Preface
Posttraumatic stress disorder (PTSD) is the only major mental disorder for which a cause is considered to be known, viz., an event that involves threat to the physical integrity of oneself or others and induces a response of intense fear, helplessness, or horror. Although PTSD is still largely regarded as a psychological phenomenon, over the past three decades the growth of the biological PTSD literature has been explosive, and thousands of references now exist. Ultimately, the impact of an environmental event, such as a psychological trauma, must be understood at organic, cellular, and molecular levels. The present review attempts to present the current state of this understanding, based upon psychophysiological, structural and functional neuroimaging, endocrinological, genetic, and molecular biological studies in humans and in animal models.
Introduction
A day scarcely passes that one does not see a mention of PTSD in the media. However, this has not always been the case. In American history, posttraumatic psychopathology has been recognized under various names following wars: soldier’s heart from the Civil War, shell shock from WW I, combat fatigue from WW II, delayed stress from the Vietnam War. However, between these wars, the condition was all but forgotten. Finally, spearheaded by Vietnam veterans and their advocates, PTSD made its way into the American psychiatric nomenclature as a formal diagnostic entity in 1980.1 Because of the political impetus behind its introduction, and the fact that PTSD is largely diagnosed on the basis of patients’ reports (which may be influenced by secondary motives), the new disorder was initially met with suspicion. Discoveries of biological markers for PTSD, however, have gone a long way to counteracting this skepticism and bolstering the now widespread acceptance of the disorder.
As currently understood, the PTSD syndrome is a blend of intrusive memories of the traumatic event, avoidance of reminders of it, emotional numbing, and hyperarousal.2 Initially, PTSD was conceptualized nearly entirely in psychological terms, and the PTSD biological literature consisted only of sparse psychophysiological observations.3 Although purely psychological research into PTSD is important, it is enhanced by an understanding of the neurobiological mechanisms underlying the disorder. The purpose of this article is to review the current state of biological research into PTSD. Such a review is timely and important if the popular understanding of PTSD is not to outstrip its scientific basis, and if PTSD is to be grounded in the field of medicine. This article will also review animal models, which have stimulated hypotheses about PTSD in humans and which point the way to future developments in the field. The ultimate goals of biological research are to identify risk factors, elucidate the mechanisms involved in the development of PTSD, establish biomarkers, and generate novel preventive and therapeutic interventions aimed at alleviating the substantial suffering and dysfunction this disorder imposes.
It is tempting to assume that because PTSD by definition is caused by a psychologically traumatic environmental event, any biological abnormality found to accompany PTSD must also have been traumatically induced. However, it is also possible that an abnormality pre-dated the traumatic event and came to be associated with PTSD because it increased the risk of this disorder’s developing upon the traumatic exposure — a psychiatric epitome of gene x environment interaction. Readers should keep this in mind as they digest the rich material that follows.
Psychophysiological Studies
Measures of heart rate, skin conductance, facial electromyogram (EMG), and cortical electroencephalographic event-related potentials (ERPs) have been extensively applied to the study of PTSD for more than 25 years.4, 5 A robust literature addresses the heightened emotional reactivity to trauma-related cues, exaggerated startle, impaired extinction, and increased sensitivity to stimulation observed in people with PTSD, as well as their use in predicting PTSD risk and assessing treatment outcome. A meta-analysis of studies comparing resting psychophysiological levels, trauma-related cue reactivity, and exaggerated startle responses between groups of individuals diagnosed with vs. individuals without PTSD attests to the maturation of this field.6
Psychophysiological markers for PTSD
The majority of psychophysiological research on PTSD has been performed in cross-sectional studies that compared individuals with PTSD to trauma-exposed or -unexposed individuals without the disorder. One of the earliest and most replicated PTSD findings is that of heightened autonomic (heart rate, skin conductance) and facial EMG reactivity to external, trauma-related stimuli such as combat sounds and film clips 7, as well as to internal, mental imagery of the traumatic event 8. Reactivity to trauma-related cues correlates with severity of the disorder.9, 10 In addition to research focused on reactions to trauma reminders, a substantial body of work has examined exaggerated startle, as measured by eyeblink EMG, to sudden loud sounds.4, 6 Although there is compelling evidence for increased startle in PTSD, it is unclear whether this represents a pre-existing trait, increased sensitivity to contextual threat, or sensitization of the nervous system. Patients with PTSD also show heightened heart rate responses to startling stimuli, which appear to be acquired.11, 12 Reduced P3b ERPs to infrequent target stimuli, and larger skin conductance responses to novel stimuli4 could reflect the PTSD symptoms of difficulty concentrating and hypervigilance respectively.
Psychophysiological assessments of treatment outcome provide more objective information than a patient’s report and may be a more sensitive measure of progress. For example, heart rate, skin conductance and facial EMG reactivity during personal traumatic imagery was lower in a group that had received post-trauma propranolol, a β-adrenergic antagonist that has been found in animal and human studies to attenuate the consolidation of stressful memories, compared to placebo, even though groups did not differ in subjective PTSD symptom severity.13 A recent study that examined psychophysiological responses before and after cognitive-behavioral therapy for PTSD found that treatment responders showed a significant reduction in eye-blink EMG, heart rate, and skin conductance responses to loud tones, whereas treatment non-responders did not.14 Understanding discordances between psychophysiological and subjective measures of treatment outcome will require further research. Pre-treatment psychophysiological assessments might be examined for their usefulness in guiding treatment selection. For example, individuals who show heightened psychophysiological reactivity to trauma-related cues might benefit from exposure-based therapies that aim to desensitize the emotional arousal associated with traumatic memories.
Conditioning and sensitization
Symptoms related to re-experiencing of the traumatic event are a defining feature of PTSD and may be conceptualized within a fear-conditioning framework. In contrast, anxiety and hyperarousal in the absence of trauma-related cues may reflect a general sensitization of the nervous system. Alterations in fear conditioning, extinction learning, extinction retention, and sensitization are likely involved in the development and/or maintenance of PTSD.15 Recent findings suggest that PTSD is associated with deficits in the ability to extinguish or maintain extinction learning of an acquired fear response, as measured by skin conductance16 and/or fear-potentiated startle.17 In addition, PTSD has been found to be associated with extinction failure of a second-order conditioned skin conductance response, after this response was established by pairing a neutral stimulus with a trauma-specific stimulus.18 A recent study in identical twins discordant for combat exposure (i.e., one member of each pair was a Vietnam combat veteran and the other had no combat exposure) found that PTSD twins showed poorer extinction retention of a conditioned skin conductance response; this appeared to be an acquired, rather than pre-existing, PTSD feature, because it was not shared by the PTSD veterans’ twins.19 Impaired extinction or extinction retention would likely interfere with recovery from PTSD symptoms that are based upon conditioned fear.
Evidence for increased nervous system sensitization comes from findings of larger heart rate responses to loud-tone stimuli and larger skin conductance orienting responses,4 as well as increased intensity dependence of the P2 ERP response.20 In a study that involved both startle and conditioned fear assessments, individuals with PTSD were found to show increased eyeblink EMG startle responses during testing that followed a previous session involving aversive fear conditioning.21 A possible explanation for this finding is that administration of the shock unconditioned stimulus several days earlier sensitized individuals with PTSD such that they showed a subsequent increase in baseline startle reactivity. Findings from identical twin11, 20 and longitudinal12, 22 studies strongly suggest that the heightened heart rate reactivity to loud tones observed in individuals with PTSD is an acquired feature of the disorder; however, it may not be specific to PTSD.23
Psychophysiological risk factors for PTSD
The possibility that some measures of psychophysiological reactivity represent pre-existing vulnerability traits has stimulated research examining potential predictors of risk for developing PTSD. A prospective study of firefighter trainees found that increased skin conductance and eyeblink EMG responses to a series of loud tones24 and slower extinction of fear-conditioned corrugator EMG responses25 predicted severity of posttraumatic stress symptoms following subsequent exposure to a traumatic event. Another prospective study also implicated slower extinction of fear-conditioned corrugator EMG responses in predicting posttraumatic stress symptoms.26 Increased physiological reactivity to trauma-related cues soon after exposure to a traumatic event is predictive of subsequent severity and/or persistence of PTSD symptoms.9, 27, 28 For example, women who showed an increased heart rate response while engaging in personal traumatic imagery two weeks after trauma exposure had higher symptom severity and were more likely than female nonresponders and men to develop PTSD six months later.10 Whether resting heart rate obtained immediately post-trauma predicts the subsequent development of PTSD is currently unclear.27, 29
Sleep
Although sleep disturbance is a PTSD diagnostic criterion, support for it in the polysomnography laboratory has not been as straightforward as might have been expected. However, more Stage 1 sleep and less slow wave sleep, indicative of shallower sleeping, as well as greater rapid-eye-movement density, have been demonstrated in PTSD subjects.30
Structural Neuroimaging Studies
Because of findings in experimental animals suggesting that chronic stress damages the hippocampus,31 this brain structure was a logical starting place for structural magnetic resonance imaging (sMRI) studies in PTSD patients. In fact, the most replicated structural abnormality found in PTSD is lower volume of the hippocampus. Lower volume of ventromedial prefrontal cortex (vmPFC), another structure damaged by chronic stress in animals, has also been reported. However, just because these structures can be damaged by chronic stress in animals does not necessarily mean that this is the origin of their diminutions in PTSD; they may also represent premorbid risk factors. To the extent that diminished volume may underlie diminished function, these findings whatever their origin are consistent with a model of PTSD that posits a reduced cortical capacity to inhibit fear and other negative emotional responses. In this view, hippocampus may fail to utilize contextual cues in the environment to signal safety, and vmPFC may fail to adaptively maintain extinction of conditioned emotional responses once traumatic learning is no longer relevant.
Hippocampus
Pioneering sMRI studies found significantly smaller hippocampi in subjects with PTSD compared to trauma- and non-trauma-exposed subjects without PTSD.32–34 Since then, a large literature has emerged, the majority of which, along with meta-analyses,35 has provided empirical support for lower hippocampal volume in PTSD. A recent study that utilized high-resolution sMRI found the most substantial hippocampal volumetric diminution in the CA3 and dentate gyrus subfields.36 Recent meta-analyses have concluded that smaller hippocampal volume in PTSD is bilateral,37, 38 and they have not provided strong evidence for a gender effect.39 The severity of PTSD symptoms may be an important factor in determining effect sizes of PTSD-related hippocampal differences;38, 40 studies of adults with PTSD that failed to replicate the finding of smaller hippocampal volumes generally employed subjects with less severe or less chronic illness.41, 42 Studies conducted in children with PTSD have often also failed to reveal smaller hippocampi, suggesting a role for neuromaturational factors in the development of hippocampal diminution.43 Numerous investigations have attempted to control for the impact of confounding comorbid conditions; most have concluded that hippocampal volume differences in PTSD are not accounted for by histories of alcohol abuse or depression.40, 44, 45 Moreover, studies using magnetic resonance spectroscopic imaging (MRSI), which quantifies N-acetylaspartate (NAA) as a marker of neuronal density, have also consistently found neuronal reduction in the hippocampus of patients with PTSD.46, 47
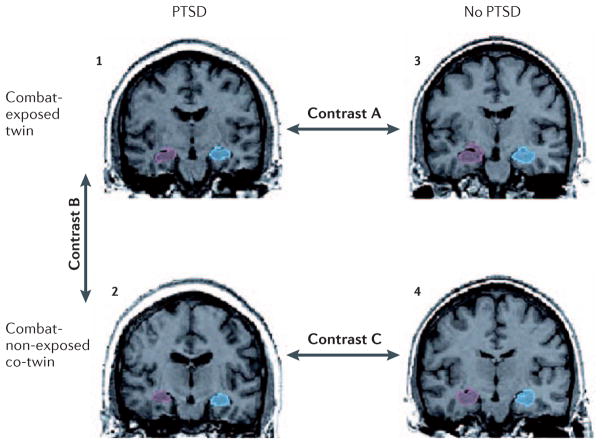
Controversy exists as to whether smaller hippocampal size in PTSD is a result of trauma exposure or rather represents a risk factor for PTSD that is of genetic and/or shared environmental origin. A study of identical twins discordant for combat exposure in Vietnam (Figure 1) found that the (high-risk) combat-unexposed, non-PTSD co-twins of combat veterans with PTSD had hippocampal volumes that were comparable that of their PTSD twins but lower than the hippocampal volume of combat veterans without PTSD and their (low-risk) unexposed co-twins.40 This finding suggests that hippocampal volume serves as a pre-trauma risk factor for PTSD. Cavum septum pellucidum (so-called “fifth ventricle”), a neurodevelopmental abnormality in part related to hippocampal maldevelopment, is found more frequently in persons with PTSD.48 Not all neuroimaging evidence is consistent with a risk factor interpretation of hippocampal diminution.49 Pharmacological treatment of PTSD with the selective serotonin reuptake inhibitor (SSRI) paroxetine has been reported to increase hippocampal volume,50 suggesting that it could be an acquired, reversible abnormality; however, even pre-existing risk factors may be malleable. In addition, although PTSD subjects were found to show diminished hippocampal volume relative to trauma-exposed subjects without PTSD, this difference was smaller than that observed in the comparison between PTSD subjects and non-trauma-exposed control subjects.37, 38 Similarly, a recent meta-analysis revealed that non-PTSD, trauma-exposed subjects have smaller hippocampi than non-PTSD, non-trauma-exposed subjects.51 These findings suggest that trauma may reduce hippocampal volume regardless of the development of subsequent PTSD, or that reduced hippocampal volume may also represent a risk factor for trauma exposure. However, trauma-exposed subjects who are not categorically classified as having PTSD may still have some PTSD symptoms, and this could also account for their lower hippocampal volume. In short, the debate between risk-factor vs. acquired origin of hippocampal diminution in PTSD has not been resolved; it is quite possible that both play a role.
