73), substantial agreement for distress disorders (ranging from dysthymia = .78 to major depressive = .86), and substantial to almost perfect agreement for externalizing disorders (ranging from cannabis abuse/dependence = .77 to cocaine abuse/dependence = .97). Prevalence rates for lifetime diagnosis were: 52% depression, 16% panic disorder, 3% agoraphobia, 12% specific phobia, 4% obsessive-compulsive disorder, 10% generalized anxiety disorder, 51%/41% alcohol abuse/dependence, 10%/9% cannabis abuse/dependence, and 4%/11% cocaine abuse/dependence.
(d) Clinician Administered PTSD Scale (Blake et al, 1995)
The CAPS is a 30-item structured diagnostic interview that assesses the frequency and severity of the 17 DSM-IV PTSD symptoms, 5 associated features, and related functional impairment. Dimensional lifetime severity scores were calculated by summing the frequency and intensity ratings (each range from 0–4) for each of the 17 items (possible range: 0–136; Weathers, Ruscio, & Keane, 1999). Inter-rater reliability for the CAPS was calculated for approximately 25% of the sample and was excellent (kappa = 0.87). The prevalence of lifetime PTSD was 55%.
Sample 2 only
Cognitive functions
We analyzed three domains of cognitive functioning: inhibitory control, working memory capacity, and episodic memory. Participants completed the color-word interference test (i.e., Stroop) from the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001) to measure inhibitory control. The inhibition subtest measures the ability to inhibit an automatic response (i.e., word reading) in order to generate a less salient incongruent response (i.e., color naming), and the inhibition/switching subtest measures the ability to flexibly switch between these response sets. We used the scaled scores from these subtests adjusted for performance on the color-naming and word-reading component tests by creating a difference score [e.g., inhibition - (color naming + word reading)/2]. To assess working memory, participants completed the digit span subtest of the Wechsler Adult Intelligence Scale—Fourth Edition (Wechsler, 2008), which measures the ability to hold and manipulate digits in working memory, and the auditory consonant trigrams (ACT) task, which measures divided attention and the ability to recall simple stimuli following a distracting task (Brown, 1958). Performance on these tasks was measured using the digit span total scaled score and ACT total number of correct responses z-score. Verbal episodic memory was assessed using performance on the long delay free recall and long-delay cued recall trials (z-scored) on the California Verbal Learning Test - 2nd edition (Delis, Kramer, Kaplan, & Ober, 2000).
We extracted factor scores via the regression method from a principal axis factor analysis with varimax rotation conducted with the six indicators of cognitive functioning described above. Three factors with eigenvalues greater than one accounted for a total of 78% of the covariance. The episodic memory indicators loaded on the first factor (36% of covariance, free recall = 0.96, cued recall = 0.91), the inhibitory control indicators loaded on the second factor (23% of covariance, inhibition = 0.70, inhibition/switching = 0.56), and the working memory indicators loaded on the third factor (18% of covariance, digit span = 0.58, ACT = 0.56). Data from 12 participants were removed from the analyses with these measures due to failure on the Green Medical Symptom Validity Test (Green, 2003), a measure of motivation to perform at optimal levels on the neuropsychological tasks.
Both samples
(a) Traumatic Life Events Questionnaire (TLEQ; Kubany et al., 2000)
A count variable was created by summing the number of different lifetime traumatic events reported by participants on the TLEQ, a self-report questionnaire. The TLEQ assesses a broad array of types of traumatic events (e.g., accidents, combat or warfare, sudden death of friend/loved one, life-threatening illness, assault, unwanted sexual contact) and demonstrates excellent convergent validity with interview-based measures of trauma exposure (Kubany et al., 2000). We used number of different types of trauma rather than total occurrences of trauma to avoid excessive skewness in this measure (Cronbach’s alphas = .78 for Sample 1 and .70 for Sample 2). Both samples were enriched for trauma exposure, with the first sample endorsing 5.7 trauma types on average (SD = 3.9, Min = 0, Max = 18) and the second sample endorsing 5.5 trauma types on average (SD = 2.9, Min = 0, Max = 15).
(b) DNA genotyping
DNA was isolated from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents; concentrations were normalized using the Quant-iT™ PicoGreen dsDNA fluorescent assay (Invitrogen). DNA quality and quantity were ascertained by the TaqMan® RNase P Detection assay (Applied Biosystems Assay, Life Technologies, Carlsbad, CA) with fluorescence detection on a 7900 Fast Real Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. DNA samples were whole-genome amplified, fragmented, precipitated and resuspended prior to hybridization on Illumina HumanOmni2.5-8 beadchips for 20 hours at 48 °C according to the manufacturer’s protocol (Illumina, San Diego, CA). After hybridization, a single-base extension followed by a multi-layered staining process was performed. Beadchips were imaged using the Illumina iScan System and analyzed with Illumina GenomeStudio v2011.1 software containing Genotyping v1.9.4 module. A GenomeStudio project was created with a custom genotyping cluster file, and call rates were > 0.994 for all samples. Technical replicates had genotyping reproducibility error rates < 0.0005 prior to SNP data cleaning.
SNP data cleaning and manipulation was performed using PLINK (Purcell et al., 2007). X-chromosome genotypes were concordant with self-reported sex in all cases. IBD analysis was used to check for cryptic relatedness in the sample. Concordance between self-reported and genetically predicted ancestry was investigated using principal components (PC) analysis as implemented in EIGENSTRAT (Price et al., 2006), based on the genotypes of 100,000 common SNPs.
