ABSTRACT
Adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, play important roles in the initial stage of atherosclerosis. Cryptotanshinone (CPT), a natural compound isolated from Salvia miltiorrhiza Bunge, exhibits anti-atherosclerotic activity although the underlying mechanisms remain elusive. In this study, the protective effect of CPT against oxidized low-density lipoprotein (ox-LDL)-induced adhesion molecule expression was investigated in human umbilical vein endothelial cells. Ox-LDL significantly induced ICAM-1, VCAM-1, and E-selectin expression at the mRNA and protein levels but reduced eNOS phosphorylation and NO generation, which were reversed by CPT pretreatment. Sodium nitroprusside, a NO donor, N-acetyl-L-cysteine (NAC), a reactive oxygen species (ROS) scavenger, and BAY117082, a NF-κB inhibitor, inhibited ox-LDL-induced ICAM-1, VCAM-1, and E-selectin expression. Ox-LDL-induced ROS production was significantly inhibited by CPT and NAC. Furthermore, ox-LDL activated the NF-κB signaling pathway by inducing phosphorylation of IKKβ and IκBα, promoting the interaction of IKKβ and IκBα, and increasing p65 nuclear translocation, which were significantly inhibited by CPT. In addition, CPT, NAC, and BAY117082 inhibited ox-LDL-induced membrane expression of ICAM-1, VCAM-1, E-selectin, and endothelial–monocyte adhesion and restored eNOS phosphorylation and NO generation. Results suggested that CPT inhibited ox-LDL-induced adhesion molecule expression by decreasing ROS and inhibiting the NF-κB pathways, which provides new insight into the anti-atherosclerotic mechanism of CPT.
KEYWORDS: cryptotanshinone, endothelial cells, endothelial dysfunction, Ox-LDL, ROS
Introduction
Adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, are transmembrane proteins that mediate adhesion and interactions between cells or a cell and extracellular matrix.1 Increased expression and activation of these molecules in vascular endothelial cells mediate the attachment of circulating monocytes/leukocytes to the surface of endothelial cells, which is an early step in atherosclerosis development.2 P- and E-selectin expressed on the surface of activated endothelial cells bind to carbohydrate ligands on leukocytes.3 The subsequent firm attachment of monocytes to the endothelium is mediated by the interaction of ICAM-1 or VCAM-1 and integrin VLA-4 on the endothelium and monocytes, respectively.2 Therefore, the important role of adhesion molecules in the initial step of atherosclerosis makes them potential drug targets for therapeutic intervention of atherosclerosis.4
Oxidized low-density lipoprotein (ox-LDL), the oxidized form of LDL caused by reactive oxygen species (ROS), has been implicated as the key risk factor in the pathogenesis of atherosclerosis. Up-regulation of endothelial surface adhesion molecules, such as ICAM-1, VCAM-1, E-, and P-selectin, and enhanced monocyte–endothelial adhesion by ox-LDL have been detemined to play important roles in atherosclerosis.5-7
Cryptotanshinone (CPT), an active ingredient isolated from traditional Chinese herb Salvia miltiorrhiza Bunge, demonstrates anti-tumor, anti-inflammation, and anti-Alzheimer's disease effects.8-10 A previous study reported that CPT decreases ox-LDL-induced secretion of soluble ICAM-1 (sICAM-1) and soluble VCAM-1 (sVCAM-1) in human umbilical vein endothelial cells (HUVECs),11 but the underlying mechanisms remain unclear. In this study, the potential mechanisms of CPT inhibiting ox-LDL-induced adhesion molecule expression were investigated.
Materials and methods
Materials
CPT (>95%) was purchased from Chengdu Pufeide Biological Technology Co., LTD. (China). MTT and 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFH2-DA) were obtained from Sigma (USA). Antibodies for NF-κB p65, phosphorylated eNOS (p-eNOS), eNOS, phosphorylated IκBα (p-IκBα), IκBα, phosphorylated IKKβ (p-IKKβ), IKKβ, Histone H3, ICAM-1, VCAM-1, E-selectin, and GAPDH were acquired from Cell Signaling Technology (USA). Antibodies for Nrf-1 and Nrf-2 were procured from Santa Cruz (USA). Sodium nitroprusside (SNP) and 3-amino,4-aminomethyl-2′7′-difluorescein diacetate (DAF-FM) were purchased from Beyotime (China). Protein A/G PLUS-Agarose was obtained from Santa Cruz (USA). Hoechst 33342 was acquired from Invitrogen (USA). Primers and other real-time PCR-related materials were purchased from Sangon Biotech (China) and TaKaRa Bio Group (Japan). BCA protein kits were purchased from Thermo Fisher (USA). Ox-LDL was obtained from Yiyuan Biotechnology Co., LTD. (China).