Figure 1. Assessing structural abnormalities in PTSD using a combat-discordant identical-twin design.
Sample coronal structural magnetic resonance images of right (red) and left (blue) hippocampi in a twin pair consisting of a combat veteran with PTSD (1) and his combat-unexposed co-twin (2), who has no PTSD but is considered “high risk” because his identical twin developed PTSD when exposed to trauma; as well as a control twin pair consisting of a combat-exposed veteran without PTSD (3) and his “low-risk” combat-unexposed co-twin (4), who also has no PTSD. Contrast A provides a replication test of studies demonstrating smaller hippocampal volume in combat veterans with vs. without PTSD. Two additional contrasts can shed light on whether this abnormality is a result of combat exposure or of having PTSD, or whether it represents a pre-existing vulnerability factor. Contrast B compares hippocampal volumes in combat-exposed PTSD veterans with their own high-risk co-twins. If the twin with PTSD (1) has smaller hippocampal volume than his co-twin (2), the trait has likely been acquired. Contrast C compares hippocampal volumes in high vs. low-risk co-twins. If the trauma unexposed, non-PTSD twin of the veteran with PTSD (2) has smaller hippocampal volume than the unexposed, non-PTSD twin of the veteran without PTSD (4), it is likely that the trait represents a pre-existing vulnerability factor. This type of design can also be used to assess the origin of other abnormalities observed in patients with PTSD.
Prefrontal cortex
The PTSD neuroimaging literature also documents volume reductions in prefrontal brain regions. Structural MRI studies have found reduced volume in individuals with PTSD in both the rostral (or pregenual) vmPFC52 and in the dorsal anterior cingulate cortex (dACC).53 Youth with more PTSD symptoms (but not full-blown PTSD) were found to have less total brain tissue and lower total cerebral gray matter volumes, with specific diminution in left ventral inferior prefrontal regions.54 In contrast to the findings in hippocampus, a sMRI study of combat-discordant twins that employed voxel-based morphometry suggested that reduction in ACC volume represents an acquired feature of PTSD rather than a pre-existing vulnerability.52 MRSI studies have also reported diminished neuronal density in the ACC of PTSD patients.46, 47 Furthermore, studies employing diffusion tensor imaging, which provides a measure of the integrity of white matter tracts, have shown aberrant white matter integrity in the cingulum bundle, a major neuronal tract that connects the ACC with the amygdala,55 which may be a basis for impaired inhibitory interaction between these two regions, as further discussed below.
A recent study capitalized on a sample of subject who had undergone sMRI prior to an earthquake; 42 subjects returned for re-scanning afterwards. Although none of these developed full-blown PTSD, higher gray matter volume in the right ventral ACC before the earthquake were negatively associated with PTSD symptoms, i.e., conferred resilience. Subjects with more PTSD symptoms showed a greater decrease in gray matter volume in the left orbitofrontal cortex from before to after the earthquake, suggestive of acquired diminution in this brain region.56
Functional Neuroimaging Studies
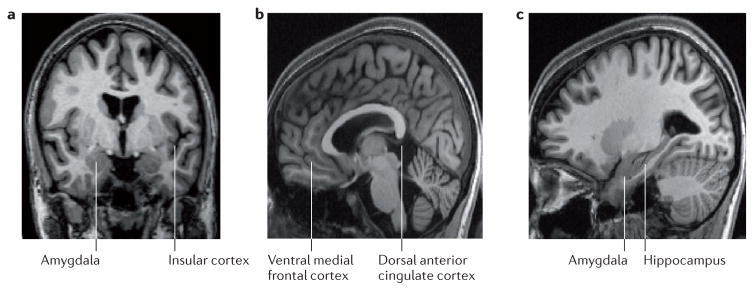
Functional neuroimaging studies using positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) have shown altered activity in individuals with PTSD in the amygdala, vmPFC and dACC, as well as in the hippocampus and insular cortex (Figure 2.) During the last two decades, rodent studies have outlined the neural networks involved in fear learning and its extinction. These have shown that the amygdala is a key structure for both the recognition of dangerous stimuli and the coordination of the fear response. Its activity is modulated by higher cortical influences — in rodents mainly by the infralimbic (IL) and prelimbic (PL) cortices. Evidence from pharmacological, electrophysiological, stimulation, and molecular approaches in animals points to PL as a brain region facilitating the expression of conditioned fear, whereas IL is critical for the consolidation and expression of fear extinction memory (i.e. extinction retention), resulting in lowered fear.57–59 Fear conditioning and extinction paradigms in humans have identified functionally homologous brain regions in the human brain, with the dACC and vmPFC being putative human homologues of PL and IL, respectively. 59
Figure 2. Brain regions implicated in PTSD functional neuroimaging studies.
The amygdala (shown in Panels A and C) is involved in recognizing both conditioned and unconditioned stimuli signaling danger, as well as in expressing the fear response. Amygdala reactivity is exaggerated in individuals with PTSD and is positively correlated with symptom severity. The insular cortex (Panel A) and dorsal anterior cingulate cortex (Panel B) are also hyperreactive in PTSD; these structures may modulate (in these cases enhance) the amygdala’s expression of fear. In contrast, activation in the ventral medial prefrontal cortex (Panel B), which also modulates (in this case reduces) the amygdala’s expression of the fear response, is diminished in PTSD; vmPFC activity is also negatively correlated with symptom severity. Functional neuroimaging findings in the hippocampus (Panel C), which is involved in recognizing both safe and dangerous contexts, have been mixed in PTSD, with both hypo- and hyperreactivity observed.
Amygdala
The amygdala plays a crucial role in the detection of threat, fear learning, fear expression, and heightening memory for emotional events. Functional neuroimaging studies have reported exaggerated amygdala activation in response to trauma-related stimuli (e.g., narratives, photographs, odors, sounds)60 as well as generic stimuli (e.g., fearful facial expressions, emotional photographs, tones) in patients with PTSD compared to control subjects.61 PTSD patients show greater amygdala activation during the acquisition of conditioned fear.62
Ventral medial prefrontal cortex
Areas in the vmPFC (including the rostral ACC), subcallosal cortex, and medial frontal gyri show decreased activation in PTSD subjects during tasks that employ either trauma-related63 or trauma-unrelated64, 65 stimuli. Ventromedial PFC activation during the recollection of personal traumatic events negatively correlates with PTSD symptom severity.66 Furthermore, degree of symptomatic improvement after cognitive behavioral therapy has been positively correlated with increase in rostral ACC activation.67 In PTSD subjects, failure to recall extinction learning is accompanied by lower vmPFC activation68 (Figure 3). PTSD subjects also show deficient vmPFC activation during emotional cognitive interference tasks.69
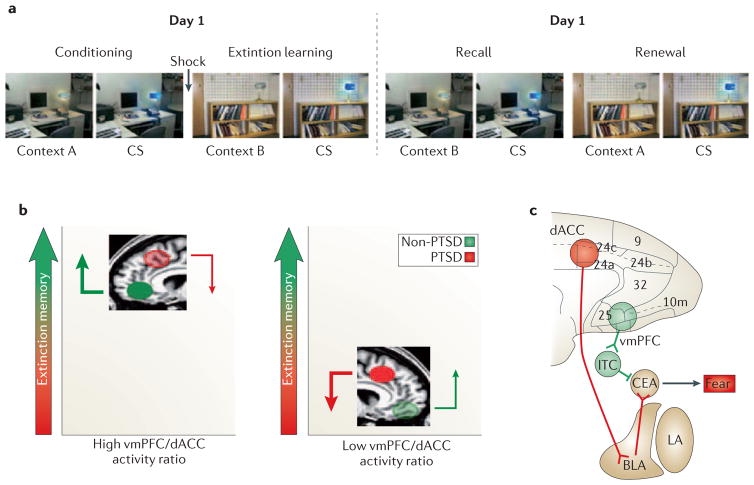
Figure 3. Contribution of prefrontal regions to fear regulation and expression.
A. Activation in the human brain during fear conditioning and extinction can be investigated in a Pavlovian fear conditioning and extinction paradigm. During conditioning, a conditioned stimulus (a colored light) is paired with a mild electric shock to the fingers in a particular context (context A). The acquisition of conditioned fear in this paradigm can be measured by the skin conductance response to the light. During extinction learning, the light is subsequently repeatedly presented without the shock in a different context (context B). This leads to extinction of the conditioned response. The next day, the light is again presented in the absence of the shock in Context B (extinction recall), and then again in Context A (fear renewal). Greater retention of extinction learning is associated with a lower fear response during extinction recall. Extinction retention is context-dependent: the hippocampus is thought to recognize whether a context is safe (B) or dangerous (A) and to communicate this information to other structures in the fear network.
B. fMRI studies have shown that activation of ventromedial prefrontal cortex (vmPFC, shown in green) during extinction recall is positively correlated with extinction retention,70 whereas activation of dorsal anterior cingulate cortex (dACC, shown in red) is negatively correlated with extinction retention.71 Ventromedial PFC sends excitatory glutamatergic projections to gamma-aminobutyric acid (GABA) -ergic intercalated cells (ITC) in amygdala, which in turn inhibit the expression of the fear response by the amygdala’s central nucleus (Ce). In contrast, dACC sends excitatory glutamatergic projections to amygdala’s basolateral nucleus, which in turns activates the expression of the fear response by Ce. LA=lateral amygdala nucleus.
C. Non-PTSD subjects (left) have a relatively high vmPFC/dACC activation ratio during extinction recall, which tips the balance toward better extinction retention and less fear expression. By contrast, PTSD subjects (right) have a lower vmPFC/dACC activation ratio, which tips the balance toward less extinction retention and more fear expression.68
Dorsal anterior cingulate cortex
The dACC subserves response selection, error detection, pain perception, and fear learning and expression. Activation in the dACC is increased in PTSD vs. control groups during fear conditioning,72 reduced recall of extinction learning68 (Figure 3), and auditory oddball tasks73, 74 (which measure responses to infrequent or novel stimuli). Such functional abnormalities are positively associated with PTSD symptom severity.75 Individuals with PTSD as well as their identical (high-risk) co-twins showed greater resting dACC glucose metabolism in a PET study,76 and greater dACC activation during a non-emotional cognitive interference task in an fMRI study,77 compared to trauma-exposed individuals without PTSD and their identical (low-risk) co-twins. These latter findings suggest that elevated dACC activity is a biomarker reflecting familial risk for developing PTSD after trauma.
Hippocampus
The hippocampus is involved in the encoding and recognition of episodic memories and environmental cues (e.g., contexts), including those that are present during fear learning and extinction. Functional neuroimaging findings in the hippocampus in patients with PTSD have been mixed, with some studies reporting less78 and others reporting more79 activation than in comparison groups. These different findings could be due to differences in the types of paradigms and/or analyses conducted across studies. In PTSD subjects, failure to recall extinction learning is associated with lower hippocampal activation.68 Whether potential functional abnormalities in the hippocampus in PTSD are linked to smaller hippocampal volume has yet to be determined.
Insular cortex
The insular cortex is involved in monitoring internal bodily states. Individuals with PTSD exhibit greater insular cortex activation, possibly reflecting heightened detection of bodily arousal, during the anticipation of aversive images and in response to fearful facial expressions, painful stimuli, and traumatic memories.80–82 Insular cortex activation appears to be positively correlated with PTSD symptom severity. 80 Elevated insular cortex activation has also been observed in other anxiety disorders61 and therefore is not specific to PTSD.