Statistical Analyses
Computation of Polygenic Risk Score
To calculate the polygenic scores used in Salvatore et al. (2015), we obtained a list of reference alleles and effect sizes for 587,378 SNPs from the Collaborative Study on the Genetics of Alcoholism (COGA) investigators. We confirmed that this list had been pruned of SNPs with ambiguous coding (i.e. A/T and G/C SNPs). Of the COGA SNPS, 480,856 were genotyped on the Illumina OMNI 2.5-8 array in our samples and available for risk-score calculations. Polygenic risk scores were calculated by PLINK1 using the --score option, which computes a linear function of the additively coded number of reference alleles weighted by the log odds ratios (betas) from the COGA sample. To limit the degree of multiple testing, we only examined polygenic risk scores computed at three different p-value cutoffs: p < .30 threshold which was most significant in Salvatore et al. (2015), as well as p < .05 and p < .50, which represent a low and high degree of polygenicity respectively. These scores were used to establish the threshold that explained the most variance in the externalizing phenotype.
Polygenic Association Analyses
All analyses with the polygenic risk score were adjusted for age, sex, and the first three ancestry principal components (reflecting population substructure within this sample of all White, non-Hispanic participants).
(a) Sample 1 analysis: Polygenic associations with externalizing and internalizing latent variables, and impulsive personality traits
First, we performed confirmatory factor analysis using Mplus 7.11 (Muthén & Muthén, 2013) to model the externalizing and internalizing latent variables using lifetime symptom severity scores for each diagnosis in Sample 1. The externalizing indicators consisted of antisocial personality disorder symptom severity, and alcohol, cannabis, and cocaine abuse/dependence symptom severity. The residuals for cannabis and cocaine abuse/dependence were allowed to correlate, because they were based on items with virtually identical structure and wording. We did not include impulsive personality traits as an indicator of the externalizing latent variable, because these data were only available for approximately 28% of participants. The indicators for internalizing consisted of lifetime posttraumatic stress disorder, depression, dysthymia, panic disorder, agoraphobia, specific phobia, obsessive-compulsive disorder, and generalized anxiety disorder symptom severity. Second, we performed hierarchical linear regression analyses with MPQ Constraint as the dependent variable, and the covariates (age, sex, and ancestry principal components; Block 1), the polygenic risk score (Block 2), trauma exposure (Block 3), and the polygenic risk score x trauma exposure interaction (Block 4) entered as independent variables in blocks.
(b) Sample 2 analysis: Polygenic associations with cognitive functions
Given that we did not have the clinical measures necessary to create a latent externalizing dimension in Sample 2, we used the polygenic risk score as a proxy for externalizing risk in this sample. We conducted three hierarchical linear regression analyses, each with a different cognitive function entered as the dependent variable (inhibitory control, working memory capacity, or episodic memory), with the covariates (age, sex, ancestry principal components, an estimate of IQ; Block 1), the polygenic risk score (Block 2), trauma exposure (Block 3), and the polygenic risk score x trauma exposure interaction (Block 4) entered as independent variables in blocks.1 Given that the cognitive variables were created to represent distinct (and uncorrelated) cognitive functions, we implemented a Bonferroni multiple-testing correction to reduce the likelihood of Type 1 error. For these analyses, p-values less than .016 were interpreted as significant.
Results
Selecting a Polygenic Risk Score Threshold
In Sample 1, we modeled the externalizing and internalizing latent variables using confirmatory factor analysis. The model showed adequate fit (RMSEA = .06; SRMR = .04; CFI = .90), and all of the diagnostic indicators loaded significantly on their respective latent variables (ps <.001). Next, we extracted the factor scores of the latent variables to establish the p-value threshold for the polygenic risk score that explained the most variance in the externalizing psychopathology dimension. A significant association between the polygenic risk score and externalizing was observed for the SNP set based on a threshold of p < .50 (p = .033), but not for sets with thresholds of p < .30 or p < .05 (ps >.85). The polygenic risk score based on a threshold p < .50 explained 0.7% of the variance in the externalizing psychopathology dimension. Because the externalizing and internalizing dimensions were moderately correlated in this sample (r = .59, p < .001), we also examined the unique variance associated with externalizing by creating a residual externalizing score with the variance shared with internalizing removed. Using this factor residual score, the polygenic risk score based on a threshold of p < .50 explained 1.2% of the variance in externalizing (p = .009). Based on these findings, we used the polygenic score derived from a p-value threshold of .50 in all subsequent analyses to maximize the variance explained in the externalizing phenotype.