Cell culture
Primary HUVECs (Gibco, Life Technologies Corp. C-003-5C) from a newborn were cultured in Ham's F-12K (Kaighn's) medium (Gibco) with endothelial cell growth supplement from bovine neural tissue (ECGS) in a humidified incubator at 37°C and 5% CO2. Cells at passages 2–4 were used.
Human monocyte cell line (THP-1) obtained from ATCC was cultured in RPMI 1640 medium containing 10% fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
MTT assay
Confluent HUVECs in 96-well microplates were treated with ox-LDL (1.0–20 μg/mL) or CPT (0–1.0 μM) for 24 h. After the removal of medium, MTT (5 mg/mL) was added to each well and incubated for another 4 h. The supernatant was then removed, and 100 μL of DMSO was added to each well to solubilize the formazan crystals. The absorbance was measured at 540 nm using an automated microplate reader (PerkinElmer).
Measurement of ROS
Approximately 1.0 × 106/well cells were seeded in a 6-well plate overnight. After ox-LDL (10 μg/mL) treatment for 2 h with or without CPT (50 nM) pretreatment, cells were incubated with CM-DCFH2-DA (10 μM) in the dark at 37°C for 40 min. Cells were washed with PBS and detached with trypsin/EDTA, and cellular fluorescence was analyzed by flow cytometry (Becton Dickinson FACS Canto™).
Immunoprecipitation
The immunoprecipitation assay was performed as described in our previous report12 with minor revisions. In brief, after ox-LDL treatment with or without CPT pretreatment, cell lysate was collected, and the protein content was determined. The cell lysate was then incubated with anti-IKKβ antibody (2 μg) for 2 h at 4°C, followed by incubation with 20 μL of protein A/G plus-agarose beads overnight with constant shaking. The beads were washed 3 times with ice-cold radio immunoprecipitation assay buffer, and bound protein was extracted by adding 40 μL of 2 × SDS sample buffer and boiling for 5 min. The complex was subjected to SDS-PAGE and visualized by Western blot.
Measurement of NO
Cells were treated with ox-LDL (10 μg/mL) for 2 h with or without CPT (50 nM) pretreatment. NO production was detected by fluorescent probe DAF-FM (5 μM) at 37°C for 30 min in the dark. Cells were rinsed with PBS, fixed in 2% paraformaldehyde (v/v) at 4°C for 5 min, and examined by fluorescent microscopy. To quantitatively determine NO formation, cells were trypsinized, and the fluorescence was detected by a flow cytometer.
Endothelial–monocyte adhesion assay
The endothelial–monocyte adhesion assay was performed as described in our previous report.13 In brief, THP-1 cells were labeled with Hoechst 33342 (10 μg/mL) for 30 min in the dark. The labeled THP-1 cells were incubated with ox-LDL-treated endothelial cells (with or without CPT pretreatment) for 3 h at 37°C. The non-adherent cells were removed with PBS. Endothelial–monocyte adhesion was observed by fluorescent microscopy.
Immunofluorescence assay
Cells (5 × 104 cells/well) were seeded on glass slides in 12-well plates. After ox-LDL treatment (with or without CPT pretreatment), the slides were fixed with 4% PFA for 30 min. The slides were then permeabilized with PBS-T (containing 0.1% Triton x-100 in PBS solution) and blocked with PBS-B (containing 4% BSA in PBS solution). After incubation with the primary (1:1000) and secondary antibodies (1:2000), the cells were stained with Hoechst 33342 in the dark for 30 min. The location and expression of adhesion molecule were observed by fluorescence microscopy.
Real-time RT-PCR
Total RNA was extracted with TRIzol Reagent. About 2 μg RNA was reverse-transcribed into cDNA using First Strand cDNA Synthesis Kit (Toyobo, Japan). Real-time PCR was performed using SYBR Green PCR reagents (Applied Biosystems). The specific primers are as follows: for ICAM-1, 5′-GGC TGG AGC TGT TTG AGA AC-3′ (forward) and 5′-ACT GTG GGG TTC AAC CTC TG′ (reverse); for VCAM-1, 5′-AAA AGC GGA GAC AGG AGA CA-3′ (forward) and 5′-AGC ACG AGA AGC TCA GGA GA′ (reverse); for E-selectin, 5′-TCT CTC AGC TCT CAC TTG-3′ (forward) and 5′-TTC TTC TTG CTG CAC CTC T′ (reverse); and for GAPDH, 5′-CGA GAT CCC TCC AAA ATC AA-3′ (forward) and 5′-TTC ACA CCC ATG GAC GAA CAT-3′ (reverse). The expression levels of target genes were determined by normalizing to GAPDH expression.