Neurocircuitry of PTSD
A recent meta-analysis of 79 functional PTSD neuroimaging studies found that mid- and dorsal anterior cingulate cortex and bilateral amygdala were the most hyperactivated regions, whereas vmPFC and inferior frontal gyrus were the most hypoactivated regions. Decreased vmPFC activity was associated with increased amygdala activity.83 Neurocircuitry models of PTSD84, 85 posit that vmPFC fails to inhibit the amygdala, leading to attentional bias toward threat, increased fear responses, impaired extinction of traumatic memories and its retention, and deficits in emotion regulation. The later neurodevelopment of prefrontal cortex relative to amygdala may help to explain the increased risk for PTSD associated with younger age in combat veterans.86 In contrast to vmPFC, dACC appears to promote fear expression. Hippocampal dysfunction may be associated with memory impairments for neutral material and deficits in recognizing safe contexts. A hyperactive insular cortex, which may reflect heightened interoceptive awareness, may confer proneness to anxiety.
Receptor imaging
A functional imaging technique that has been less utilized in PTSD is the use of selective, exogenously administered radioligands to assess the binding and distribution of various neurotransmitter receptors in the brain via PET. One such study found a reduction in GABAA binding throughout the cortex, hippocampus, and thalamus in veterans with compared to without PTSD,87 suggestive of globally diminished inhibitory brain function. The fact that the changes were so widespread prohibits relating this finding to any specific PTSD neurocircuitry. Another study of non-combat-related PTSD found decreased serotonin (also known as 5-hydroxytryptamine or 5-HT) transporter binding in the amygdala,88 which the authors suggested is consistent with findings of amygdala hyperactivity upon exposure to trauma- or fear-related stimuli in PTSD reviewed above. However, the absence of a trauma-exposed, non-PTSD control group limited inferences regarding the specificity of this finding to PTSD. Indeed, in two other exogenous ligand studies — one that found reductions in μ-opioid receptor binding potential in various limbic and paralimbic brain regions,89 and another that found reduced serotonin-1B receptor expression in caudate, amygdala, and ACC90 — abnormalities occurred both in PTSD subjects and in trauma-exposed, non-PTSD subjects, raising the possibility that they reflect the effect of trauma exposure on the brain rather than specific aspects of PTSD pathophysiology. Finally, interpretation of results of exogenous ligand studies is often obscured by incomplete knowledge of the underlying microanatomy (e.g., neuron is an excitatory projection neuron vs. an inhibitory interneuron; receptor is a post-synaptic receptor vs. an autoreceptor vs. a heteroceptor) and physiology (e.g., binding is lower because receptor number/affinity is reduced vs. binding is lower because receptor is already occupied with endogenous ligand; down (or up) -regulation is primary vs. compensatory). Variations in these details can have different, or even opposite, implications.
Neuroendocrinological Studies
Abnormalities in catecholamines and cortisol were among the first findings in individuals with PTSD. Subsequent studies have revealed changes in many other hormonal and neuroregulatory factors among populations of patients with PTSD as well.
Catecholamines
Numerous studies have provided compelling evidence for the presence of sympathetic system hyperreactivity in PTSD.91 It has been suggested that an excessively strong adrenergic response to the traumatic event may mediate the formation of the durable traumatic memories that in part characterize the disorder.92 Factors that may contribute to the increased release of norepinephrine (NE, also known as nordrenaline) in response to sympathetic system activation in PTSD include genetic or stress-induced decrements in neuropeptide Y (NPY),93 which inhibits norepinephrine release, as well as a lower number94 or affinity95 of alpha-2-adrenergic autoreceptors. Noradrenergic hyperreactivity in patients with PTSD is associated with hyperarousal and re-experiencing symptoms, including trauma-related nightmares, flashbacks, intrusive memories, and emotional and physiological reactions to traumatic cues.96–99 In addition, sympathetic system activation induced by administration of yohimbine (an alpha-2-adrenergic autoreceptor antagonist) decreased orbitofrontal and prefrontal cortical metabolic activity in subjects with PTSD, whereas it increased metabolic activity in these regions in control subjects.100 Studies demonstrating the efficacy of prazosin, a post-synaptic alpha-1-noradrenergic receptor inhibitor, for the treatment of nightmares or daytime hyperarousal and re-experiencing symptoms of PTSD101, 102 are consistent with these findings. Results of early studies suggested that propranolol, a beta-adrenergic antagonist, had value in the acute prevention of PTSD,13, 103 but these results have not been consistently replicated.104, 105
Indoleamines
The serotonin system also appears to be implicated in both the acute mediation of PTSD symptoms and the modulation of PTSD risk, as neuropharmacological, treatment, and genetic epidemiological studies have indicated. Administration of meta-chlorophenylpiperazine (mCPP), which acts as a 5-HT agonist, resulted in acute anxiety, panic attacks, and PTSD symptoms (including flashbacks) in a subgroup of male combat veterans with PTSD.106 Interaction of mCPP with the 5-HT transporter107 and multiple 5-HT receptor subtypes results in increased extracellular 5HT, as well as behavioral, psychological, and cognitive effects reminiscent of PTSD that are reversible by administration of mixed 5HT1c/5HT2 antagonists.108 This suggests that a phasic increase in serotonin acting at post-synaptic 5HT1c/5HT2 receptors may induce PTSD symptom expression, which is supported by findings in the rodent single prolonged stress model of PTSD (see below). It also accords with the potential clinical benefits of chronic use of selective serotonin reuptake inhibitor (SSRI) drugs, which appear to result in 5HT2c receptor desensitization.109 Further evidence for a role of the serotonin system in PTSD is provided by studies of the serotonin transporter gene (see below).
Neuropeptide Y
Research has demonstrated that NPY has protective effects during stress, likely mediated by the modulation of sympathetic responses93 and antagonism of the anxiogenic effects of corticotropin-releasing hormone (CRH, also known as corticotropin releasing factor, or CRF).110 Humans possessing NPY gene variants associated with increased gene expression showed lower levels of trait anxiety and less amygdala reactivity to emotionally provocative stimuli.111 Similarly, male military personnel who exhibited higher plasma NPY levels during intense training stress showed less distress and dissociation and superior performance.112 In contrast, lower cerebrospinal fluid (CSF) and resting plasma NPY levels and/or a blunted NPY response to sympathetic activation have been associated with more PTSD symptoms.93, 113 In addition, a retrospective study in male veterans showed that lower plasma NPY levels were associated with less improvement in PTSD symptoms over time.114 Efforts to develop pharmacological agents with clinically relevant, circumscribed NPY receptor-mediated effects have so far been unsuccessful. Manipulating the NPY system epigenetically may have greater promise as a strategy for the development of NPY-based therapeutics.
Corticotropin-releasing hormone
Corticotropin-releasing hormone has anxiogenic physiological and behavioral effects.115 Increased CRH levels have been found in the CSF and/or blood of several different cohorts of patients with PTSD.116, 117 However, a recent study found that CSF CRH levels decreased among PTSD patients watching a trauma-related video,118 indicating that much is yet to be learned about the dynamics of CRH in the CNS during stress. Although recent clinical treatment trials of newly developed CRH antagonists have had to be aborted due to hepatic toxicity, interest in pharmacological treatments for PTSD directed at the CRH system continues.
Cortisol
Early research produced the paradoxical finding of abnormally low cortisol levels in PTSD,119 although this finding has not been consistently replicated in large studies. Nevertheless, sufficient evidence has accumulated to conclude that PTSD is not characterized by elevated tonic cortisol levels,120 as might be expected in a state of chronic stress. In contrast, greater suppression of plasma cortisol after a low dose of dexamethasone, reported in some studies of PTSD,121 is consistent with excessive shutting down of the hypothalamic-pituitary-adrenal cortical (HPA) axis due to enhanced sensitivity to negative feedback. Research has supported a higher number of glucocorticoid receptors (GRs) in lymphocytes of such PTSD subjects.122 The foregoing findings are thought to represent, at least in part, a pre-trauma risk factor for the disorder.123 A gene of current high interest related to PTSD risk, viz., FKBP5, is a co-chaperone of the glucocorticoid receptor, and polymorphisms in this gene have been related to GR supersensitivity and risk for PTSD.124 An exception to the absence of elevated cortisol in PTSD patients may presented by persons, mainly women, with comorbid major depressive disorder.108, 125, 126 Premenopausal women with PTSD also exhibited elevated phasic cortisol responses to CRH and adrenocorticotropin.127
The proposition that a cortisol deficit may play a role in the pathogenesis of PTSD has been supported by findings that administration of supplemental doses of cortisol to acutely ill medical patients reduces the PTSD outcome.128 A recent preliminary study found that high-dose cortisol administration within hours of a traumatic event reduced the subsequent development of PTSD;129 these encouraging therapeutic results call for testing in larger samples. Another study found beneficial effects of cortisol administered to patients who already had PTSD, which was attributed to the well-recognized inhibitory effect of cortisol on memory retrieval (in this case traumatic memory retrieval).130 However, the possibility that cortisol could worsen PTSD in some cases, e.g., in patients without a deficit, should not be dismissed.
Despite the above, there still exists preclinical evidence suggesting the possibility that increased responses of this stress hormone could play a role in PTSD pathogenesis. Cortisol, like epinephrine, potentiates memory consolidation;131 an acute cortisol response to the traumatic event could contribute to formation of a durable traumatic memory. Whether this possibility is relevant to PTSD remains to be directly tested. Baseline levels, phasic responses to stress, dosage (physiological vs. pharmacological), target of action, and the possibility of an inverted U-shaped, dose-response curve may all be important parameters to address in resolving the mixed cortisol findings in PTSD. Indeed, both too high and too low a level of glucocorticoids can interfere with frontal lobe-mediated working memory and long-term potentiation (LTP) in the hippocampus, in part through dose- and time-dependent effects on glutamatergic neurotransmission during learning.132 On the other hand, glucocorticoids are critical to stress adaptation, and many genes with relevance to resilience, including those encoding enzymes involved in the synthesis of allopregnanolone (see below) and NPY, contain glucocorticoid response elements.133
In future clinical research, it will be important to evaluate cortisol (and CRH) levels in relation to other neuroendocrinological factors that it regulates (e.g., allopregnanolone and NPY), or that confer protection from its potentially deleterious effects (e.g., dehydroepiandrosterone, or DHEA). It will also be important to take into account specific psychiatric comorbidities such as depression and nicotine134 and other substance dependence; gender, reproductive and menstrual phase; and genetic polymorphism known to affect HPA axis regulation. (These considerations may apply to other endocrinological variables as well.) It also will be important to study central as well as peripheral cortisol regulation in relation to PTSD symptoms. Results of one study suggest that cortisol regulation in the CNS may differ from that in the periphery. Plasma cortisol levels were comparable between male veterans with PTSD and healthy comparison subjects, whereas CSF cortisol levels (arguably a better reflection of the level of brain exposure to cortisol) were higher in the veterans with PTSD and were correlated with CSF CRH levels116 Recent work has also highlighted the possible importance of performing cortisol measurements by gas chromatography with mass spectrometry (GC-MS) because standard cortisol measurement by radioimmunoassay (RIA) does not discriminate between cortisol and its inactive metabolites. For example, a relationship between low total glucocorticoid species (measured simply as “cortisol” by RIA) and resistance to prolonged exposure therapy for PTSD was attributable to low levels of an inactive cortisol metabolite synthesized by 5-alpha reductase rather than to low levels of cortisol per se.135
Dehydroepiandrosterone
Adrenally derived dehydroepiandosterone (DHEA), the immediate precursor of the androgens, is secreted synchronously with cortisol and is also thought to be the source of its active sulfated metabolite, DHEAS.136 In the brain, DHEA and DHEAS both antagonize GABAA receptors and facilitate NMDA receptor function, which in the amygdala is essential to both fear conditioning and fear extinction. In the hippocampus, DHEA reverses cortisol-induced impairments in LTP, protects against excitatory amino acid- and oxidative stress-induced neuronal damage, regulates programmed cell death, and promotes neurogenesis.136 Such regional neuroprotective effects may be conferred by the anti-glucocorticoid properties of DHEA.137, 138 Although clinical studies have demonstrated increases in adrenal DHEA release and increased plasma DHEA(S) levels in individuals with PTSD, they paradoxically have also shown negative relationships between these indices and general severity of PTSD and comorbid negative mood symptoms.139–142 In addition, there are studies showing a positive relationship between DHEA(S) levels, or the ratio of DHEA to cortisol, and stress resilience in active duty military personnel143, 144 and long-term recovery from PTSD in male veterans.145 These findings could suggest that stress-associated increases in DHEA confer resilience, but such an interpretation may need qualification. For example, sleep disturbance in the context of PTSD has been associated with high DHEA responses to adrenal activation141 and with high baseline blood DHEAS levels.140 It may be that the balance between levels of this neuroprotective, excitatory neuroactive steroid and other neuroendocrine factors such as cortisol or the inhibitory GABAergic neurosteroids such as allopregnanolone146 (see below) is relevant. In humans, administration of DHEA reduces blood levels of cortisol and increases levels of allopregnanolone, testosterone and estrogen, effects that may be variously beneficial in PTSD.147 Chronic DHEA administration has also been found to have antidepressant effects in multiple clinical trials,148 but to date, no trials have assessed its therapeutic effects in PTSD.