Externalizing and Internalizing Psychopathology
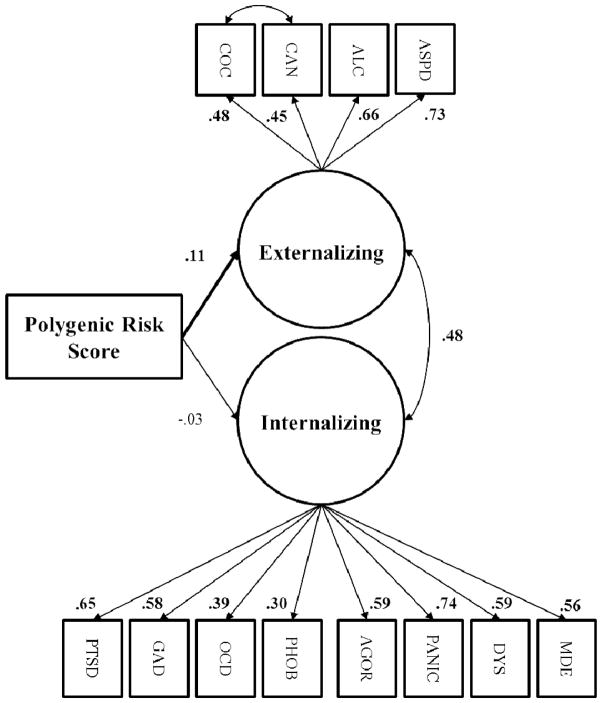
We used structural equation modeling to simultaneously model the associations of the polygenic risk score with the latent externalizing and internalizing psychopathology variables, because the factor scores are not perfect reflections of the latent traits, and we wanted to simultaneously test multivariate associations between the risk score and the latent internalizing and externalizing dimensions, as well as the associations among these variables.2 Results of the structural equation model are depicted in Figure 1. As hypothesized, and consistent with the initial factor score results, the polygenic risk score predicted greater externalizing psychopathology severity (p = .034), but it was unrelated to the internalizing psychopathology latent variable (p > .57). 3,4
Figure 1. Polygenic Risk Score Associations with Externalizing and Internalizing Psychopathology.
Structural equation model of polygenic risk score predicting externalizing and internalizing latent variables. ASPD = antisocial personality disorder. ALC= alcohol abuse/dependence. CAN = cannabis abuse/dependence. COC = cocaine abuse/dependence. MDE = major depressive episode. DYS = dysthymia. PANIC = panic disorder. AGOR = agoraphobia. PHOBIA = specific phobia. OCD = obsessive-compulsive disorder. GAD = generalized anxiety disorder. PTSD = posttraumatic stress disorder. Paths for age, sex, and ancestry principal components were included in the model, but are not depicted. RMSEA = .06; SRMR = .06; CFI = .80. Significant standardized parameter estimates depicted in bold (p < .05).
To assess the effects of environmental influences, lifetime trauma exposure and polygenic risk x trauma exposure were added to the model as predictors of externalizing psychopathology. Trauma exposure positively predicted the externalizing latent variable (β = .24, p < .001), and the polygenic score continued to predict the externalizing dimension with trauma in the model (β = .11, p = .044). The polygenic risk score did not interact with trauma exposure to predict externalizing, however (p > .76).
Impulsive Personality Traits
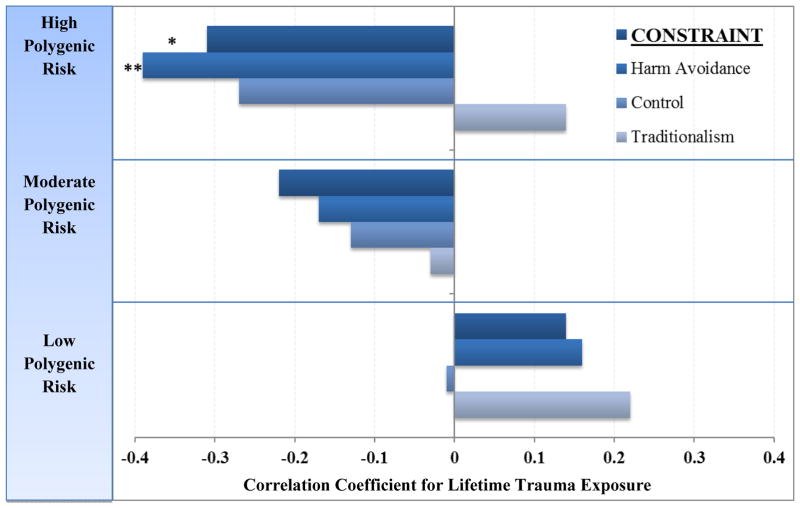
Next, we conducted linear regressions to examine polygenic associations with impulsivity in the subset of veterans in Sample 1 with MPQ-BF data. The polygenic score did not evidence a main effect with MPQ-BF Constraint (p > .40). When lifetime trauma exposure was added to the model, a significant polygenic risk score x trauma exposure interaction effect was present (β = −1.3, p = .015, R2 = 3.8%). To decompose this interaction, we examined associations between lifetime trauma exposure and Constraint at varying levels of polygenic risk. As illustrated in Figure 2, the effect of trauma exposure on impulsivity was greatest at high levels of polygenic risk. To aid in the interpretation of this finding, we also examined the primary trait scales that contribute to the higher-order dimension of Constraint. Results of these follow-up analyses indicated that the Constraint effect was driven primarily by the Harm Avoidance scale (β = −1.3, p = .012, R2 = 4.1%). Results for the Control (β = −0.7, p > .18, R2 = 1.2%) and Traditionalism (β = −0.2, p > .65, R2 = 0.1%) primary scales were not significant, though the pattern of associations was similar for the Control scale.
Figure 2. Polygenic Risk x Lifetime Trauma Exposure Predicts Impulsive Personality Traits.