Western blot
Total proteins were extracted from treated cells, and the protein contents were quantified by BCA Protein Assay Kit. About 30 μg of proteins was subjected to SDS-PAGE and transferred onto a PVDF membrane. After blocking with 5% non-fat milk in TBST (20 mM Tris-HCl, 500 mM NaCl, and 0.1% Tween 20) at room temperature for 2 h, membranes were incubated with primary antibodies (1:2000) overnight at 4°C followed by secondary antibodies (1:10,000). The protein–antibody complexes were detected by an ECL Advance Western Blot Detection Kit. The intensity of the bands was quantitated with QuantityOne software (Bio-Rad).
Statistical analysis
Data were expressed as the mean ± SD from at least 3 separate experiments. The differences between groups were analyzed using Prism 5.0 (Graph Pad Software Inc., San Diego, CA, USA) by one-way ANOVA, followed by Student–Newman–Keuls test. p < 0.05 was considered statistically significant.
Results
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production
Given that both ox-LDL and CPT were cytotoxic to a panel of cells, we first tested their effect on HUVEC viability. Both ox-LDL and CPT were cytotoxic to endothelial cells. However, ox-LDL at 10 μg/mL and CPT at 50 nM revealed no obvious cytotoxicity (Figs. 1A and B). Ox-LDL remarkably induced ICAM-1, VCAM-1, and E-selectin expression at both protein and mRNA levels, which were significantly reversed by CPT pretreatment (Figs. 2A and B). Furthermore, ox-LDL treatment decreased the protein expression of p-eNOS and intracellular NO production, which were also restored by CPT pretreatment (Figs. 2C, D, and E). SNP, a NO donor, also dramatically reversed the ox-LDL-induced down-regulation of p-eNOS expression (Fig. 2F) and upregulation of ICAM-1, VCAM-1, and E-selectin expression (Fig. 2G). CPT alone showed no effect on the protein expression of eNOS, p-eNOS, ICAM-1, VCAM-1, and E-selectin (Supplementary Fig. 1).
Figure 1.
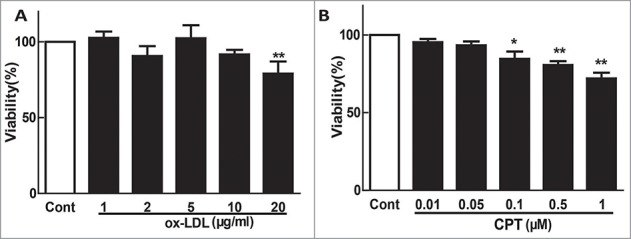