Allopregnanolone and pregnanolone
CSF levels of the adrenal- and brain-derived neuroactive steroids allopregnanolone and its equipotent enantiomer pregnanolone (together termed ALLO) relate strongly and negatively to PTSD re-experiencing and depressive symptoms.146 The ratio of allopregnanolone level to the level of its progesterone precursors is also low in the CSF and serum of patients with PTSD, suggesting that ALLO synthesis is deficient. ALLO is the most potent and selective positive endogenous modulator of GABA action at brain GABAA receptors. At extrasynaptic GABAA receptors, ALLO maintains a tonic inhibitory conductance that moderates gain in neuronal output during periods of increased excitation, such as occurs during stress.149 Functionally, ALLO provides long-loop negative feedback at the HPA axis and confers anxiolytic, sedative, anesthetic, neuroprotective, and regenerative effects. Results of an animal study suggest that the effects of SSRIs (the current drug treatment of choice for PTSD) may be due to ALLO increases rather than to serotonin reuptake blockade.150 It is possible that a deficiency in ALLO synthesis accounts for the substantial rate of SSRI treatment resistance in PTSD, but this remains to be determined.
Putative brain-state shift in PTSD
The development of PTSD may involve a shift in brain state from high-level processing of multimodal contextual and mnemonic stimuli (dependent on hippocampus and PFC-mediated working memory) (Figure 4A) — to more primitive amygdala-mediated formation of time-locked sensory associations and expression of the species-specific defense response (Figure 4B). Individual constitutional and personality (e.g., intelligence, neuroticism) differences may influence the stimulus threshold at which this shift occurs and thereby impact vulnerability. A number of neuroregulatory and neuroendocrinological factors discussed above may influence this ‘brain state shift;’ these factors vary across individuals due to genetic and epigenetic influences, as well as within individuals over time due to environmental influences such as intervening stressful events. These neuroendocrine factors probably interact in both counter-regulatory and synergistic manners that are likely to influence PTSD risk, symptom profiles, and severity, as well as capacity for recovery, thus providing potentially exploitable pharmacological and epigenetic targets for the development of new PTSD treatments.
Figure 4. Putative brain-state relevant to PTSD.
Panel A (resilience): The response of either a) previously nontraumatized individuals exposed to mild or moderately arousing unconditioned threat stimuli, b) resilient individuals who are resistant to the arousing effects of more extreme unconditioned threat, or c) individuals with PTSD after undergoing extinction and recovery so that conditioned threat stimuli are no longer highly arousing. Panel B (risk): The response of a) previously nontraumatized individuals exposed to unconditioned threat that is highly arousing, b) individuals with PTSD who are re-exposed to trauma-related cues (conditioned threat) prior to extinction and recovery, or c) individuals with PTSD who are resistant to recovery.
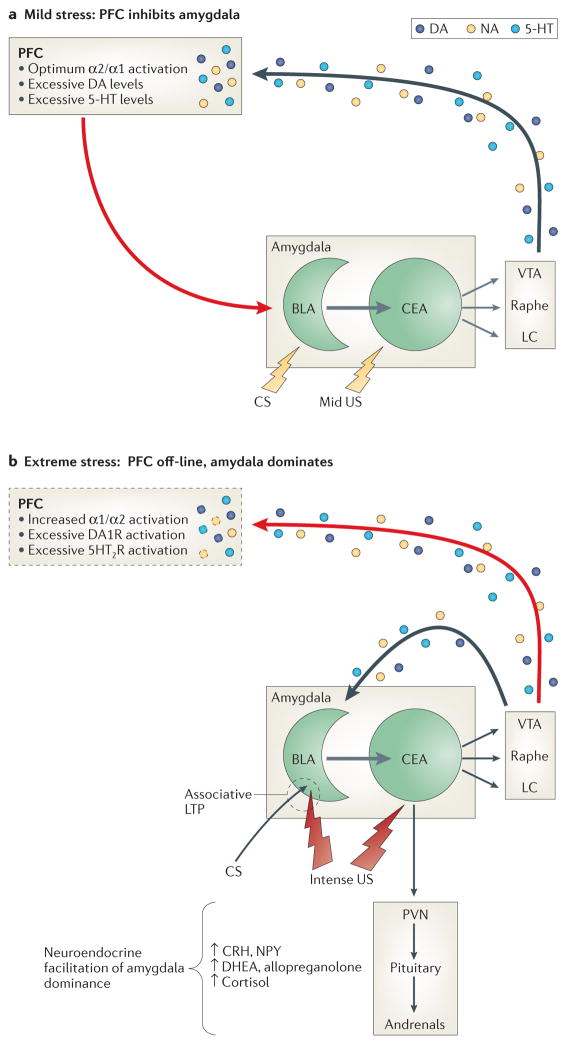
A. Neuromodulation contributing to relative prefrontal cortical dominance (resilience). Mild to moderately arousing sensory stimuli activate the central nucleus (CE) of the amygdala, which projects both directly and indirectly to brainstem monoaminergic cell body regions to activate mesocorticolimbic dopamine (DA) pathways emanating from the ventral tegmental area (VTA), as well as more widely disseminating NE and serotonergic (5-HT) pathways emanating from the locus coeruleus (LC) and median/dorsal raphe nuclei, respectively.151 In the prefrontal cortex (PFC), the resulting mild to moderate increases in synaptic levels of these monoamines engage high-affinity (e.g., noradrenergic alpha-2) receptors to enhance working memory152 and activate glutamatergic pyramidal output neurons that project back to the amygdala. There, glutamatergic activation of GABAergic interneurons in the basolateral (BLA) and/or intercalated nuclei suppresses associative learning and inhibits excitatory BLA pyramidal cell projections to the CE and the expression of the species-specific defense response (see below).153
B. Neuromodulation contributing to relative amygdala dominance (risk). Strongly arousing unconditioned threat stimuli (due to objective threat characteristics or an individual’s increased sensitivity to objective threat) or conditioned threat stimuli in persons with PTSD activate the amygdala to a greater degree, which in turn excites brainstem mesocorticolimbic monoaminergic neurons more vigorously57, 151—a process likely facilitated by reductions in GABAergic neurotransmission within the amygdala.154, 155 In the PFC, higher synaptic levels of these monoamines engage low-affinity noradrenergic alpha-1, as well as DA1 and 5HT2 receptors, resulting in working memory impairment and a reduction in PFC inhibition of amygdala.152 Consequent lifting of the PFC “brake” reduces GABAergic tone within the BLA and intercalated nuclei of the amygdala to enable associative pairing of unconditioned threat stimuli (US) and convergent contextual stimuli (CS) or later reconsolidation of conditioned stimuli, as well as activation of the species-specific defense response (SSDR(, which includes increases in blood pressure and heart rate, HPA axis activation, engagement in reflexive defensive behaviors (fight, flight, freezing), and restriction of high-level information processing to enable efficient focus on survival-relevant phenomena. Direct catecholamine effects in the amygdala also facilitate defensive responding: activation of D1 receptors on PFC projection neuron terminals inhibits glutamate activation of GABAergic interneurons, whereas activation of post-synaptic D2 receptors on BLA to CE pyramidal projection neurons increases their excitation by convergent US-CS inputs, as well as adventitious sensory stimuli. This likely facilitates or maintains associative fear conditioning and may contribute to generalization of fear.156 In contrast, neuronal activity in the BLA is generally suppressed by NE alpha-2 and alpha-1 receptor stimulation, though the latter effect, mediated by enhanced terminal release of GABA, is reduced by chronic stress.157 In contrast NE beta-receptor activation is excitatory and enhances US-CS pairing.158
Genetic Studies
Genetic influences account for 30%159, 160 to 72%161 of vulnerability to PTSD. These estimates take into account genetic factors that may contribute to exposure to traumatic events such as combat or interpersonal violence. 160,162, 163 Genetic influences on exposure to trauma are thought to function largely through heritable personality traits. Genetic risk factors that are common to major depression, generalized anxiety disorder, and panic disorder also account for the majority of genetic variation in PTSD identified to date. Thus, genes that affect risk for PTSD also influence risk for other psychiatric disorders and vice versa. As with other mental disorders,163–166 influences on PTSD are likely polygenic; at least 17 gene variants have been associated with PTSD in at least one published study (Supplementary Table 1). These include genes involved in dopaminergic and serotonergic systems, HPA axis regulation, the locus coeruleus/noradrenergic system, and neurotrophins.
Recent studies have attempted to identify the mechanisms by which gene variants influence PTSD risk. For example, a single nucleotide polymorphism (SNP) in a putative estrogen response element within the gene encoding adenylate cyclase activating polypeptide 1 (pituitary) receptor type I (ADCYAP1R1, also known as PACAPR1) predicted PTSD diagnosis and symptoms in females only.167 This SNP was associated with brain ADCYAP1R1 mRNA expression and fear discrimination. However, the genetic association reported in this paper was not replicated in two large independent samples.168 Similar to other psychiatric disorders, the candidate gene approach has not yet been successful in leading to the identification of robust genetic variants that confer vulnerability to (or resilience against) PTSD.
Gene expression
A growing literature has provided evidence for gene expression patterns that distinguish between individuals with and without PTSD. An early microarray study of RNA derived from peripheral blood cells identified 656 transcripts involving immune and hormonal systems that were differentially expressed in acutely traumatized persons who went on to develop PTSD.169 Another study found 19 differentially expressed transcripts involving immune functions or reactive oxygen species, 5 of which were upregulated and 14 downregulated in people with PTSD compared to individuals without PTSD who had been exposed to the same traumatic event almost 20 years earlier.170 More recently, 16 distinct genes involved in signal transduction, neuronal signaling and survival, and immune cell function, and HPA axis activity were found to be differentially expressed in individual with PTSD vs. individuals without PTSD who had been exposed to the 9/11 World Trade Center attack.171 The gene encoding mannosidase, alpha, class 2C, member 1 (MAN2C1) showed the largest expression difference and had not previously been linked to PTSD. Another recent study found methylation differences in this gene in blood cells of patients with PTSD.172
Epigenetic mechanisms
The lack of consistency of associations between specific genetic variants and PTSD may be explained by epistatic effects (modification of genetic effects by other genes) and/or by environmental factors not accounted for across studies. For example, some studies have found significant effects for specific genetic variants only under conditions of extreme traumatic stress173 or a history thereof.174 The environment modifies genetic effects through epigenetic mechanisms such as DNA methylation. PTSD has been distinguished by methylation profiles suggesting upregulation in immune system-related genes and downregulation in genes involved in neurogenesis and the startle response.175, 176 Methylation of ADCYAP1R1 in peripheral blood was associated with PTSD.167 ADCYAP1R1 appears to be an important gene in fear learning.167
A joint consideration of genotype and methylation patterns may clarify inconsistencies in the PTSD genetics literature. SLC6A4 (also called SERT, HTT, 5HTT, 5-HTTLPR), which regulates synaptic serotonin reuptake and thereby influences emotional behavior, has been the most studied gene in relation to PTSD. Published findings for this locus have been contradictory, with most studies implicating the short (S) allele, which is associated with decreased gene expression, but others implicating the long (L) allele, associated with increased expression. Several studies have found significant genotype by environment interactions.164 Childhood adversity appears to be a particularly potent modifier of genetic risk for PTSD.174, 177, 178 Social context also appears to modify PTSD risk, e.g., the S allele was associated with decreased PTSD risk in environments with low crime and unemployment rates, but with increased PTSD risk in opposite environments.179 Emerging evidence suggests that methylation at downstream CpG sites modifies SLC6A4 expression.180 A recent study showed that SLC6A4 methylation modified the effect of the number of traumatic events on PTSD after controlling for SLC6A4 genotype.181 Specifically, persons who had a greater number of experienced traumatic events were at greater risk for PTSD only if SLC6A4 methylation levels were low. By contrast, high SLC6A4 methylation levels seemed to protect people who had experienced a greater number of traumatic events from developing PTSD. These findings suggest that both genotype and gene-specific methylation patterns contribute to either PTSD vulnerability or resilience. Histone deacetylation is another molecular epigenetic mechanism that may be involved in PTSD pathogenesis.182
Collectively, the above studies suggest that genotype, methylation/histone deacetylation, and gene expression differences influence or accompany the development of PTSD. However, we are unaware of any study that has incorporated all three forms of genetic information into one study. Nor are there definitive findings for any one gene or gene system in the etiology of PTSD. Importantly, epigenetic changes and gene expression in living humans presently can only be assessed in peripheral blood cells, which severely limits the interpretation of the findings. For example, the frequent finding of genetic immune system alterations in PTSD could be an artifact of the tissue used in these analyses, viz., white blood cells, a chief function of which is to regulate the immune response. The most important tissue, viz., that from brain regions reviewed in this article, in which variations in molecular epigenesis and gene transcription are most likely to be relevant to PTSD, is currently off limits (except in post-mortem tissue, which is not without problems183). Technological developments that could make live human brain tissue accessible for methylation/histone deacetyation and gene transcript research could lead to breakthroughs, but such developments may be a long way off. Meanwhile, this points to the need for animal models.