Correlations between impulsive personality traits on the Multidimensional Personality Questionnaire-Brief and lifetime trauma exposure as a function of polygenic risk score liability. Lifetime trauma exposure predicted greater impulsive traits as polygenic risk load increased. Bars represent partial correlations accounting for age, sex, and ancestry principal components. N = 151. Polygenic Risk Score: Low n = 50, Moderate n = 50, High n = 51. *p < .05. **p < .01.
Cognitive Functioning
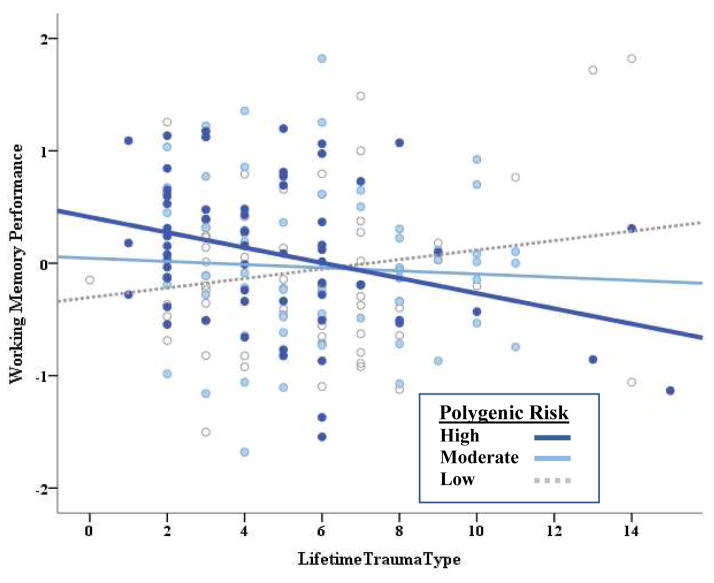
Next, we performed linear regression analyses to examine executive dysfunction as a potential intermediate phenotype of polygenic risk for externalizing using the second sample. Results of these analyses are presented in Table 1. We observed a main effect of polygenic risk on inhibitory control, such that greater genetic susceptibility for externalizing predicted poorer performance on these tasks, with genetic effects accounting for 3.8% of the variance in the inhibitory control factor. This genetic effect remained significant after the addition of lifetime trauma exposure to the model, and the polygenic score did not interact with trauma exposure. In contrast to inhibitory control, the polygenic risk score did not show main effects for working memory or episodic memory. However, lifetime trauma exposure did interact with polygenic risk to predict working memory performance. We decomposed this interaction by examining the effects of trauma exposure on working memory at low, moderate, and high polygenic risk. As illustrated in Figure 3, lifetime trauma exposure predicted increasingly poorer working memory performance as polygenic susceptibility to externalizing increased.
Table 1.
Hierarchical Linear Regressions of Polygenic Risk Score and Lifetime Trauma Exposure Predicting Cognitive Functioning
| Inhibitory Control
|
Working Memory
|
Episodic Memory
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β/SE | P-value | R2 | β/SE | P-value | R2 | β/SE | P-value | R2 | |
|
|
|
|
|||||||
| Block 1 | 2.1% | 14.0% | 4.5% | ||||||
| Age | .05/0.01 | .52 | .06/0.01 | .37 | −.01/0.01 | .90 | |||
| Sex | .05/0.20 | .50 | −.14/0.17 | .06 | −.04/0.25 | .59 | |||
| Est. IQ | −.07/0.01 | .37 | .33/0.00 | <.001 | .21/0.01 | .007 | |||
| PC1 | .07/0.87 | .37 | −.04/0.75 | .62 | .04/1.09 | .60 | |||
| PC2 | −.02/0.86 | .84 | −.04/0.74 | .62 | .02/1.08 | .80 | |||
| PC3 | −.09/0.88 | .27 | .08/0.75 | .28 | −.04/1.10 | .64 | |||
|
|
|
|
|||||||
| Block 2 | 3.8% | 0.7% | 0.0% | ||||||
| Polygenic Score | −.20/0.53 | .009 | .09/0.46 | .23 | .01/0.67 | .92 | |||
|
|
|
|
|||||||
| Block 3 | 0.5% | 0.0% | 0.1% | ||||||
| Trauma Exposure | −.07/0.02 | .34 | .01/0.2 | .95 | .03/0.03 | .75 | |||
|
|
|
|
|||||||
| Block 4 | 0.9% | 5.1% | 0.0% | ||||||
| Polygenic Score x Trauma Exposure | .59/0.19 | .19 | −1.38/0.16 | .001 | .07/0.24 | .88 | |||
Note. Est. IQ = Estimated intelligence quotient from Wechsler Test of Adult Reading (Wechsler, 2001). PC = principal component. Trauma exposure = total types of different traumas over the lifespan. R2 = R2 change for blocks 2–4.
Figure 3. Polygenic Risk x Lifetime Trauma Exposure Predicts Working Memory Performance.
Lifetime trauma exposure predicting working memory performance as a function of polygenic risk. To decompose the interaction, polygenic risk score was grouped into low, moderate, and high categories reflecting tertiles. N = 182. Polygenic Risk Score: Low n = 60, Moderate n = 59, High n = 63.