Cytotoxic effect of ox-LDL and CPT on HUVECs. Confluent HUVECs (1.0 × 104 cells/well) in 96-well plates were exposed to different concentrations of ox-LDL or CPT for 24 h, and the cell viability was examined with MTT assay. *p < 0.05,**p < 0.01 vs. Cont. Cont, control group.
Figure 2.
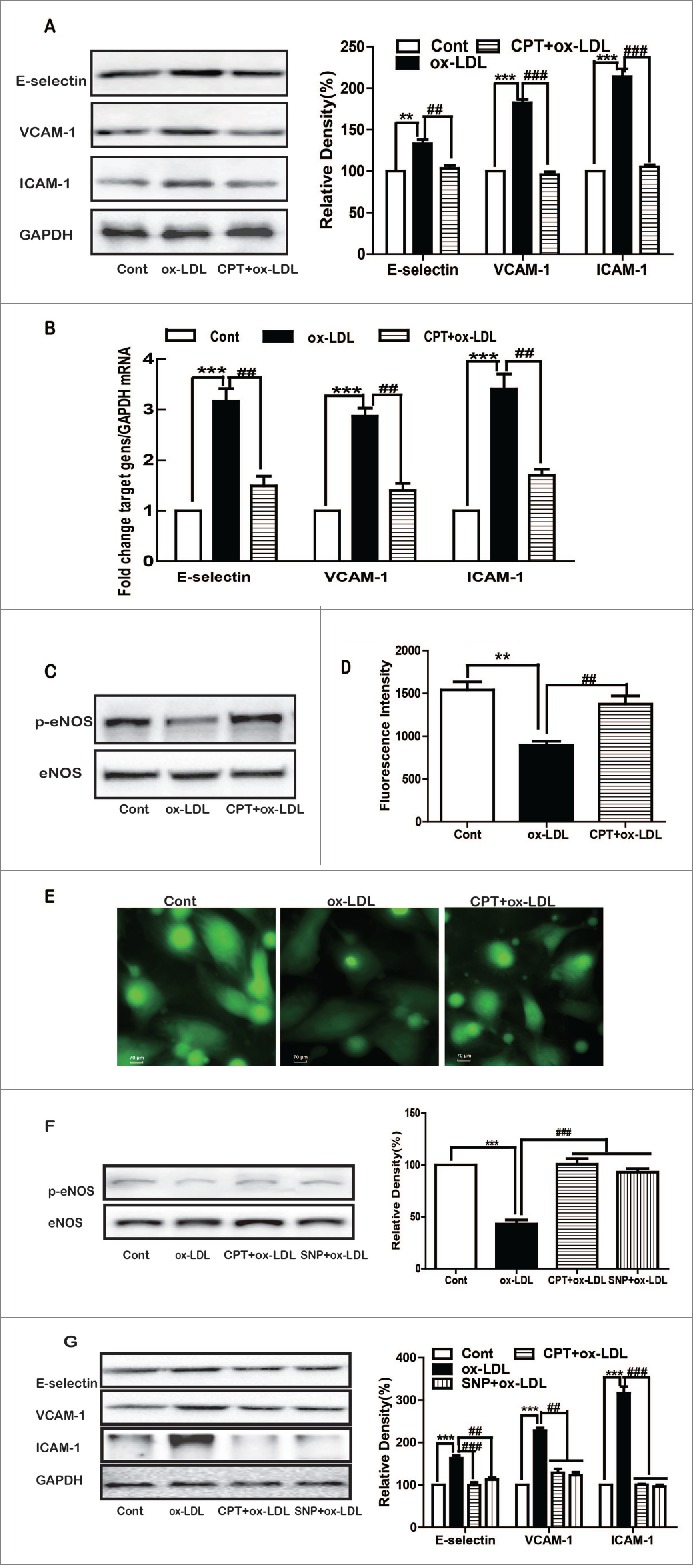
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production. HUVECs were treated with ox-LDL (10 µg/mL) for 24 h with or without CPT pretreatment (50 nM) for 1 h. The protein (A, C) and mRNA (B) expression levels were determined by Western blot and RT-PCR, respectively. HUVECs were incubated with ox-LDL for 2 h with or without CPT (50 nM) pretreatment for 1 h. Intracellular NO production was determined by a flow cytometer (D) and detected by fluorescence microscope (60×) (E) after DAF-FM staining. HUVECs were stimulated with ox-LDL (10 µg/mL) for 24 h with or without CPT (50 nM) or SNP (1 mM) pretreatment, and the protein expression was measured by Western blot (F and G). SNP, sodium nitroprusside. **p < 0.01, ***p < 0.001 vs. Cont. Cont, control group;##p < 0.01,###p < 0.001 vs. ox-LDL group.
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production via reducing ROS
Compared with the untreated group, ox-LDL treatment induced a significant right shift of ROS peaks, suggesting increased ROS formation (Fig. 3A). CPT and NAC pretreatment reversed the right shift of ROS peaks, indicating the inhibition of ROS. Statistical analysis demonstrated that ox-LDL induced more than twofold of ROS production, which was significantly suppressed by CPT or NAC pretreatment in HUVECs (Fig. 3B). Furthermore, NAC pretreatment, similar to CPT, dramatically inhibited ox-LDL-induced protein (Fig. 3C) and mRNA (Fig. 3D) expression levels of ICAM-1, VCAM-1, and E-selectin. In addition, ox-LDL-induced decreased expression of p-eNOS and NO production were also partly restored by CPT and NAC (Figs. 3E, F, and G).
Figure 3.