Animal Models of PTSD
A full explanation of the mechanisms involved in the development of PTSD requires prospective studies. Experimentally inducing stressors of the magnitude capable of causing PTSD is ethically impermissible in humans, so researchers have relied on animal models. Such studies have identified candidate brain circuits and cellular/molecular processes involved in PTSD pathophysiology and tested molecular therapeutic targets and specific interventions.
Early animal models of PTSD were based largely on face validity. Some utilized experimental manipulations that seemed as if they would be “traumatic” to the animals, and some were non-specific stress models such as inescapable shock. Although these approaches were useful, they provided limited PTSD-specific information. Similarly, exposure to fear conditioning alone, being a normative animal and human phenomenon, is insufficient to produce the PTSD phenotype.184 Newer models have incorporated construct validity by capitalizing on the increasing understanding of the pathophysiology of PTSD. These models have employed one or more “PTSD-specific” end-points, such as abnormal fear learning, exaggerated acoustic startle response, enhanced glucocorticoid negative feedback, and exaggerated catecholamine release. Here we review only studies that have utilized models with both satisfactory face and construct validity. These models include predator exposure, exposure to single prolonged stress, and exposure to foot shock with additional stressors.
Predator exposure (PredEx) models expose animals (e.g., rats) to a natural predator (e.g., cat, ferret) or to predator scents (e.g., cat litter, fox urine) in an environment from which the animal cannot escape. Exposed animals develop enhanced acoustic startle and show increased behavioral manifestations of anxiety.185, 186 Some studies have added “cut-off behavioral criteria” that capitalize on individual variability in stress reactivity in order to identify animals that exhibit extreme responses thought to be analogous to PTSD.187 In some instances, a subsequent psychosocial stress is added to the predator exposure.188
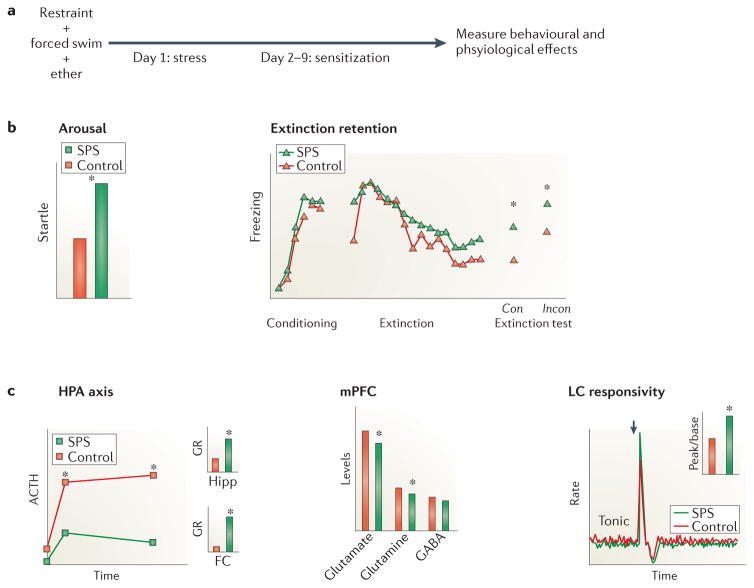
Single prolonged stress (SPS) paradigms involve serial exposure to multiple stressors, e.g., restraint, cold swim or ether anesthesia, that independently activate the HPA axis, followed by a one-week ‘no-touch’ sensitization period. The sensitization period is necessary for the development of the enhanced glucocorticoid negative feedback189 and increased acoustic startle response190 reported in PTSD (See Figure 5). Time-dependent sensitization and stress–restress can be seen as variants of the SPS model, with stress–restress adding another stressful exposure (or electric shock) at the end of the sensitization period.
Figure 5. Behavioral and physiological changes in a PTSD animal model.
A. A prototypic rodent model of PTSD: (single prolonged stress (SPS). B. Behavioral effects of exposure to SPS include increased arousal as reflected in startle response (left) and decreased extinction retention in contexts that are either consistent (con) or inconsistent (incon) with the extinction context (right). C. Physiological effects of exposure to SPS include enhanced glucocorticoid receptor (GR) expression in the hippocampus (Hipp) and frontal cortex (FC) and negative feedback in the HPA axis (left), decreased excitatory tone in the medial prefrontal cortex (mPFC) (middle), and decreases in tonic as well as increases in phasic responses to stimulation (arrow) of locus coeruleus (LC) neurons (right). Note: data are illustrative.
Foot shock with additional stress (here called foot shock plus, or FS+) models use a single session of exposure to an electric shock (sometimes paired with a conditioned stimulus, in which case it is a fear conditioning paradigm) followed by additional shocks191 or contextual reminders.192 A variant of this model pre-exposes animals to repeated stress prior to fear conditioning. Using post-conditioning reminders leads to enhancement of acoustic startle,191, 192 whereas using pre-conditioning stress leads to enhanced fear conditioning and sensitization.193
These animal models have been important in identifying the PTSD-specific neurocircuitry described in the sections above. Animal learning studies lead to hypotheses regarding fear conditioning and/or extinction abnormalities in PTSD, and sensitization studies lead to hypotheses regarding abnormal unconditioned response in PTSD such as startle. Results of animal models have also implicated cellular processes such as protein synthesis and apoptosis194 in brain regions within this circuitry, suggesting novel interventions targeting these processes. However, the major contribution of animal models to date has been the identification of molecular biological processes that may be involved in PTSD pathophysiology, which too may be targets for interventions. These include alterations in neuroendocrine (HPA), neurotransmitter (glutamatergic, serotonergic, catecholaminergic), and cellular, brain-derived neurotrophic factor (BDNF)/TrkB receptor systems.
HPA axis
Elevated hippocampal glucocorticoid receptor (GR) levels have been found in SPS189 and PredEx (using cut-off behavioral criteria)195 models, as has an increased hippocampal GR/MR ratio in SPS.189, 196 Pretreatment with a GR antagonist (RU40555) prevented SPS-induced changes in fear conditioning and LTP in the hippocampal CA1 region.190 Similarly, GR antagonism (in combination with a beta-noradrenergic blocker),197 CRF1 antagonism,198 and early treatment with high-dose corticosterone199 prevented the development of a PTSD-like phenotype in a PredEx model. Together, the foregoing findings implicate hippocampal glucocorticoid receptors in the development of posttraumatic psychopathology, a role that is supported by recent evidence that high-dose glucocorticoid administration shifts appropriate contextual conditioned responding to generalized, context-inappropriate responding. Moreover, this shift was accompanied by c-fos expression changes in the dorsal CA1and ventral dentate gyrus regions of hippocampus.200 These animal data are consistent with GR hypersensitivity found in PTSD patients and support further exploration of GR-targeted interventions as potential PTSD prevention and/or treatment approaches.
Glutamate and NMDA
Abnormal vmPFC glutamate levels and hippocampal NMDA receptor levels have been found in SPS201 and stress-restress202 models, respectively. In addition, SPS models showed alterations in the level of the NMDA receptor modulator glycine and glycine transporter mRNA in the hippocampus.203 Because glutamatergic projections from the vmPFC and the hippocampus are critical in the modulation of fear extinction and contextual fear conditioning, decreased glutamatergic signaling could contribute to impaired extinction recall or enhanced contextual fear. Indeed, the NMDA receptor modulator D-cycloserine prevented SPS-induced changes in fear extinction and NMDA receptor mRNA expression,204 and the NMDA antagonist CPP blocked the effects of predator stress on pCREB-like immunoreactivity in brain areas implicated in fear behavior.205 These findings offer specific molecular targets for therapeutic strategies aimed at addressing fear memories and intrusive recollection in PTSD patients, in addition to shedding light on pathophysiology
Serotonin
The serotonin system plays a key role in regulating mood, anxiety, and stress responses. Serotonin transporter knockout mice show an enhanced vulnerability to predator stress.206 Changes in hippocampal serotonin levels have been implicated in SPS/stress–restress models,207 and a serotonin reuptake inhibitor reversed behavioral changes in a PredEx model.208 Increases in 5-HT1A receptor levels in the dorsal raphe nucleus209 and hippocampus210 have been reported in SPS and time-dependent sensitization models, respectively, and 5-HT1A receptor antagonist (WAY-100635) inhibited SPS-induced increases in GR and CRF expression in hippocampus and hypothalamus.211 In an SPS model, amygdala 5HT2c receptor gene expression was increased, and administration of a 5HT2c receptor antagonist decreased SPS-induced contextual fear-related freezing.212 Although the exact role of serotonin systems in trauma-related psychopathology awaits further clarification, the animal data suggest that targeting potential upregulation of 5-HT1A and 5HT2c receptors might prove a useful strategy in expanding PTSD treatment/prevention arsenal.
Catecholamines
Noradrenergic transmission plays a key role in arousal regulation and, together with glucocorticoids, in memory processes. Abnormal hippocampal NE levels have been implicated in SPS.207 Animals exposed to PredEx and chronic stress show an abnormal behavioral and endocrine response to the α2-adrenergic autoreceptor antagonist yohimbine.186 Noradrenergic abnormalities have also been reported in a modified FS+reminders model.213 Beta-adrenergic blockers prevent the anxiogenic effects of PredEx,197 and blockade of postsynaptic alpha-1-adrenoreceptors normalizes the behavioral abnormalities in a modified FS+reminders model.213 Together with human data, these findings suggest that noradrenergic signaling might be a useful target for the development of treatment strategies aimed at decreasing arousal and stress reactivity.
BDNF–TrkB
BDNF–TrkB signaling is critical for hippocampal-dependent memory, so associated abnormalities could contribute to PTSD symptomatology. Changes in BDNF levels (mRNA and protein) and levels of the BDNF receptor TrkB have been reported in SPS and PredEx models. Interestingly, hippocampal TrkB levels were increased in both models, whereas BDNF levels were increased in SPS214 but down-regulated in PredEx.215 BDNF DNA methylation may be differentially regulated in hippocampal subfields,216 which might explain this discrepancy. These findings are particularly interesting because there have been no identified effective neurotrophic-factor-based treatment strategies to date. As these novel approaches become available, their applicability to PTSD will need to be examined.
Data from the above animal models suggest that multiple molecular pathways may be involved in PTSD pathophysiology. This is hardly surprising considering the fact that on the one hand PTSD symptomatology is heterogeneous, and on the other hand, three of the five systems identified (glucocorticoid, catecholamine and serotonin systems) interact and often regulate or modulate each other’s activity. These models also have identified intriguing changes in glutamatergic and BDNF–TrkB systems that warrant further exploration as potential novel targets for prevention and intervention. Further research is needed to narrow these foci and translate potential targets into effective therapeutic strategies.
General Conclusions
The research findings reviewed in this article suggest that PTSD has become one of the better understood psychiatric disorders from the biological standpoint, although much work remains. A substantial achievement has been the identification of core biological abnormalities that cut across a wide variety of traumatic events, ranging from child abuse to military combat. Part of the reason for the impressive progress in biological PTSD research has been the fact that, unlike in many psychiatric conditions, the causal environmental event and hence the onset of the pathophysiological process are considered to be known. This has provided a jump-start for investigating in both human patients and animal models the organic, cellular, and molecular pathophysiological processes set in motion by the traumatic event.