Discussion
The purpose of this study was to validate the externalizing polygenic risk score by examining its associations with psychopathology symptoms, impulsive traits, and cognitive functions. As expected, we replicated the polygenic association with externalizing psychopathology reported by Salvatore et al. (2015) in a sample of adults with high levels of trauma exposure. We also demonstrated that the polygenic risk score does not predict internalizing symptoms. Greater polygenic risk was associated with impaired performance on tasks of executive functioning, specifically inhibitory control (main effect) and working memory capacity (interaction with trauma exposure), but not performance on a task of episodic memory. Finally, cumulative lifetime trauma exposure interacted with genetic predispositions to confer risk for impulsive traits and working memory dysfunction. These findings extend previous research by linking polygenic risk for externalizing to deficits in executive functioning – a possible cognitive mechanism by which genes confer vulnerability for externalizing psychopathology – and point to trauma exposure as an important environmental moderator of polygenic risk for externalizing.
The present findings converge with previous research showing that an inherited liability for executive dysfunction is a core susceptibility for externalizing psychopathology (e.g., Bogg & Finn, 2010; Young et al., 2009). Intact inhibitory control and working memory capacity are essential for maintaining self-control and regulating impulsive urges (Nigg, 2000). Deficits in inhibitory control can disrupt one’s ability to control impulsive motor responses and manage intense emotional reactions (e.g., Nigg, 2000; Miyake & Friedman, 2004). Similarly, impaired working memory capacity can interfere with one’s ability to keep long-term goals in mind when confronted with salient short-term rewards or other motivationally-relevant stimuli (Barkley, 1997). Thus, in situations where cognitive resources are taxed, reduced inhibitory control and working memory capacity may lead to impulsive decision-making and reckless behavior, which are hallmarks of the externalizing spectrum. Interestingly, our findings suggest that trauma exposure interacts with polygenic risk to predict decrements in working memory capacity, a cognitive mechanism that may contribute to elevated rates of externalizing problems (e.g., substance/alcohol use, antisocial behavior) in trauma-exposed samples. In contrast, episodic memory problems are not typically associated with externalizing psychopathology, and we did not observe genetic effects for this cognitive indicator. Our pattern of findings suggests that polygenic predispositions for externalizing confer risk for executive dysfunction, but not necessarily cognitive problems more generally.
A fine-grained assessment of polygenic associations with multiple domains of cognitive functioning and across key developmental stages (e.g., childhood, adolescence) would help to clarify the role genetic vulnerability for executive dysfunction plays in the etiology of externalizing. We were unable to test mediation models (e.g., executive dysfunction mediating the effects of polygenic risk on externalizing psychopathology) in this study, because information about externalizing was not available in the sample with the cognitive performance indicators. However, investigating executive dysfunction as a mediator of relationships between the polygenic risk score and externalizing phenotypes will be critical to determine in future studies to validate these etiological pathways. To functionally connect genes to externalizing psychopathology, future research needs to examine other core characteristics of externalizing, such as reward sensitivity and affective reactivity, as potential heritable intermediate phenotypes. Moreover, future research on the neurobiological correlates of this risk score could provide valuable insight into the heritability of neural susceptibilities (e.g., reduced P3 amplitude, disrupted neural circuits; Hicks, Krueger, Iacono, McGue, & Patrick, 2004) for externalizing outcomes. Armed with these data, it would be possible to disentangle the contributions of heritable versus acquired susceptibilities for the cognitive dysfunction and affective dysregulation that characterizes externalizing and parse heterogeneity in the etiology of externalizing psychopathology based on different patterns of cognitive and affective vulnerability (e.g., Baskin-Sommers, Curtin, & Newman, 2015).
Examination of polygenic risk-by-trauma interactions produced mixed results in our samples. Polygenic risk did not interact with cumulative trauma exposure to predict the latent externalizing factor or inhibitory control task performance, but it did moderate trauma associations with impulsive personality traits and working memory capacity. It is not clear why moderation was evident for certain externalizing phenotypes and not others. One possibility is that the genetic predispositions for externalizing and inhibitory control deficits are present regardless of environmental conditions, representing a core cognitive vulnerability for externalizing, and the influence of environmental risk factors, like multiple trauma exposures, becomes more important at higher levels of genetic liability for impulsivity and working memory capacity. Another possibility is that our sample characteristics influenced the likelihood of observing gene-by-environment interactions for trauma. On average, participants reported high levels of trauma in both samples (e.g., ~5.5 types of traumatic events on average), which may have restricted variance to the high end of the trauma exposure continuum. Thus, moderation effects may appear more consistently in samples with a broader range of trauma exposure, including greater representation of those with no trauma exposure. A third possibility is that genetic liability for behavioral disinhibition increases the likelihood that an individual will be exposed to high-risk environments where they will be exposed to traumatic events (i.e., indicative of a gene-environment correlation), which in turn potentiates externalizing psychopathology. Such a model is consistent with research showing that impulsivity leads to greater trauma and life stress exposure prospectively (e.g., Sadeh, Miller, Wolf, & Harkness, 2015). However, a relationship between the polygenic risk score and lifetime trauma exposure was not evident in our samples, suggesting that gene-environment correlation is unlikely.5
As with any study, there are limitations to consider when interpreting the findings. First, although comparable to other studies examining polygenic effects (e.g., Salvatore et al., 2015), the samples sizes in this study were modest for a genetic association analysis. Consequently, null findings need to be interpreted with caution and replication in larger and more diverse samples is warranted. Second, the military veteran composition of the sample may limit the generalizability of the findings, for example, to males with high levels of trauma exposure. Despite this, military veterans represent a clinically-relevant sample in which to examine externalizing phenotypes, given that antisocial behavior, violence, and alcohol/substance dependence are present at higher rates than the general population (Elbogen et al., 2010; Elbogen et al., 2014; Miller, Vogt, Mozley, Kaloupek, & Keane, 2006; National Institute on Drug Abuse, 2013). They also provide a unique opportunity to examine the impact of trauma exposure on genetic influences, as traumatic events are well represented. Third, analyses were limited to individuals of White, non-Hispanic ancestry, which limits the generalizability of the findings to this population. Development of a polygenic risk score for externalizing that can be applied to other ancestry groups is an important direction for future research. Finally, given that we did not have a measure of externalizing psychopathology in Sample 2, we used the polygenic risk score as a proxy for externalizing risk in this sample. This methodological limitation prevented us from testing the replicability of the polygenic associations with externalizing psychopathology and from directly linking externalizing psychopathology with deficits in executive functioning. Thus, the novel polygenic associations with executive functions that we observed need to be replicated and their relevance for explaining externalizing psychopathology needs to be established in future research.