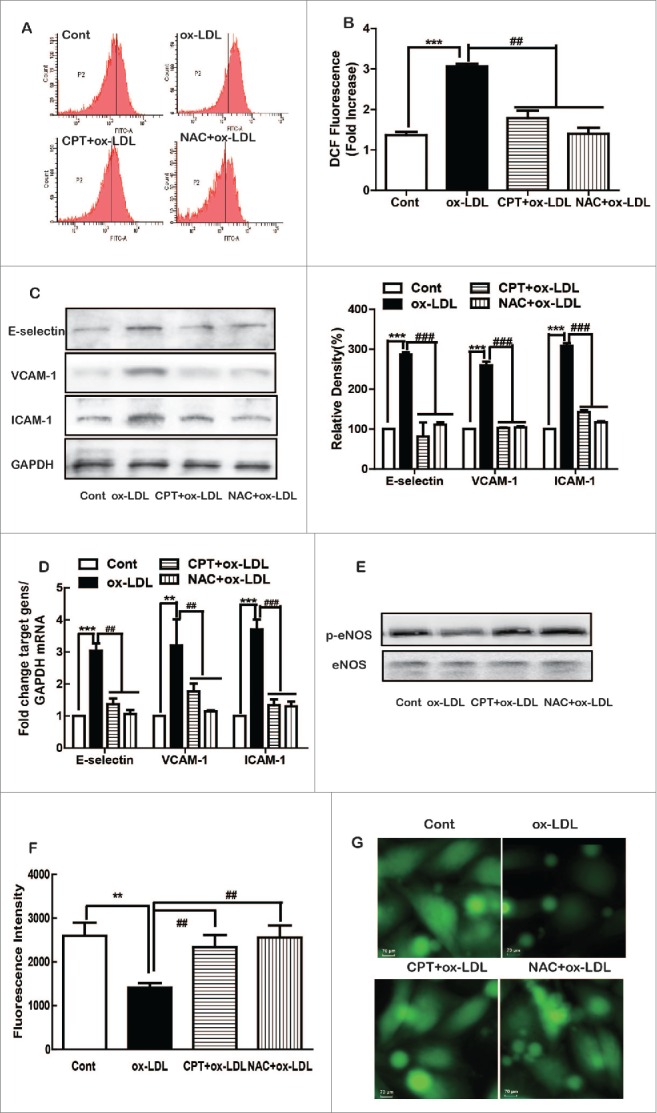
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production via ROS. HUVECs were pretreated with CPT (50 nM) or NAC (5 mM) for 1 h, followed by ox-LDL (10 µg/mL) treatment for 2 h. ROS generation was measured by flow cytometry (A, B). HUVECs were pretreated with CPT or NAC for 1 h and then treated with ox-LDL for 24 h. The protein expression was measured by Western blot (C, E), and mRNA expression was determined by RT-PCR (D). Intracellular NO production was determined by a flow cytometer (F) and detected with fluorescence microscope (60×) (G) after DAF-FM staining. **p < 0.01, ***p < 0.001 vs Cont. Cont, control group;##p < 0.01,###p < 0.001 vs. ox-LDL group.
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production via NF-κB signaling
Ox-LDL treatment significantly increased the nuclear expression of NF-κB p65, which was inhibited by CPT (Fig. 4A). Ox-LDL displayed no effect on either Nrf-1 or Nrf-2 protein expression. The IKK kinase complex, which is essentially composed of 2 kinases, namely, IKKα and IKKβ, is the core element of the NF-κB cascade. IKKβ plays a critical role in the phosphorylation of IκBα and subsequent activation of NF-κB. Thus, the effect of CPT on IKKβ was determined. Ox-LDL induced the phosphorylation of IKKβ and IκBα, which was inhibited by CPT (Fig. 4B). Furthermore, immunoprecipitation assay showed that ox-LDL enhanced the binding of IKKβ with IκBα, which was also decreased by CPT (Fig. 4C). Both CPT and BAY117082 (a NF-κB inhibitor) obviously reversed ox-LDL-induced ICAM-1, VCAM-1, and E-selectin expression (Figs. 4D and E), as well as restored p-eNOS expression and NO production (Figs. 4F and G). In addition, compared with SNP treated group, combination with BAY117082 further decreased the expression ICAM-1, VCAM-1, and E-selectin induced by ox-LDL (Fig. 4H).
Figure 4.