PTSD biomarkers
As reviewed above, a number of biological abnormalities have been found statistically to discriminate PTSD from non-PTSD control groups in various studies; on this basis, they may loosely be regarded as biomarkers. However, none of them possesses the specificity and sensitivity that is necessary to be used as a stand-alone diagnostic test for PTSD. The current “gold standard” for PTSD is the DSM-IV-TR diagnostic criteria, which rely heavily on patients’ subjective reports. The reliability of subjectively reported symptoms directly impacts the sensitivity and specificity of PTSD biomarkers, because the latter can be no more reliable or accurate than the diagnostic standard. Someday a biomarker (or combination of biomarkers) may be the gold standard for PTSD, against which the accuracy of subjective measures will be judged.
From an applied standpoint, biomarkers that are likely to represent pre-existing risk factors might be used to identify individuals who are at especially high risk for PTSD for preventive intervention. However, no putative biomarker has yet progressed to the point of practical utility. Acquired biomarkers (that is, biomarkers of the disorder itself rather than of vulnerability for the disorder) offer promise as targets of therapeutic intervention. Importantly, however, blood, CSF, and/or brain neuroimaging markers that are collected during a resting state may turn out to be inadequate to fully characterize PTSD, given that a central feature of the PTSD diagnosis is an abnormal reactivity to both trauma-related and -unrelated stimuli. Measures acquired under conditions of symptom provocation, e.g., responses to traumatic reminders, may offer more promise as PTSD diagnostic biomarkers.217
Translational PTSD research
It is scarcely possible to read a grant proposal nowadays without encountering the buzz word “translational.” If translational is taken to mean the cross-fertilization that can be achieved when results from animal research are used to inform human studies and vice versa, then PTSD research has been highly fruitful in this regard. However, if translational is taken to mean the conversion of research results into novel and effective treatments, the story is different. The currently most effective treatment for PTSD, viz., cognitive behavioral therapy, was conceived entirely on psychological grounds. Trials of the most effective drugs for PTSD, viz., SSRIs, were based upon these drugs’ observed anti-depressant effect; the recognition that the 5-HT system in is involved in the biology of PTSD did not come until afterwards. Indeed, despite the abundance of biological insights into PTSD that have been achieved, it is difficult to think of examples of any of these having improved treatment.
A recent example of translational research that could potentially lead to a clinical application is the preliminary finding, described above, that a high dose of cortisol given shortly after the traumatic event reduced the probability of developing PTSD.129 This study was inspired by observations that the natural cortisol response to a traumatic event may be lower in persons who go on to develop PTSD.218, 219 If the results of this preliminary study are replicated, high-dose cortisol may become part of psychiatrists’ and emergency room physicians’ armamentarium for preventing this disorder.
An example of a potential translational treatment for PTSD is blockade of traumatic memory reconsolidation.220, 221 This approach is based upon animal findings that a fear memory may not necessarily last forever but rather may be susceptible to pharmacological intervention after it has been activated. However, insufficient human studies exist to conclude that this intervention is efficacious in PTSD. Non-pharmacological memory updating procedures based upon reconsolidation may also offer promise, but these have only been studied pre-clinically.222 To date, the development of PTSD treatments has not been different from the history of much of medicine: effective agents are discovered by serendipity, and their biological mechanisms of actions clarified later.
Future directions
A conspicuous limitation in PTSD research to date has been reliance on cross-sectional, i.e., correlational, designs in the overwhelming majority of studies in PTSD patients. There is presently a dearth of pre-trauma prospective studies, which are required to establish causation. That such studies are expensive and difficult to perform is the likely reason they are few in number, but they will be required if biological PTSD research is to advance above the plateau posed by cross-sectional designs. Especially useful will be prospective treatment studies attempting to identify biomarkers that inform prognosis and aid in treatment selection, about which little guidance currently exists. Novel molecular targets implicated by validated animal models, e.g., neurotrophic factors, should be examined as potential biomarkers, as well as used to further dissect molecular processes involved. It will be necessary to continue to move from serendipitous discovery to testing translational hypotheses derived from preclinical animal and human work, as guided for example by the National Institute of Mental Health’s recent emphasis on “research domain criteria.” Progress may be also expected from capitalizing on technological advances, e.g., utilization of mass spectography in place of radioimmunoassay to better characterize critical molecules, the development of radioligands with greater receptor specificity, improved neuroimaging resolution capable, for example, of measuring activity in functionally different amygdala substructures, finding a way to perform DNA analyses on live brain tissue, and the application of new fusion algorithms derived from bioengineering to complex, multivariable data.
Table 1.
Summary of Candidate Genes Studied in Relation to Posttraumatic Stress Disorder*
| Gene | Common name(s) | Location | Total Number of Published Reports | Significant Findings | Null Findings |
|---|---|---|---|---|---|
| RD2 (D2R, D2DR) | Dopamine receptor DR | 11q23 | 6 | 4 | 2 |
| DRD4 (D4DR) | Dopamine receptor D4 | 11p15.5 | 1 | 1 | 0 |
| SLC6A3 (DAT1) | Dopamine transporter | 5p15.3 | 4 | 3 | 1 |
| DBH | Dopamine beta-hydroxylase | 9q34 | 1 | 0 | 1 |
| SLC6A4 (HTT, 5HTT, SERT, 5- HTTLPR) | Serotonin transporter | 17q11 | 16 | 12 | 4 |
| HTR2 (5-HT2A) | 5-hydroxytryptamine (serotonin) receptor 2A | 13q14-q21 | 1 | 1 | 0 |
| FKBP5 | FK506 binding protein 5 | 6p21 | 4 | 4 | 0 |
| GCCR (NR3C1) | Glucocorticoid receptor | 5q31.3 | 1 | 0 | 1 |
| CRHR1 | Corticotropin-releasing hormone receptor 1 | 17q12-22 | 1 | 1 | 0 |
| RGS2 | Regulator of G-protein signaling 2 | 1q31 | 1 | 1 | 0 |
| CNR1 (CB1, CNR) | Cannabinoid receptor 1 (brain) | 6q14-q15 | 1 | 0 | 1 |
| APOE | Apolopoprotein E | 19q13 | 1 | 1 | 0 |
| BDNF | Brain-derived neurotrophic factor | 11p13 | 3 | 0 | 3 |
| NPY | Neuropeptide Y | 7p15.1 | 1 | 0 | 1 |
| GABRA2 | GABAA | 4p12 | 1 | 1 | 0 |
| COMT | Catechol-O-methyltransferase | 22q11 | 2 | 2 | 0 |
| ADCYAP1R1 | Receptor for adenylate cyclase-activating polypeptide 1 | 7p14 | 2 | 1 | 1 |
| DTNBP1 | Dystrobrevin-binding protein 1 | 6p22 | 1 | 1 | 0 |
| CRNA5 | Cholinergic receptor, neuronal nicotinic, alpha polypeptide 5 | 15q25.1 | 2 | 1 | 1 |
| PRKCA | Protein kinase C alpha | 17q22-q23.2 | 1 | 1 | 0 |
| TPH1 | Tryptophan hydroxylase 1 | 11p15.3-p14 | 1 | 1 | 0 |
| TPH2 | Tryptophan hydroxylase 2 | 12q21.1 | 1 | 1 | 0 |
A referenced version of this Table appears as Table 1S in Supplemental Material.
At-a-Glance Summary.
Perhaps the best replicated biological finding in PTSD is higher autonomic (heart rate, skin conductance) and facial EMG responding during internal, mental imagery of the traumatic event, and upon exposure to external, trauma-related cues.
Higher heart rate responding to sudden loud tones in PTSD likely reflects an acquired sensitization of the nervous system.
Diminished volumes of hippocampus and anterior cingulate cortex represent the most frequently replicated neuroanatomic findings in patients with PTSD. These do not appear to be fully explained by co-morbid conditions such as substance abuse and depression.
Some evidence exists to support both pre-existing vulnerability and neurotoxicity and as origins of brain volume reductions in PTSD. Based upon present data, it is going too far to say that stress damages the brain, but there is no doubt that it changes it.
Functional neuroimaging studies suggest that amygdala and dorsal anterior cingulate cortex are hyper(re)active, whereas ventral medial prefrontal cortex is hypo(re)active, in PTSD. These abnormalities likely underlie the attentional bias toward threat, impaired emotion regulation, and persistence of fear memories in this disorder.
The classic model of stress based upon chronic hyperactivity of the hypothalamic-pituitary-adrenal cortical axis does not characterize PTSD.
A number of neurotransmitters and neuroendocrinological factors interact to influence PTSD risk, symptom profiles, and severity. These factors vary across individuals due to genetic and epigenetic factors, as well as within individuals over time in response to environmental influences including exposure to psychological trauma.
As with other mental disorders, genetic vulnerability to PTSD likely involves the sum of contributions from multiple alleles each with small effects.
The full range of molecular genetic factors, which include genotype, methylation/histone deacetylation, and gene expression, likely influence or accompany the development of PTSD. However, at this time there are no definitive findings for any one gene or gene system in the disorder’s etiology.
Animal models have identified important molecular pathways that likely contribute to PTSD’s pathophysiology and may constitute promising therapeutic targets.
Glossary
- Allele
one or more alternate forms of a genetic locus or a gene
- Brain-derived neurotrophic factor (BDNF)
A protein, often released from a neuron, that is involved in growth and the differentiation of new neurons and synapses
- CA3 subfield
A sector of the cornu ammonis subfield of the hippocampus and a major target of glucocorticoids
- Conditioned fear response
A fear response that is elicited by a conditioned stimulus, or cue, following fear conditioning. Typical measures include freezing in rodents, skin conductance in humans, and potentiated startle in both.
- Construct validity
The degree to which a model or a term corresponds to or reflects an underlying theory
- Corrugator EMG
A measure of electromyographic activity associated with contraction of the corrugator supercilii muscle, which draws the inner brow inward and downward during negatively valenced emotion
- DNA methylation
The modification of a strand of DNA in which a methyl group is added to a cytosine molecule that stands directly before a guanine molecule in the same chain. It has the effect of reducing gene expression.
- Dentate gyrus
A subfield of the hippocampus, which contains adult neural stem cells and is an important site of neurogenesis
- Diffusion tensor imaging (DTI)
A structural magnetic resonance imaging-based technique that tracks the diffusion of water molecules within a closed space, usually a tube such as a neural axon. It is useful in revealing white matter fiber structure and providing information regarding regional brain connectivity.
- Dorsal anterior cingulate cortex (dACC)
A cortical area that roughly corresponds to Brodmann area (BA) 24. May also be called anterior mid cingulate cortex (aMCC)
- Epigenesis
One or more mechanisms that regulate gene function without altering underlying DNA sequence
- Extinction
A procedure by which a conditioned stimulus is repeatedly presented in the absence of the unconditioned stimulus, resulting in diminution of the conditioned response.
- Extinction recall (or retention)
Memory that a conditioned stimulus has been previously been extinguished, expressed as a continuing reduction of the conditioned response.
- Extrasynaptic receptor
A neuronal receptor located outside the synapse. Extrasynaptic receptors can be accessed by neuromodulatory factors derived from the periphery and circulating in the cerebrospinal fluid.
- Face validity
The degree to which a model or a term appears to measure what it is supposed to measure.
- Fear conditioning
An experimental paradigm that teaches an animal or human to associate a previously neutral conditioned stimulus (CS, e.g., a light or a tone) with an aversive unconditioned stimulus (US, e.g., an electric shock), the latter of which produces an aversive unconditioned response (UR). Eventually because of the association the CS alone comes to elicit a fear response.
- Fear-potentiated startle
Increased electromyographic responsiveness to a startling stimulus that occurs when an animal or human is afraid.
- Functional magnetic resonance imaging (fMRI)
A functional neuroimaging technique that uses a magnetic field and radio waves to measure blood oxygenation level dependent (BOLD) signal, which serves as an index of regional brain activation.
- Glucocorticoid negative feedback
A negative feedback phenomenon by which cortisol reduces its own release through inhibition of the hypothalamic-pituitary-adrenal cortical (HPA) axis.
- Heritable
Capable of being passed from one generation to the next via DNA.
- Magnetic resonance spectroscopic imaging (MRSI)
A non-invasive research and diagnostic technique that is similar to magnetic resonance imaging but uses a stronger field to detect regional body chemistry at the cellular level. Also called 1H-nuclear magnetic resonance spectroscopic imaging and proton magnetic resonance spectroscopic imaging.
- N-acetylaspartate (NAA)
A putative marker of neuronal integrity thought to be present predominantly in neuronal cell bodies. It emits the largest signal in magnetic resonance spectroscopic imaging of the human brain.
- Neuromodulation
An alteration in the response of a neuron induced by a substance that would not, by itself, affect neuronal firing rate
- P2
An electroencephalographic event-related potential response that is positive and reaches its maximum deflection approximately 200 miliseconds after a stimulus is presented. It is thought to reflect “tuning” of a mechanism that regulates the amount of sensory input to the cortex.
