ABSTRACT
Receptor tyrosine kinases, such as the epidermal growth factor (EGF) receptor (EGFR) and Met lead to activation of intracellular signals including Akt, a critical regulator of cell survival, metabolism and proliferation. Upon binding their respective ligands, each of these receptors is recruited into clathrin coated pits (CCPs) eventually leading to endocytosis. We have recently shown that phosphorylation of Gab1 and Akt following EGFR activation requires clathrin, but does not require receptor endocytosis. We examined whether clathrin regulates Akt signaling downstream of Met, as it does for EGFR signaling. Stimulation with the Met ligand Hepatocyte Growth Factor (HGF) leads to enrichment of phosphorylated Gab1 (pGab1) within CCPs in ARPE-19 cells. Perturbation of clathrin using the inhibitor pitstop2 decreases HGF-stimulated Akt phosphorylation. These results indicate that clathrin may regulate Met signaling leading to Akt phosphorylation similarly as it does for EGFR signaling.
KEYWORDS: clathrin, endocytosis, membrane traffic, phosphorylation, receptor tyrosine kinase, signaling adaptor, signaling scaffold, signaling microdomain, TIRF microscopy
Introduction
Receptor tyrosine kinases (RTKs) control many aspects of cell physiology, including cell proliferation, survival, metabolism, migration and differentiation.1 Many RTKs exhibit increased intrinsic tyrosine kinase activity upon binding ligand, thus activating numerous secondary signaling intermediates and pathways, such as PI3K-Akt-mTOR and Ras-Erk.2
Importantly, many RTKs also exhibit changes in cell surface membrane traffic by undergoing internalization via clathrin-mediated endocytosis (CME) upon ligand binding. These RTKs are recruited to clathrin-coated pits (CCPs), eventually leading to receptor internalization. CCPs are 50–100 nm structures associated with the inner leaflet of the plasma membrane, and are comprised of a lattice-like assembly of the protein clathrin, the adaptor protein AP2, and ∼30–50 other specific proteins recruited from the cytosol.3 Some CCPs undergo scission from the plasma membrane by the GTPase dynamin 2, leading to formation of intracellular vesicles; however, most CCPs undergo abortive turnover at the plasma membrane without producing vesicles,4 suggesting that these structures may have other role(s) directly at the plasma membrane.2,5
For some RTKs, the activation of receptor-proximal signaling intermediates occurs simultaneously with the residence of the receptor within CCPs.2,5 We have recently shown that upon activation of the epidermal growth factor (EGF) receptor (EGFR), the phosphorylation of the signaling adaptor Gab1, as well as the downstream phosphorylation of Akt, requires clathrin.5 Importantly, this requirement for clathrin did not reflect a requirement for EGFR internalization from the plasma membrane, as blocking EGFR endocytosis by perturbation of dynamin 2 (which allowed EGFR enrichment within plasma membrane clathrin structures) did not impact EGFR signaling.5 Thus, we proposed that some plasma membrane clathrin structures function as cell surface signaling microdomains, enriched in specific factors required for activation of EGFR signals (e.g. Gab1-Akt), but dispensable for others (e.g., Erk).5 Indeed using total internal reflection fluorescence microscopy (TIRF-M) and computational image analysis, we resolved that EGF stimulation increased the levels of phosphorylated Gab1 (pGab1) within CCPs.
We also showed that the requirement for clathrin for the activation of Akt signaling was specific for signaling by EGFR, such that clathrin was not required for EGF-stimulated Akt phosphorylation in cells that also express ErbB2.5 EGFR and ErbB2 preferentially form heterodimers; these EGFR-ErbB2 heterodimers exhibit differences in the activation of c-Src6 and c-Cbl7 compared to EGFR homodimers. Hence, the requirement for clathrin for the phosphorylation of Gab1 and Akt was specific for signaling by EGFR homodimers but not that by EGFR-ErbB2 heterodimers.5 Whether this novel function of clathrin extends to other receptor tyrosine kinases beyond EGFR is an important question that has not yet been examined. Met is another RTK which has some similarities to EGFR in terms of the signaling pathways activated by the ligand-bound receptors.8 In contrast to EGFR, Met is activated upon binding to its ligand hepatocyte growth factor (HGF). Like in EGFR signaling, activated Met elicits the phosphorylation of Gab1, which contributes to activation of many downstream signals, including PI3K-Akt.8 While EGFR requires the signaling adaptor Grb2 to recruit and elicit phosphorylation of Gab1, Met can also bind directly to Gab1 upon phosphorylation of Y1349 within Met.9,10 Furthermore, Met undergoes recruitment to CCPs and clathrin-mediated endocytosis following binding to HGF.11,12 Whether Met may have a similar requirement for clathrin as does EGFR for the activation of Akt is not known. We have thus used similar methods as we have recently done for EGFR5 to examine whether HGF stimulation (i) leads to enrichment of phosphorylated Gab1 within CCPs, and (ii) elicits Akt phosphorylation that is dependent on clathrin.