By reducing the number of genetic parameters from millions of possible SNPs in genome-wide association studies to a single genome-wide polygenic risk score, this state-of-the-art metric provides a powerful tool for identifying biological vulnerabilities for externalizing and disentangling pathways to antisocial behavior. Mapping the connections from polygenic risk to the manifestation of problem behaviors across multiple levels of analysis (e.g., epigenetic mechanisms, neural susceptibilities, emotional intelligence, social skills), as a function of environmental context (e.g., early adversity, peer contexts, violence exposure), and across developmental periods would be incredibly valuable for delineating antisocial and externalizing trajectories (e.g., Hyde, 2015; Javdani, Sadeh, & Verona, 2011). This type of research is imperative for moving the field beyond piecemeal characterizations of risk for these etiologically complex outcomes and towards a holistic picture of how risk and resiliency processes interact across different types of risk factors over time. The polygenic risk score also provides a unique opportunity to discover the neural mechanisms by which the genome confers susceptibility for antisocial behavior and externalizing psychopathology, which has proven challenging in past research due to the staggering number of potentially relevant genetic loci and neuroimaging parameters. Studying the neurobiology of genetic risk for externalizing at multiple levels of neural analysis (e.g., cortical thickness, functional and structural brain networks, task-based functional activation) is much more feasible using the polygenic risk score than genome-wide association studies or even candidate gene studies. This type of neurogenetic analysis would provide a rich characterization of the neural bases of disinhibition-related pathology and unprecedented insight into how heritable neural mechanisms contribute to different pathways of antisocial behavior. A study with this wide a range of neural and genetic data would have been impossible 10 years ago, but the identification of polygenic risk scores for complex diseases and efforts like the Human Connectome Project (e.g., Chiang et al., 2009), have set the stage for this type of groundbreaking work.
Acknowledgments
This research was supported in part by a National Institute of Mental Health award RO1MH079806, a Department of Veterans Affairs, Clinical Science Research & Development Program award 5I01CX000431-02, a Department of Veterans Affairs, Biomedical Laboratory Research & Development Program award 1I01BX002150-01, the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B9254-C), and the Cooperative Studies Program, Department of Veterans Affairs. This research is the result of work supported with resources and the use of facilities at the Pharmacogenomics Analysis Laboratory, Research and Development Service, Central Arkansas Veterans Healthcare System, Little Rock, Arkansas. This work was also supported by a Career Development Award to E.J. Wolf from the Department of Veterans Affairs, Clinical Sciences Research and Development Program. We would like to thank Jeffrey M. Spielberg for his comments on the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
We would like to thank the Collaborative Study on the Genetics of Alcoholism (COGA) who supplied data for calculating the polygenic risk scores used in this study. COGA Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); University of Texas Medical Center in San Antonio (L. Almasy), Virginia Commonwealth University (D. Dick), Mount Sinai School of Medicine (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, S. O’Connor, L. Wetherill, X. Xuei (Indiana University); Grace Chan (University of Iowa); D. Chorlian, N. Manz, K. Chella, A. Pandey (SUNY Downstate); J-C Wang (Mount Sinai School of Medicine) and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. COGA continues to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owes a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Footnotes
An estimate of IQ derived from the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) was also included as a covariate in analyses of cognitive functioning to ensure results were not accounted for by individual differences in general intelligence.
We also conducted analyses with the factor scores (rather than the latent psychopathology variables in SEM) and found that the results do not change, with the exception that the p-value for the polygenic association with externalizing is slightly stronger (β = .09, p = .009); it remained non-significant for internalizing (β = −.05, p = .13).
To examine whether the paths from polygenic risk to externalizing and internalizing can be equated, we set each path to 0 in separate models and examined changes in model fit. Setting the path from polygenic risk to the latent externalizing dimension to 0 significantly degraded model fit (Wald test = 4.4, p =.035), whereas setting the path from polygenic risk to the internalizing dimension to 0 did not degrade model fit (Wald test = 0.3, p =.58). However, the 95% confidence intervals for the path parameter estimates overlapped, indicating that the path parameters for externalizing and internalizing were not significantly different.