- P3b
An electroencephalographic event-related potential response that is positive and reaches its maximum deflection approximately 300 miliseconds after a stimulus is presented. It is thought to reflect the amount of attentional resources allocated to a particular stimulus.
- Phasic
Designating intermittent signaling, usually in response to a stimulus
- Positron emission tomography (PET)
A functional neuroimaging technique that uses radioactive isotopes to quantify regional cerebral blood flow, glucose metabolism, or receptor occupancy.
- Single nucleotide polymorphism (SNP)
A variation in a DNA sequence in which a single nucleotide (A, C, G, or T) at a specific locus differs between members of the same biological species or between paired chromosomes of an individual
- Skin conductance
A measure of sweat activity recorded from two adjacent fingers and/or the thenar and hpothenar eminences of the palm of the hand. It is thought to be primarily under sympathetic nervous system influence.
- Structural magnetic resonance imaging (sMRI)
A non-invasive diagnostic and research procedure that utilizes a magnetic field and radio waves to create detailed sectional images of the internal structure of the body, including the brain
- Tonic
Designating continuous, steady, or baseline signaling
- TrkB
A membranous tyrosine kinase receptor that binds BDNF and other neurotrophic factors, or neurotrophins
- Ventromedial prefrontal cortex (vmPFC)
A region within the medial wall of prefrontal cortex that roughly corresponds to Brodmann area (BA) 10. Some studies treat portions of adjacent Brodmann areas as part of vmPFC
- Voxel-based morphometry (VBM)
An automated neuroimaging analytic technique that allows the investigation of focal differences in brain anatomy using the statistical approaches of statistical parametric mapping and smoothing applied to structural images
Biographies
Roger K. Pitman, M.D. is Professor of Psychiatry at Harvard Medical School and Psychiatrist at Massachusetts General Hospital. He is a recipient of the International Society for Traumatic Stress Studies (ISTSS) Award for Outstanding Scientific Achievement in the Field of PTSD and the ISTSS Lifetime Achievement Award. He is a Distinguished Life Fellow of the American Psychiatric Association and a Fellow of the American College of Neuropsychopharmacology (ACNP). His PTSD research has included psychophysiological, neuroimaging, and other biological approaches to PTSD and has also employed identical twins discordant for combat exposure in Vietnam.
Ann M. Rasmusson, M.D. is Associate Professor of Psychiatry at Boston University School of Medicine, Psychiatry Liaison for PTSD Research and Education at the VA Boston Healthcare System, and an affiliate of the National Center for PTSD Women’s Health Science Division. Her research focuses on the neuroendocrinology of PTSD and PTSD-comorbid medical and other psychiatric disorders, as well as the development of new PTSD therapeutics.
Karestan Chase Koenen, M.A., Ph.D., is Associate Professor of Epidemiology at Columbia University’s Mailman School of Public Health. She is a recipient of the ISTSS Young Professional Award and the American Psychopathological Association Young Investigator Award. Dr. Koenen is currently President-Elect of the ISTSS. Her research interests include the interplay of genetic and environmental factors in the etiology of stress-related mental disorders such as PTSD and depression.
Lisa M. Shin, Ph.D. is Professor of Psychology at Tufts University and Associate Researcher in the Department of Psychiatry at the Massachusetts General Hospital. She is a recipient of the Anxiety Disorders Association of America Young Investigator ‘s Research Award and the ISTSS Young Professional Award. Her research involves using functional neuroimaging to study brain abnormalities in PTSD and whether these are acquired characteristics of the disorder or familial vulnerability factors, as well as determining how such abnormalities predict treatment response.
Scott P. Orr, Ph.D. is Associate Professor of Psychology in the Department of Psychiatry at Harvard Medical School and Massachusetts General Hospital. He is a recipient of the ISTSS Award for Outstanding Scientific Achievement in the Field of Traumatic Stress. His major research interest is the psychophysiology of PTSD, including the identification or risk factors and predictors of treatment response.
Mark W. Gilbertson, Ph.D. is Instructor in the Department of Psychiatry at Harvard Medical School and Brigham & Women’s Hospital. He is Research & Development Coordinator for the Manchester, NH VA Medical Center. Dr. Gilbertson’s published work has centered on structural neuroimaging and neurocognitive function. His current interests include the role of hippocampus in contextual processing as it relates to the development and maintenance of chronic PTSD.
Mohammed R. Milad, Ph.D. is Associate Professor of Psychiatry at Harvard Medical School and Director of the Behavioral Neuroscience Lab at Massachusetts General Hospital. He is the recipient of the Positive Neuroscience Award from the Templeton Foundation and was an invited Kavli Frontier of Science Speaker in 2009. His research involves the use of neuropharmacological and molecular tools in rodents, and structural and functional neuroimaging and psychophysiological tools in humans, to examine the function of the fear extinction network across mental disorders. He also studies the influence of sex hormones on fear extinction.
Israel Liberzon, M.D. is Professor of Psychiatry, Psychology and Neuroscience at the University of Michigan and Associate Chair for Academic Development at the Department of Psychiatry. He is an ACNP Fellow and a Past President of the Psychiatric Research Society. His research focus is on the neurobiology and the neuroanatomy of stress, negative emotions, and cognitive-emotional interactions in PTSD patients and healthy individuals. His research program encompasses both basic science (genetic, molecular biology and animal models) and clinical/translational projects (fMRI, neuroendocrinology, and treatment outcome studies).
Footnotes
All authors have numerous publications in the psychiatry, clinical psychology, and/or neuroscience literature, are recipients of National Institute of Mental Health (NIMH), Department of Veterans Affairs (VA) Department of Defense (DOD) and/or other institutional research grants, and have served on various NIMH, VA, and/or DOD study sections and on various journal editorial boards.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition. American Psychiatric Association; Washington, D.C: 1980. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. American Psychiatric Press; Washington, D.C: 2000. [Google Scholar]
- 3.Dobbs D, Wilson WP. Observations on persistence of war neurosis. Dis Nerv Syst. 1960;21:686–691. [PubMed] [Google Scholar]
- 4.Orr SP, Metzger LJ, Miller MW, Kaloupek DG. Psychophysiological assessment of PTSD. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD: A Handbook for Practicioners. 2. Guilford Publications; New York: 2004. pp. 289–343. [Google Scholar]
- 5.Metzger LJ, Gilbertson MW, Orr SP. Electrophysiology of PTSD. In: Vasterling J, Brewin C, editors. Neuropsychology of PTSD: Biological, Clinical, and Cognitive Perspectives. Guilford Publications; New York: 2005. pp. 83–102. [Google Scholar]
- 6.Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. A comprehensive review and meta-analysis of the most important psychophysiological research in PTSD as of that date. [DOI] [PubMed] [Google Scholar]
- 7.Keane TM, et al. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 1998;66:914–923. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 8.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. Introduced a novel symptom provocation technique for PTSD which has come into widespread psychophysiologic, neuroimaging, and other research use. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard EB, et al. Psychophysiology of posttraumatic stress disorder related to motor vehicle accidents: replication and extension. J Consult Clin Psychol. 1996;64:742–751. doi: 10.1037//0022-006x.64.4.742. [DOI] [PubMed] [Google Scholar]
- 10.Kleim B, Wilhelm FH, Glucksman E, Ehlers A. Sex differences in heart rate responses to script-driven imagery soon after trauma and risk of posttraumatic stress disorder. Psychosom Med. 2010;72:917–924. doi: 10.1097/PSY.0b013e3181f8894b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr SP, et al. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 12.Shalev AY, et al. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am J Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- 13.Pitman RK, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MG, Resick PA, Galovski TE. Does physiologic response to loud tones change following cognitive-behavioral treatment for posttraumatic stress disorder? J Trauma Stress. 2012;25:25–32. doi: 10.1002/jts.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Lissek S, Grillon C. Learning models of PTSD. In: Beck JG, Sloan DM, editors. The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; New York: 2012. pp. 175–190. [Google Scholar]
- 18.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 19.Milad MR, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger LJ, Pitman RK, Miller GA, Paige SR, Orr SP. Intensity dependence of auditory P2 in monozygotic twins discordant for Vietnam combat: associations with posttraumatic stress disorder. J Rehabil Res Dev. 2008;45:437–449. doi: 10.1682/jrrd.2007.07.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grillon C, Morgan CA., III Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 22.Griffin MG. A prospective assessment of auditory startle alterations in rape and physical assault survivors. J Trauma Stress. 2008;21:91–99. doi: 10.1002/jts.20300. [DOI] [PubMed] [Google Scholar]
- 23.Buhlmann U, et al. Physiologic responses to loud tones in individuals with obsessive-compulsive disorder. Psychosom Med. 2007;69:166–172. doi: 10.1097/PSY.0b013e31802f2799. [DOI] [PubMed] [Google Scholar]
- 24.Guthrie RM, Bryant RA. Auditory startle response in firefighters before and after trauma exposure. Am J Psychiatry. 2005;162:283–290. doi: 10.1176/appi.ajp.162.2.283. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 26.Orr SP, et al. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biol Mood Anxiety Disord. 2012;2:8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell ML, Creamer M, Elliott P, Bryant R. Tonic and phasic heart rate as predictors of posttraumatic stress disorder. Psychosom Med. 2007;69:256–261. doi: 10.1097/PSY.0b013e3180417d04. [DOI] [PubMed] [Google Scholar]
- 28.Suendermann O, Ehlers A, Boellinghaus I, Gamer M, Glucksman E. Early heart rate responses to standardized trauma-related pictures predict posttraumatic stress disorder: a prospective study. Psychosom Med. 2010;72:301–308. doi: 10.1097/PSY.0b013e3181d07db8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalev AY, et al. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry. 1998;55:553–559. doi: 10.1001/archpsyc.55.6.553. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bremner JD, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurvits TV, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 35.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. First study in humans to employ high resolution sMRI to determine more specific volume diminutions within selected hippocampal subfields, and to delineate those regions specific to PTSD versus aging effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 38.Karl A, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Woon F, Hedges DW. Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: a meta-analysis. Hippocampus. 2011;21:243–252. doi: 10.1002/hipo.20746. [DOI] [PubMed] [Google Scholar]
- 40.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. Used data from monozygotic twins discordant for combat exposure and PTSD to suggest that smaller hippocampal volume in PTSD represents a pre-existing vulnerability factor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonne O, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 43.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 44.Emdad R, et al. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother Psychosom. 2006;75:122–132. doi: 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- 45.Bonne O, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69:1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuff N, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karl A, Werner A. The use of proton magnetic resonance spectroscopy in PTSD research--meta-analyses of findings and methodological review. Neurosci Biobehav Rev. 2010;34:7–22. doi: 10.1016/j.neubiorev.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Myslobodsky MS, et al. Changes of brain anatomy in patients with posttraumatic stress disorder: a pilot magnetic resonance imaging study. Psychiatry Res. 1995;58:259–264. doi: 10.1016/0165-1781(95)02708-5. [DOI] [PubMed] [Google Scholar]
- 49.Bremner JD. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder. Hippocampus. 2001;11:75–81. doi: 10.1002/hipo.1023. [DOI] [PubMed] [Google Scholar]
- 50.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Kasai K, et al. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biol Psychiatry. 2010;68:491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SJ, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- 56.Sekiguchi A, et al. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.51. [DOI] [PubMed] [Google Scholar]
- 57.Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 58.Herry C, et al. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 59.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liberzon I, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 61.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bremner JD, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin LM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 64.Gold AL, et al. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychol Med. 2011:1–10. doi: 10.1017/S0033291711000730. [DOI] [PubMed] [Google Scholar]
- 65.Felmingham K, et al. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- 66.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 67.Felmingham K, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. Used fMRI to reveal functional brain changes in response to cognitive behavioral therapy in PTSD. [DOI] [PubMed] [Google Scholar]
- 68.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin LM, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 70.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Milad MR, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 72.Rougemont-Bucking A, et al. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryant RA, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 74.Pannu HJ, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonzo GA, et al. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin LM, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin LM, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bremner JD, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 79.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmons AN, et al. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strigo IA, et al. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biol Psychiatry. 2010;68:442–450. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 82.Aupperle RL, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. Nicely linked functional brain activation patterns with neuropsychological test performance in PTSD. [DOI] [PubMed] [Google Scholar]
- 83.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Pitman RK. Combat effects on mental health: the more things change, the more they remain the same. Arch Gen Psychiatry. 2006;63:127–128. doi: 10.1001/archpsyc.63.2.127. [DOI] [PubMed] [Google Scholar]