Results and discussion
To examine the role of clathrin in Met signaling, we used retinal pigment epithelial cells (ARPE-19, RPE henceforth) as we had previously used to study EGFR signaling.5 RPE cells stimulated with HGF exhibited an increase in phosphorylation of Gab1 and Akt, but not that of EGFR (Fig. 1A), showing that this cell line effectively and specifically responds to HGF stimulation.
Figure 1.
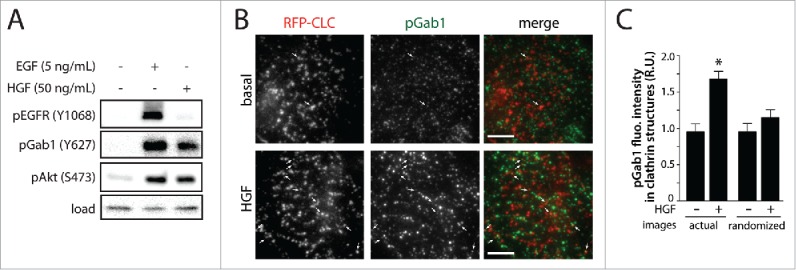
HGF stimulation increases phosphorylation of Gab1 and enrichment of pGab1 within CCPs. (A) RPE cells were stimulated with either 5 ng/mL EGF or 50 ng/mL HGF for 5 minutes. Whole-cell lysates were prepared, resolved by immunoblotting, and probed with anti-phospho-EGFR (Y1068), anti-phospho-Gab1 (Y627) and anti-phospho-Akt (S473) antibodies. (B-C) RPE cells stably expressing Tag-RFP-T fused to clathrin light chain (RFP-CLC) were stimulated with 50 ng/mL HGF and immunostained for pGab1. Shown in (B) are representative micrographs obtained by TIRF-M (scale 5 μm, arrowheads indicate pGab1-positive CCPs, selected manually). Images obtained by TIRF-M were subjected to automated detection of clathrin structures followed by quantification of pGab1 and RFP-CLC in each detected object, as in.5 Shown in (C) is the mean pGab1 fluorescence intensity detected within clathrin structures in the presence and absence of HGF compared to randomized control (> 35 cells per condition from 3 independent experiments).
To determine if phosphorylated Gab1 (pGab1) is enriched in clathrin structures upon HGF stimulation, we performed immunostaining to detect pGab1 in cells stably expressing fluorescently-labeled clathrin and imaged these samples using TIRF-M followed by automated detection of CCPs and measurement of total pGab1 within these structures (Fig. 1B). Similarly to what we had observed for EGFR signaling, HGF stimulation elicited an increase in pGab1 signal within CCPs (Fig. 1C). Randomizing the position of the pGab1 image relative to that of clathrin ablated the HGF-stimulated increase in pGab1 detected within CCPs; this shows that the overlap of pGab1 and CCPs in actual image sets was due to specific enrichment of this signaling protein in CCPs and not due to random overlap of these signals (Fig. 1C).5 Thus, like we observed upon EGF stimulation, HGF-stimulated Gab1 phosphorylation is enriched within clathrin structures.
To determine if HGF-stimulated Gab1-Akt signaling requires clathrin, we treated RPE cells with the pharmacological clathrin inhibitor pitstop2 and probed for phosphorylated Akt (pAkt) by immunoblotting. Indeed HGF-simulated Akt phosphorylation was similarly inhibited by pitstop2 treatment as was seen in the EGF-stimulated condition (Fig. 2). We previously complemented the inhibition of clathrin by pitstop2 using clathrin siRNA gene silencing and knocksideways silencing, confirming a requirement for clathrin in EGF-stimulated Akt phosphorylation.5 We also previously showed that pitstop2 treatment was without effect on EGF-stimulated Akt phosphorylation in cells expressing ErbB2,5 demonstrating that pitstop2 is not directly inhibiting Akt phosphorylation. These observations strengthen the interpretation that pitstop2 inhibits Akt phosphorylation by perturbation of clathrin instead of through off-target effects.5 Hence, our results indicate that clathrin is similarly required for HGF-stimulated Akt phosphorylation as was seen for activation of this signaling pathway by EGFR.
Figure 2.