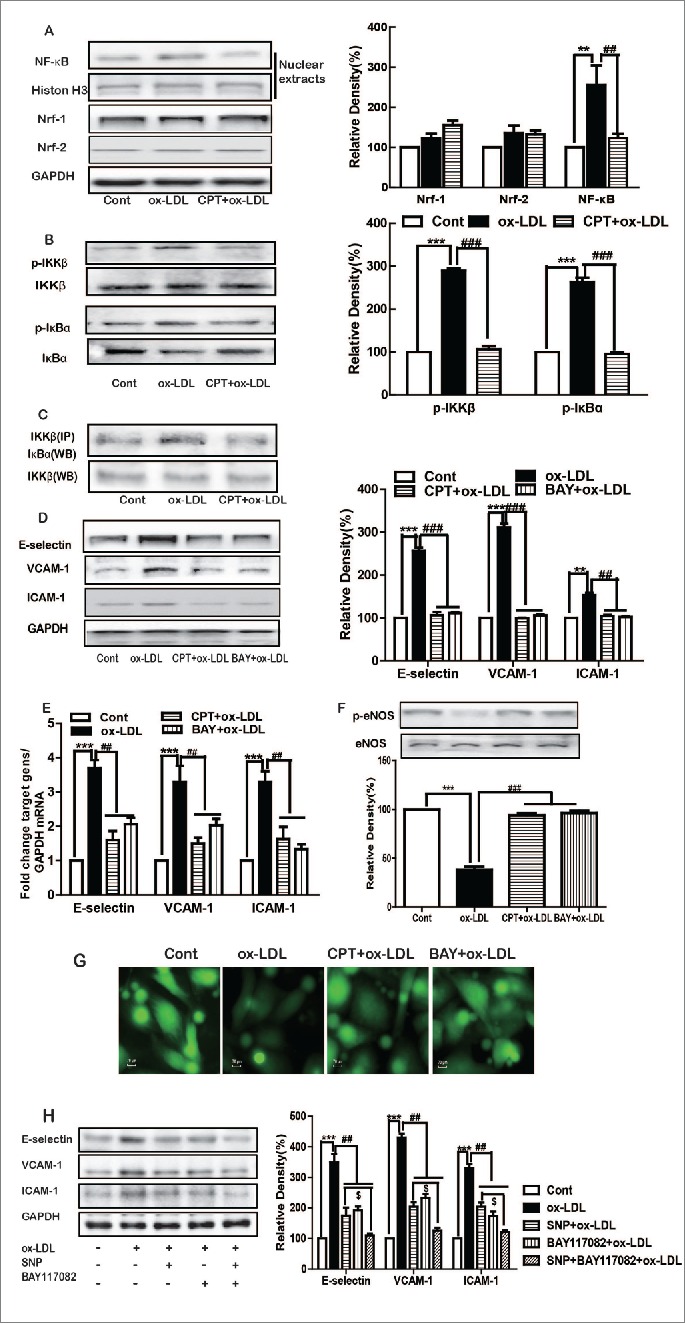
CPT inhibited ox-LDL-induced adhesion molecule expression and restored NO production via NF-κB. HUVECs were treated with ox-LDL (10 µg/mL) for 24 h with or without CPT (50 nM) SNP (1 mM) or BAY117082 (10 μM) pretreatment. The protein expression was measured by Western blot (A–D, F, H), and mRNA expression was determined by RT-PCR (E). Intracellular NO production was detected with fluorescence microscope after DAF-FM staining (60×) (G). **p < 0.01, ***p < 0.001 vs. Cont. Cont, control group; ##p < 0.01 vs. ox-LDL group;#$ < 0.05 vs. SNP + ox-LDL group.
CPT inhibited ox-LDL-induced membrane adhesion molecule expression and endothelial–monocyte adhesion
Endothelial cells expressed/secreted 2 forms of ICAM-1 and VCAM-1: membranous ICAM-1 and sICAM-1 and membranous VCAM-1 and sVCAM-1.14-16 Membranous ICAM-1 and VCAM-1 and transmembrane protein E-selectin mediate adhesion and interactions between cells or a cell and extracellular matrix 17; thus, their expression levels were determined. Ox-LDL treatment induced intensive membrane fluorescence, suggesting the increased membrane expression of ICAM-1, VCAM-1, and E-selectin, which was significantly inhibited by CPT, NAC, or BAY117082 pretreatment (Fig. 5A). Furthermore, ox-LDL stimulation increased the adhesion of THP-1 cells to HUVECs, which was also dramatically inhibited by CPT, NAC, or BAY117082 pretreatment (Fig. 5B).
Figure 5.
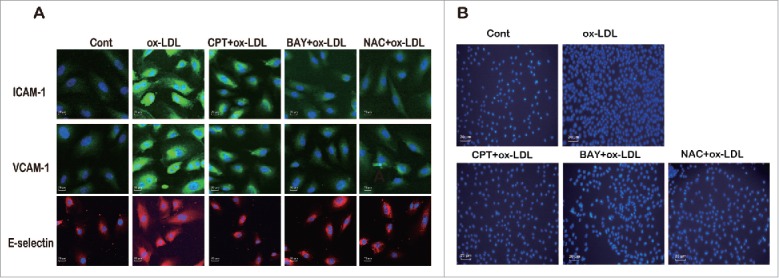
CPT inhibited ox-LDL-induced membrane expression of adhesion molecules and THP-1-HUVEC adhesion. HUVECs were treated with ox-LDL (10 µg/mL) for 24 h with or without CPT (50 nM), BAY117082 (10 μM), or NAC (5 mM) pretreatment. The membrane expression of adhesion molecules was examined by immunofluorescence assay (60×) (A). HUVECs were treated with ox-LDL (10 µg/mL) for 24 h with or without CPT (50 nM), BAY117082 (10 μM), or NAC (5 mM) pretreatment. The cells were then incubated with labeled THP-1 cells for 3 h. The attached THP-1 cells were visualized by inverted fluorescent microscopy (20×) (B).