We examined whether excluding the diagnoses with the lowest reliability estimates, specifically agoraphobia, specific phobia, panic, and obsessive-compulsive disorder, from the latent model changed the SEM findings. Removing these indicators only slightly improved model fit (RMSEA = .07; SRMR = .07; CFI = .82) and did not change the magnitude of the polygenic effects on externalizing (β = .11, p = .035) or internalizing (β = −.02, p = .77).
We examined polygenic associations with lifetime trauma exposure in both samples and did not find significant associations in either sample (rs < |.12|, ps > .09).
Author Contributions
N. Sadeh formulated the study questions and drafted the manuscript. N. Sadeh, M.W. Miller, E.J. Wolf, M.W. Logue, J.P. Hayes, R.E. McGlinchey, W.P. Milberg, and J. Lusk assisted with the study design. N. Sadeh, E. J. Wolf, and M. W. Logue conducted statistical analyses. A. Stone and S.A. Schichman conducted genotyping. All authors critically reviewed the manuscript and approved the final version for publication.
References
- Babinski LM, Hartsough CS, Lambert NM. Childhood conduct problems, hyperactivity-impulsivity, and inattention as predictors of adult criminal activity. Journal of Child Psychology and Psychiatry. 1999;40(03):347–355. http://dx.doi.org/ [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. http://dx.doi.org/10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Altering the cognitive-affective dysfunctions of psychopathic and externalizing offender subtypes with cognitive remediation. Clinical Psychological Science. 2015;3(1):45–57. doi: 10.1177/2167702614560744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35(05):637–648. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10(1):12–21. doi: 10.1080/17470215808416249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Finn PR. A self-regulatory model of behavioral disinhibition in late adolescence: Integrating personality traits, externalizing psychopathology, and cognitive capacity. Journal of Personality. 2010;78(2):441–470. doi: 10.1111/j.1467-6494.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Thompson PM. Genetics of brain fiber architecture and intellectual performance. The Journal of Neuroscience. 2009;29(7):2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisanti AS, Frueh BC. Risk of trauma exposure among persons with mental illness in jails and prisons: What do we really know? Current Opinion in Psychiatry. 2011;24(5):431–435. doi: 10.1097/YCO.0b013e328349bbb8. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Over BA. California Verbal Learning Test. 2. The Psychological Corporation; New York: 2000. CVLT-II. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; New York: 2001. [Google Scholar]
- Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. Current Directions in Psychological Science. 2007;16(6):331–335. doi: 10.1111/j.1467-8721.2007.00530.x. [DOI] [Google Scholar]
- Douglas KR, Chan G, Gelernter J, Arias AJ, Anton RF, Weiss RD, Kranzler HR. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addictive Behaviors. 2010;35(1):7–13. doi: 10.1016/j.addbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Perspectives. 2011;168(10) doi: 10.1176/appi.ajp.2011.11020191. http://dx.doi.org/10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbogen EB, Fuller S, Johnson SC, Brooks S, Kinneer P, Calhoun PS, Beckham JC. Improving risk assessment of violence among military veterans: An evidence-based approach for clinical decision-making. Clinical Psychology Review. 2010;30(6):595–607. doi: 10.1016/j.cpr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbogen EB, Johnson SC, Wagner HR, Sullivan C, Taft CT, Beckham JC. Violent behaviour and post-traumatic stress disorder in U.S. Iraq and Afghanistan veterans. The British Journal of Psychiatry. 2014;204(5):368–375. doi: 10.1192/bjp.bp.113.134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, Cantrell H. Reduced cognitive ability in alcohol dependence: Examining the role of covarying externalizing psychopathology. Journal of Abnormal Psychology. 2009;118(1):100–116. doi: 10.1037/a0014656. http://dx.doi.org/10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research; New York: 1994. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part I: Description. Journal of Personality Disorders. 1995;9:83–91. doi: 10.1521/pedi.1995.9.2.92. [DOI] [Google Scholar]
- Frick PJ, White SF. Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Green P. Green’s Medical Symptom Validity Test (MSVT) for Windows: User’s Manual. Edmonton, Canada: Green’s Publishing; 2003. [Google Scholar]
- Greenwald R. The role of trauma in conduct disorder. Journal of Aggression, Maltreatment & Trauma. 2002;6(1):5–23. doi: 10.1300/J146v06n01_02. [DOI] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61(9):922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66(6):640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW. Developmental psychopathology in an era of molecular genetics and neuroimaging: A developmental neurogenetics approach. Development & Psychopathology. 2015;27(02):587–613. doi: 10.1017/S0954579415000188. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11(04):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Javdani S, Sadeh N, Verona E. Expanding our lens: Female pathways to antisocial behavior in adolescence and adulthood. Clinical Psychology Review. 2011;31:1324–1348. doi: 10.1016/j.cpr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. http://dx.doi.org/10.1037/0021-843X.111.3.411. [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany ES, Leisen MB, Kaplan AS, Watson SB, Haynes SN, Owens JA, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12(2):210–224. doi: 10.1037//1040-3590.12.2.210. http://dx.doi.org/10.1037/1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, Miller MW. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular Psychiatry. 2013;18(8):937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Solovieff N, Leussis MP, Wolf EJ, Melista E, Baldwin C, Miller MW. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology. 2013;38(10):2249–2257. doi: 10.1016/j.psyneuen.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger AW. IPDE: International Personality Disorder Examination: DSM-IV and ICD-10 interviews. PARS Psychological Assessment Resources 1999 [Google Scholar]