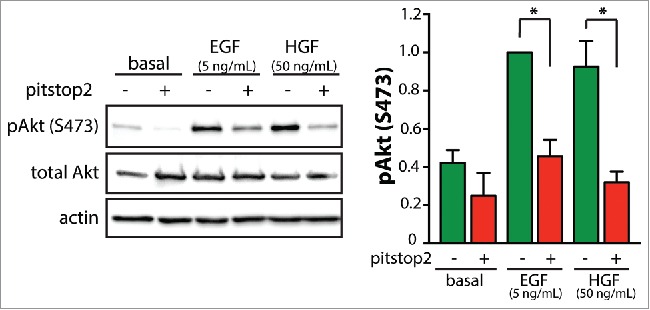
Pitstop2 treatment inhibits HGF-stimulated Akt phosphorylation. RPE cells were treated with 10 μM pitstop2 or vehicle control (0.1% DMSO) for 30 min. Following drug treatment, cells were stimulated with either 5 ng/mL EGF or 50 ng/mL HGF for 5 minutes in the continued presence or absence of the inhibitor, or left unstimulated (basal). Whole-cell lysates were prepared, resolved by immunoblotting, and probed with anti-phospho-Akt (pS473) anti-total-Akt or anti-actin antibodies. All methods were performed as described previously.5 HGF was obtained from Life Technologies (Carlsbad, CA). Shown in the left panel are representative immunoblots, and in the right panel the mean ± SE of the pAkt values in each condition (n = 4, *, p < 0.05).
The similar requirement for clathrin for EGF- and HGF-stimulated Akt phosphorylation, as well as the enrichment of pGab1 within CCPs upon stimulation with either hormone suggests that clathrin may play a similar role in signaling by each of these receptors (Fig. 3). Notably, this function of clathrin is not required for all RTKs, as clathrin is dispensable for activation of Akt upon EGF stimulation in cells expressing ErbB2 and thus signaling from EGFR-ErbB2 heterodimers (Fig. 3). Nonetheless, we have shown that clathrin controls signaling by a number of RTKs, demonstrating that this function of clathrin in controlling signaling at the cell surface is broader than the regulation of EGFR. We propose that clathrin may function as a signal scaffold, forming a clathrin signaling microdomain enriched in specific signals. Thus, recruitment of RTKs to these clathrin signaling microdomains places them in close proximity to required signal regulators; alternatively, clathrin signaling microdomains may function to exclude specific negative regulators such as phosphatases.2,5
Figure 3.
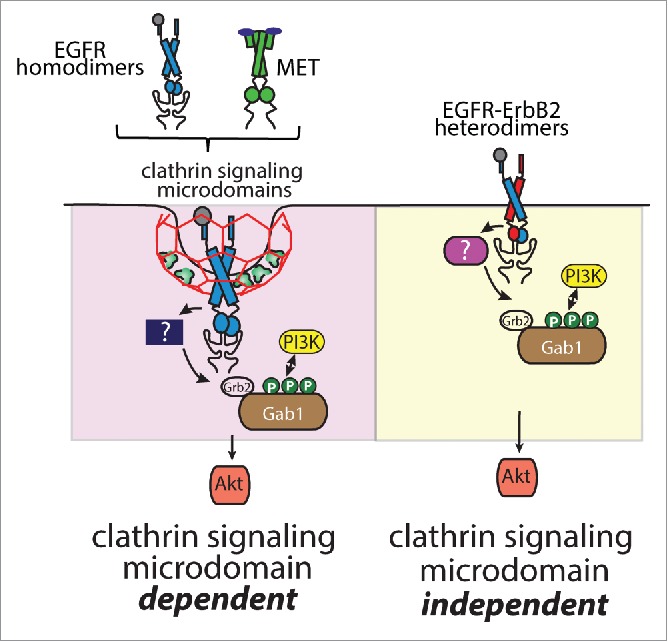
Specific receptor tyrosine kinases require clathrin for activation of Gab1-Akt signaling upon ligand binding. Shown is a diagram depicting EGFR homodimers and Met as RTKs that require plasma membrane clathrin signaling microdomains for the phosphorylation of Gab1 leading to activation of phosphatidylinositol-3-kinase (PI3K)-Akt signaling. The phosphorylation of Gab1 may require a clathrin microdomain-enriched kinase. In contrast, EGFR-ErbB2 heterodimers do not require clathrin signaling microdomains for activation of Gab1-PI3K-Akt signaling.