Discussion
Ox-LDL plays a crucial role in the initiation and progression of atherosclerosis by inducing endothelial activation and dysfunction, promoting the proliferation and migration of SMCs, facilitating the formation of foam cells, and enhancing endothelial–monocyte adhesion.18-20 Accumulated studies revealed that a panel of natural products, such as curcumin, berberine, and tanshinone IIA,21-23 can inhibit ox-LDL-induced adhesion molecule expression. A previous study observed that CPT improves ox-LDL-induced adhesion molecule expression,11 but the underlying mechanisms remain elusive. In the current study, we revealed that CPT inhibited ox-LDL-induced adhesion molecule expression via the ROS-dependent pathway mediated by NF-κB.
MTT assay showed that CPT demonstrated significant cytotoxicity to HUVECs with a high concentration (>0.1 μM). Thus, its effect was tested at non-cytotoxic concentrations. Endothelial dysfunction (ED) contributes to a panel of cardiovascular diseases, including hypertension, atherosclerosis, and coronary artery disease. The characteristics of ED include a lack of NO such as inhibiting and decreasing NO bioavailability 24 and/or overexpression of adhesion molecules, such as ICAM-1, VCAM-1, E-, and P-selectin.25,26 A previous study demonstrated that CPT inhibits ox-LDL-induced sICAM-1 and sVCM-1 secretion in HUVEC culture medium.11 In the present study, we determined that ox-LDL induced the up-regulation of ICAM-1, VCAM-1, and E-selectin expression at both protein and mRNA levels and decreased NO production, thereby suggesting that ox-LDL caused ED. CPT pretreatment reversed these alterations, indicating that CPT protected ox-LDL-induced ED.
ROS is a major target of oxidant stress, playing a critical role in the pathophysiology of several vascular diseases and disorders.27 Ox-LDL has been established to induce intracellular ROS formation in endothelial cells.28,29 Consistent with these reports, elevated ROS levels were observed after ox-LDL exposure under our experimental conditions. Similar to our recent report that CPT inhibited TNF-α-induced ROS formation in HUVECs,30 CPT also dramatically inhibited ox-LDL-induced ROS formation in endothelial cells. Furthermore, NAC, a ROS scavenger, exhibited a similar effect on ox-LDL-induced adhesion molecule expression and ROS production. Collectively, these data suggested that CPT inhibited ox-LDL-induced adhesion molecule expression in a ROS-dependent manner.
Several lines of evidence indicated that ox-LDL suppresses NF-κB and incapacitates the protection from apoptosis in activated endothelial cells,28,31 whereas other studies oppositely highlighted that ox-LDL induces NF-κB activation and the subsequent expression of pro-inflammatory genes. Nrf-1 and Nrf-2 are 2 important oxidative sensitive transcription factors involved in regulating the expression of adhesion molecules.32-35 We found that ox-LDL had no influence on either Nrf-1 or Nrf-2 protein expression, but it dramatically increased the unclear expression of p65, suggesting that ox-LDL induced p65 nuclear translocation. Ox-LDL induced the phosphorylation of IKKβ and IκBα and enhanced their interactions, indicating that ox-LDL activated the NF-κB pathway by promoting p65 nuclear translocation mediated by enhancing IKKβ-IκBα interactions and their phosphorylation.36 The inhibitory effect of CPT suggested that CPT regulated IKKβ and IκBα, the upper stream factors of the NF-κB pathways. Thus, potential targets for CPT may exist in the NF-κB system. The inhibitory effect of BAY117082, a NF-κB inhibitor, on ox-LDL-induced adhesion molecule expression further confirmed the involvement of NF-κB. Collectively, these data indicated that CPT suppressed ox-LDL-induced NF-κB activation and the subsequent expression of adhesion molecules.