- 87.Geuze E, et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry. 2008;13:74–83. 3. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- 88.Murrough JW, et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry. 2011;70:1033–1038. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liberzon I, et al. Altered central mu-opioid receptor binding after psychological trauma. Biol Psychiatry. 2007;61:1030–1038. doi: 10.1016/j.biopsych.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 90.Murrough JW, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry. 2011;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Southwick SM, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 92.Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 93.Rasmusson AM, et al. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 94.Perry BD, Giller EL, Jr, Southwick SM. Altered platelet alpha 2-adrenergic binding sites in posttraumatic stress disorder. Am J Psychiatry. 1987;144:1511–1512. doi: 10.1176/ajp.144.11.1511a. [DOI] [PubMed] [Google Scholar]
- 95.Maes M, et al. Serotonergic and noradrenergic markers of post-traumatic stress disorder with and without major depression. Neuropsychopharmacology. 1999;20:188–197. doi: 10.1016/S0893-133X(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 96.Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis. 1991;179:371–373. doi: 10.1097/00005053-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 97.Southwick SM, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 98.Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 99.Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 100.Bremner JD, et al. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 101.Taylor FB, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 102.Raskind MA, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 103.Vaiva G, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 104.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 105.Hoge EA, et al. Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neurosci Ther. 2012;18:21–27. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Southwick SM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 107.Baumann MH, Mash DC, Staley JK. The serotonin agonist m-chlorophenylpiperazine (mCPP) binds to serotonin transporter sites in human brain. Neuroreport. 1995;6:2150–2152. doi: 10.1097/00001756-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 108.Murphy DL, Lesch KP, Aulakh CS, Pigott TA. Serotonin-selective arylpiperazines with neuroendocrine, behavioral, temperature, and cardiovascular effects in humans. Pharmacol Rev. 1991;43:527–552. [PubMed] [Google Scholar]
- 109.Kennett GA, et al. Effect of chronic administration of selective 5-hydroxytryptamine and noradrenaline uptake inhibitors on a putative index of 5-HT2C/2B receptor function. Neuropharmacology. 1994;33:1581–1588. doi: 10.1016/0028-3908(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 110.Britton KT, Akwa Y, Spina MG, Koob GF. Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides. 2000;21:37–44. doi: 10.1016/s0196-9781(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morgan CA, III, et al. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry. 2002;52:136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 113.Sah R, et al. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 115.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 116.Baker DG, et al. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- 117.de Kloet CS, et al. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:287–291. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- 118.Geracioti TD, Jr, et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 119.Yehuda R, et al. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. Reviewed a highly influential body of research involving hyperresponsiveness of glucocorticoid receptors, enhanced negative feedback of the hypothalamic-pituitary-adrenal cortical axis, and lower circulating cortisol levels in PTSD. [DOI] [PubMed] [Google Scholar]
- 121.Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- 122.Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL., Jr Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- 123.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 124.Mehta D, et al. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- 126.Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 127.Rasmusson AM, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 128.Schelling G, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 129.Zohar J, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 130.de Quervain DJ. Glucocorticoid-induced inhibition of memory retrieval: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:216–220. doi: 10.1196/annals.1364.016. [DOI] [PubMed] [Google Scholar]
- 131.McIntyre CK, Roozendaal B. Adrenal stress hormones and enhanced memory for emotionally arousing experiences. In: Bermúdez-Rattoni F, editor. Neural Plasticity and Memory: From Genes to Brain Imaging. CRC Press; Boca Raton (FL): 2007. [PubMed] [Google Scholar]
- 132.Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci. 2011;34:165–176. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Hou YT, Lin HK, Penning TM. Dexamethasone regulation of the rat 3alpha-hydroxysteroid/dihydrodiol dehydrogenase gene. Mol Pharmacol. 1998;53:459–466. doi: 10.1124/mol.53.3.459. [DOI] [PubMed] [Google Scholar]
- 134.Rasmusson AM, Picciotto MR, Krishnan-Sarin S. Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: a review. J Psychopharmacol. 2006;20:693–707. doi: 10.1177/0269881106060193. [DOI] [PubMed] [Google Scholar]
- 135.Yehuda R, et al. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009;34:1304–1313. doi: 10.1016/j.psyneuen.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rasmusson AM, Vythilingam M, Morgan CA., III The neuroendocrinology of posttraumatic stress disorder: new directions. CNS Spectr. 2003;8:651–657. doi: 10.1017/s1092852900008841. [DOI] [PubMed] [Google Scholar]
- 137.Chalbot S, Morfin R. Dehydroepiandrosterone metabolites and their interactions in humans. Drug Metabol Drug Interact. 2006;22:1–23. doi: 10.1515/dmdi.2006.22.1.1. [DOI] [PubMed] [Google Scholar]
- 138.Balazs Z, Schweizer RA, Frey FJ, Rohner-Jeanrenaud F, Odermatt A. DHEA induces 11 -HSD2 by acting on CCAAT/enhancer-binding proteins. J Am Soc Nephrol. 2008;19:92–101. doi: 10.1681/ASN.2007030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spivak B, et al. Elevated circulatory level of GABA(A)--antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychol Med. 2000;30:1227–1231. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- 140.Sondergaard HP, Hansson LO, Theorell T. Elevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: findings from a longitudinal study of refugees in Sweden. Psychother Psychosom. 2002;71:298–303. doi: 10.1159/000064806. [DOI] [PubMed] [Google Scholar]
- 141.Rasmusson AM, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- 142.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morgan CA, III, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 144.Morgan CA, III, Rasmusson A, Pietrzak RH, Coric V, Southwick SM. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatry. 2009;66:334–340. doi: 10.1016/j.biopsych.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 145.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 146.Rasmusson AM, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. Reported deficits in the synthesis of GABAergic neuroactive steroids in PTSD, suggesting a mechanism that may confer resistance to serotonin reuptake inhibitor (SSRI) treatment and contribute to comorbidities such as depression and chronic pain. [DOI] [PubMed] [Google Scholar]
- 147.Genazzani AD, et al. Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril. 2003;80:1495–1501. doi: 10.1016/j.fertnstert.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 148.Schmidt PJ, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 149.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 150.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- 158.Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.True WR, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 160.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 161.Sartor CE, et al. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lyons MJ, et al. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 163.Jang KL, Stein MB, Taylor S, Asmundson GJ, Livesley WJ. Exposure to traumatic events and experiences: aetiological relationships with personality function. Psychiatry Res. 2003;120:61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 164.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sartor CE, et al. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ressler KJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang SC, et al. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- 169.Segman RH, et al. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–13. 425. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- 170.Zieker J, et al. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol Psychiatry. 2007;12:116–118. doi: 10.1038/sj.mp.4001905. [DOI] [PubMed] [Google Scholar]
- 171.Yehuda R, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 172.Uddin M, et al. Gene expression and methylation signatures of MAN2C1 are associated with PTSD. Dis Markers. 2011;30:111–121. doi: 10.3233/DMA-2011-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kilpatrick DG, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. First study to document a genotype by environment interaction in risk for PTSD. The data suggested that the low expression variant of the serotonin transporter gene increases risk of PTSD under conditions of high stress and low social support but not under low stress conditions. [DOI] [PubMed] [Google Scholar]
- 174.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Uddin M, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. Provided evidence of a biologic model of PTSD etiology in which an externally experienced traumatic event induces downstream alterations in immune function by reducing methylation levels of immune-related genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith AK, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xie P, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xie P, Kranzler HR, Farrer L, Gelernter J. Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood adversity on posttraumatic stress disorder risk: A replication study. Am J Med Genet B Neuropsychiatr Genet. 2012 doi: 10.1002/ajmg.b.32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koenen KC, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Philibert RA, et al. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koenen KC, et al. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety. 2011;28:639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Trollope AF, et al. Stress, epigenetic control of gene expression and memory formation. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 183.El-Sayed AM, Halossim MR, Galea S, Koenen KC. Epigenetic modifications associated with suicide and common mood and anxiety disorders: a systematic review of the literature. Biol Mood Anxiety Disord. 2012;2:10. doi: 10.1186/2045-5380-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pitman RK, Orr SP, Shalev AY. Once bitten, twice shy: beyond the conditioning model of PTSD. Biol Psychiatry. 1993;33:145–146. doi: 10.1016/0006-3223(93)90132-w. [DOI] [PubMed] [Google Scholar]
- 185.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. An early and influential study of the predator exposure animal model for PTSD. [DOI] [PubMed] [Google Scholar]
- 186.Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- 188.Mesches MH, Fleshner M, Heman KL, Rose GM, Diamond DM. Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci. 1999;19:RC18. doi: 10.1523/JNEUROSCI.19-14-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. Demonstrated the construct validity of the SPS animal model of PTSD. [DOI] [PubMed] [Google Scholar]
- 190.Kohda K, et al. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 191.Servatius RJ, Ottenweller JE, Bergen MT, Soldan S, Natelson BH. Persistent stress-induced sensitization of adrenocortical and startle responses. Physiol Behav. 1994;56:945–954. doi: 10.1016/0031-9384(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 192.Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 193.Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- 194.Li X, Han F, Liu D, Shi Y. Changes of Bax, Bcl-2 and apoptosis in hippocampus in the rat model of post-traumatic stress disorder. Neurol Res. 2010;32:579–586. doi: 10.1179/016164110X12556180206194. [DOI] [PubMed] [Google Scholar]
- 195.Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. A distinct pattern of intracellular glucocorticoid-related responses is associated with extreme behavioral response to stress in an animal model of post-traumatic stress disorder. Eur Neuropsychopharmacol. 2009;19:759–771. doi: 10.1016/j.euroneuro.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 196.Zhe D, Fang H, Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem. 2008;41:89–95. doi: 10.1267/ahc.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 198.Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 200.Kaouane N, et al. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- 201.Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480:16–20. doi: 10.1016/j.neulet.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Harvey BH, Oosthuizen F, Brand L, Wegener G, Stein DJ. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl) 2004;175:494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- 203.Yamamoto S, et al. Alterations in the hippocampal glycinergic system in an animal model of posttraumatic stress disorder. J Psychiatr Res. 2010;44:1069–1074. doi: 10.1016/j.jpsychires.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 204.Yamamoto S, et al. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 205.Blundell J, Adamec R. The NMDA receptor antagonist CPP blocks the effects of predator stress on pCREB in brain regions involved in fearful and anxious behavior. Brain Res. 2007;1136:59–76. doi: 10.1016/j.brainres.2006.09.078. [DOI] [PubMed] [Google Scholar]
- 206.Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: sex, serotonin and other factors-relevance to PTSD. Neurosci Biobehav Rev. 2008;32:1287–1292. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Harvey BH, Brand L, Jeeva Z, Stein DJ. Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav. 2006;87:881–890. doi: 10.1016/j.physbeh.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 208.Kesner Y, et al. WFS1 gene as a putative biomarker for development of post-traumatic syndrome in an animal model. Mol Psychiatry. 2009;14:86–94. doi: 10.1038/sj.mp.4002109. [DOI] [PubMed] [Google Scholar]
- 209.Luo FF, Han F, Shi YX. Changes in 5-HT1A receptor in the dorsal raphe nucleus in a rat model of post-traumatic stress disorder. Mol Med Report. 2011;4:843–847. doi: 10.3892/mmr.2011.516. [DOI] [PubMed] [Google Scholar]
- 210.Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- 211.Wang HT, Han F, Shi YX. Activity of the 5-HT1A receptor is involved in the alteration of glucocorticoid receptor in hippocampus and corticotropin-releasing factor in hypothalamus in SPS rats. Int J Mol Med. 2009;24:227–231. doi: 10.3892/ijmm_00000225. [DOI] [PubMed] [Google Scholar]
- 212.Harada K, Yamaji T, Matsuoka N. Activation of the serotonin 5-HT2C receptor is involved in the enhanced anxiety in rats after single-prolonged stress. Pharmacol Biochem Behav. 2008;89:11–16. doi: 10.1016/j.pbb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 213.Olson VG, et al. The role of norepinephrine in differential response to stress in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2011;70:441–448. doi: 10.1016/j.biopsych.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Takei S, et al. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res. 2011;45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 215.Kozlovsky N, et al. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol. 2007;10:741–758. doi: 10.1017/S1461145707007560. [DOI] [PubMed] [Google Scholar]
- 216.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pitman RK, Orr SP. Forensic laboratory testing for post-traumatic stress disorder. In: Simon RI, editor. Posttraumatic Stress Disorder in Litigation: Guidelines for Forensic Assessment. American Psychiatric Press; Washington, D.C: 2003. pp. 207–223. [Google Scholar]
- 218.Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- 219.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 220.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 221.Brunet A, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 222.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]