We observed quantitative enrichment of pGab1 within CCPs; however, pGab1 signal was also observed outside of CCPs in TIRF-M images in HGF-stimulated cells (Fig. 1), as we observed for EGF-stimulated cells.5 This may result from movement of pGab1 to other parts of the plasma membrane subsequent to phosphorylation within CCPs, perhaps as a result of interaction of the PH domain of Gab1 with phosphatidylinositol-3,4,5-trisphosphate. It should also be noted that both EGFR and Met are recruited to structures other than clathrin under some circumstances, such as actin-dependent dorsal ruffles and invadosomes.2,13-17 Whether these receptor signaling modes also require plasma membrane clathrin signaling microdomains or whether these are clathrin-independent signaling profiles is an important question that remains to be answered.
In conclusion, we find that Met signaling requires clathrin and leads to enrichment of certain signaling intermediates within CCPs in a manner similar to that of EGFR signaling. This suggests that the role of clathrin to form signaling microdomains at the plasma membrane may not be unique for EGFR but also functions in this manner for other RTKs such as Met.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Funding for this research was provided by an Operating Grant from the Canadian Institutes of Health Research to C.N.A. (grant no. 125854) and by Ryerson University.
References
- [1].Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010; 141:1117-34; PMID:20602996; http://dx.doi.org/ 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Delos Santos RC, Garay C, Antonescu CN. Charming neighborhoods on the cell surface: Plasma membrane microdomains regulate receptor tyrosine kinase signaling. Cell Signal 2015; 27:1963-76; PMID:26163824; http://dx.doi.org/ 10.1016/j.cellsig.2015.07.004 [DOI] [PubMed] [Google Scholar]
- [3].McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011; 12:517-33; PMID:21779028; http://dx.doi.org/ 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- [4].Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 2013; 26:279-91; PMID:23891661; http://dx.doi.org/ 10.1016/j.devcel.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garay C, Judge G, Lucarelli S, Bautista S, Pandey R, Singh T, Antonescu CN. Epidermal growth factor-stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol Biol Cell 2015; 26:3504-19; PMID:26246598; http://dx.doi.org/ 10.1091/mbc.E14-09-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marcotte R, Zhou L, Kim H, Roskelly CD, Muller WJ. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol 2009; 29:5858-71; PMID:19704002; http://dx.doi.org/ 10.1128/MCB.01731-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol 1999; 19:6845-57; PMID:10490623; http://dx.doi.org/ 10.1128/MCB.19.10.6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011; 3:S7-S19; PMID:22128289; http://dx.doi.org/ 10.1177/1758834011422556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lock LS, Royal I, Naujokas MA, Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem 2000; 275:31536-45; PMID:10913131; http://dx.doi.org/ 10.1074/jbc.M003597200 [DOI] [PubMed] [Google Scholar]
- [10].Lock LS, Frigault MM, Saucier C, Park M. Grb2-independent recruitment of Gab1 requires the C-terminal lobe and structural integrity of the Met receptor kinase domain. J Biol Chem 2003; 278:30083-90; PMID:12766170; http://dx.doi.org/ 10.1074/jbc.M302675200 [DOI] [PubMed] [Google Scholar]
- [11].Li N, Lorinczi M, Ireton K, Elferink LA. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the cMet-tyrosine kinase. J Biol Chem 2007; 282:16764-75; PMID:17449471; http://dx.doi.org/ 10.1074/jbc.M610835200 [DOI] [PubMed] [Google Scholar]
- [12].Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 2002; 416:187-90; PMID:11894096; http://dx.doi.org/ 10.1038/416187a [DOI] [PubMed] [Google Scholar]
- [13].Abella JV, Vaillancourt R, Frigault MM, Ponzo MG, Zuo D, Sangwan V, Larose L, Park M. The Gab1 scaffold regulates RTK-dependent dorsal ruffle formation through the adaptor Nck. J Cell Sci 2010; 123:1306-19; PMID:20332103. [DOI] [PubMed] [Google Scholar]
- [14].Abella JV, Parachoniak C, Sangwan V, Park M. Dorsal ruffle microdomains potentiate Met receptor tyrosine kinase signaling and down-regulation. J Biol Chem 2010; 285:24956-67; PMID:20529867; http://dx.doi.org/ 10.1074/jbc.M110.127985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res 2006; 66:3603-10; PMID:16585185; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2916 [DOI] [PubMed] [Google Scholar]
- [16].Rajadurai CV, Havrylov S, Zaoui K, Vaillancourt R, Stuible M, Naujokas M, Zuo D, Tremblay ML, Park M. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. J Cell Sci 2012; 125:2940-53; PMID:22366451; http://dx.doi.org/ 10.1242/jcs.100834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williams KC, Coppolino MG. SNARE-dependent interaction of Src, EGFR and β1 integrin regulates invadopodia formation and tumor cell invasion. J Cell Sci 2014; 127:1712-25; PMID:24496451; http://dx.doi.org/ 10.1242/jcs.134734 [DOI] [PubMed] [Google Scholar]