- Luengo MA, Carrillo-De-La-Pena MT, Otero JM, Romero E. A short-term longitudinal study of impulsivity and antisocial behavior. Journal of Personality and Social Psychology. 1994;66(3):542. doi: 10.1037//0022-3514.66.3.542. http://dx.doi.org/10.1037/0022-3514.66.3.542. [DOI] [PubMed] [Google Scholar]
- Luntz BK, Widom CS. Antisocial personality disorder in abused and neglected children grown up. American Journal of Psychiatry. 1994;151(5):670–674. doi: 10.1176/ajp.151.5.670. [DOI] [PubMed] [Google Scholar]
- Miller MW, Greif JL, Smith AA. Multidimensional Personality Questionnaire profiles of veterans with traumatic combat exposure: externalizing and internalizing subtypes. Psychological Assessment. 2003;15:205. doi: 10.1037/1040-3590.15.2.205. [DOI] [PubMed] [Google Scholar]
- Miller MW, Vogt DS, Mozley SL, Kaloupek DG, Keane TM. PTSD and substance-related problems: the mediating roles of disconstraint and negative emotionality. Journal of Abnormal Psychology. 2006;115(2):369–379. doi: 10.1037/0021-843X.115.2.369. http://dx.doi.org/10.1037/0021-843X.115.2.369. [DOI] [PubMed] [Google Scholar]
- Miller MW, Fogler JM, Wolf EJ, Kaloupek DG, Keane TM. The internalizing and externalizing structure of psychiatric comorbidity in combat veterans. Journal of Traumatic Stress. 2008;21(1):58–65. doi: 10.1002/jts.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolf EJ, Logue MW, Baldwin CT. The retinoid-related orphan receptor alpha (RORA) gene and fear-related psychopathology. Journal of Affect Disorder. 2013;151(2):702–708. doi: 10.1016/j.jad.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of Mental Health and Well-Being. The American Journal of Psychiatry. 2006;163(4):652–658. doi: 10.1176/ajp.2006.163.4.652. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus 7.11. Los Angeles, CA: Muthén & Muthén; 2013. [Google Scholar]
- National Institute on Drug Abuse. Substance Abuse in the Military. Retrieved from http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military on February 16, 2015.
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. http://dx.doi.org/10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. http://dx.doi.org/10.1037/1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Hesselbrock M, Dick DM. Polygenic risk for externalizing disorders gene-by-development and gene-by-environment effects in adolescents and young adults. Clinical Psychological Science. 2015;3(2):189–201. doi: 10.1177/2167702614534211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Verona E. Analysis of monoaminergic genes, childhood abuse, and dimensions of psychopathy. Journal of Abnormal Psychology. 2013;122(1):167–179. doi: 10.1037/a0029866. http://dx.doi.org/10.1037/a0029866. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Miller MW, Wolf EJ, Harkness KL. Negative emotionality and disconstraint influence PTSD symptom course via exposure to new major adverse life events. Journal of Anxiety Disorders. 2015;31:20–27. doi: 10.1016/j.janxdis.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A. Brief manual for the multidimensional personality questionnaire. University of Minnesota; Minneapolis: 1982. –1031.pp. 1010 Unpublished manuscript. [Google Scholar]
- Vassos E, Collier DA, Fazel S. Systematic meta-analyses and field synopsis of genetic association studies of violence and aggression. Molecular Psychiatry. 2014;19(4):471–477. doi: 10.1038/mp.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment. 1999;11:124–133. http://dx.doi.org/10.1037/1040-3590.11.2.124. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. Psychological Corporation; San Antonio, TX, USA: 2001. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-IV. San Antonio, TX: Psychological Corporation; 2008. [Google Scholar]
- Widom CS. The cycle of violence. Science. 1989;244(4901):160–166. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- Wilson HW, Stover CS, Berkowitz SJ. Research Review: The relationship between childhood violence exposure and juvenile antisocial behavior: a meta-analytic review. Journal of Child Psychology and Psychiatry. 2009;50(7):769–779. doi: 10.1111/j.1469-7610.2008.01974.x. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. Journal of Abnormal Psychology. 2010;119(2):320. doi: 10.1037/a0019035. http://dx.doi.org/10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N, Frueh BC, Shi J, Gerardi D, Fabrikant N, Schumann BE. Trauma exposure and mental health characteristics of incarcerated females self-referred to specialty PTSD treatment. Psychiatric Services. 2011;62(8):954–958. doi: 10.1176/ps.62.8.pss6208_0954. [DOI] [PubMed] [Google Scholar]
- Wolff N, Shi J. Childhood and adult trauma experiences of incarcerated persons and their relationship to adult behavioral health problems and treatment. International Journal of Environmental Research and Public Health. 2012;9(5):1908–1926. doi: 10.3390/ijerph9051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Foran HM, Wood MD, Eckford RD, McGurk D. Alcohol problems, aggression, and other externalizing behaviors after return from deployment: understanding the role of combat exposure, internalizing symptoms, and social environment. Journal of Clinical Psychology. 2012;68(7):782–800. doi: 10.1002/jclp.21864. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118(1):117. doi: 10.1037/a0014657. http://dx.doi.org/10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]