Several studies suggested that NO can suppress the expression of VCAM-1, ICAM-1, and E-selectin in response to various pro-inflammatory cytokines.37 In the present study, we found that SNP, a NO donor, dramatically reversed the ox-LDL-induced expression of adhesion molecules, eNOS phosphorylation, and NO generation. Furthermore, the ox-LDL-induced downregulation of eNOS phosphorylation and decrease of NO generation were reversed by NAC and BAY117082. These data proved that NO negatively regulated adhesion molecule expression mediated by NF-κB in response to ox-LDL, which formed a branch for the regulation of adhesion molecules. The decreased intracellular NO partly resulted from the decreased expression of eNOS because eNOS was the main source of NO production in endothelial cells. However, this phenomenon might also be due to the overproduction of ROS, because certain types of ROS, especially superoxide anion, can react with NO to produce highly reactive and cytotoxic products, such as peroxynitrite (ONOO-) and peroxynitrous.29,38 Thus, the inhibitory effect of CPT on ox-LDL-induced adhesion molecule expression might be due to its direct effect on the NF-κB system or/and indirectly mediated by eNOS/NO. To make clear whether NO was located in NF-κB pathway or worked independent of NF-κB pathway, combined treatment with SNP and BAY117082 was performed. Decreased expression of adhesion molecules in response to SNP was further decreased by BAY117082. This suggested that NO was located in downstream of NF-κB. However, the exact role of eNOS/NO in the regulation of adhesion molecule expression in response to ox-LDL requires further investigation.
Monocyte adhesion to endothelial cells was an important early event in atherogenesis, which was partly controlled by expression of adhesion molecules on the endothelial cell surface.39,40 The immunofluorescence results indicated that ox-LDL induced the increased membrane protein expression of ICAM-1, VCAM-1, and E-selectin. Furthermore, ox-LDL induced enhanced THP-1 cell adhesion to endothelial cells, suggesting that these adhesion molecules played an important role in this process. The inhibitory effect of NAC and BAY117082 provided further evidence for the involvement of ROS and NF-κB in ox-LDL-induced adhesion molecule expression. Thus, the inhibitory effect of CPT indicated that CPT may enhance atherosclerosis treatment in the early stage.
In conclusion, the present work provided evidence that CPT, a natural compound, inhibited adhesion molecule expression upon ox-LDL stimulation by regulating the NF-κB pathway mediated through decreasing ROS formation (Fig. 6). This data provides new insights into the beneficial effect of CPT in cardiovascular diseases.
Figure 6.
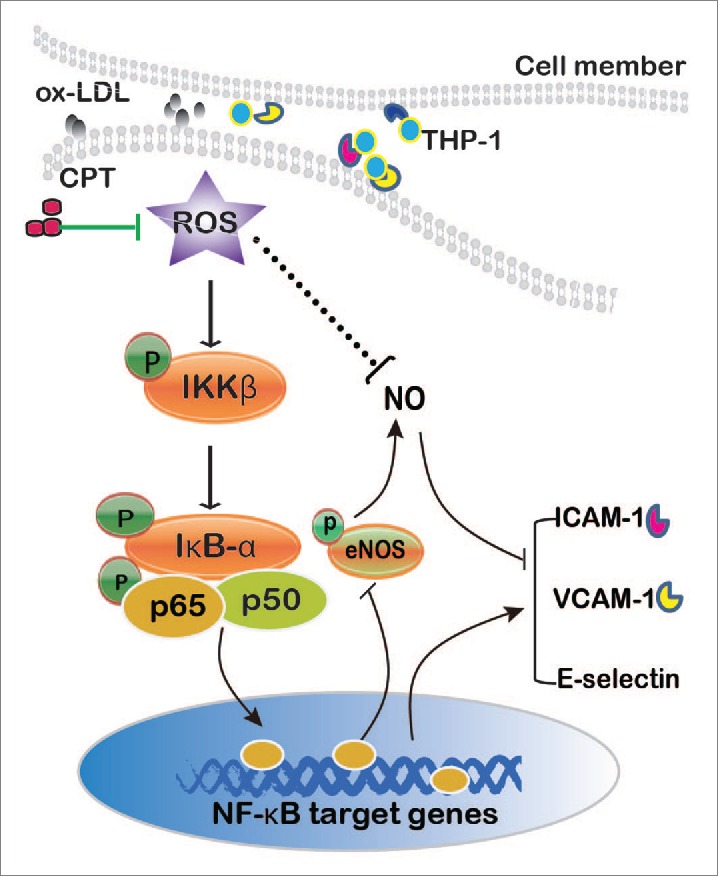
Mechanisms underlying CPT inhibited ox-LDL-induced adhesion molecule expression in HUVECs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National Natural Science Foundation of China (No 81160048), the Science and Technology Development Fund, Macao S.A.R (FDCT) (No. 021/2012/A1), and the Research Fund of University of Macau (MRG007/CXP/2013/ICMS).
References
- [1].Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The Expression of the Adhesion Molecules Icam-1, Vcam-1, Pecam, and E-Selectin in Human Atherosclerosis. J Pathol 1993; 171:223–9; PMID:7506307; http://dx.doi.org/ 10.1002/path.1711710311 [DOI] [PubMed] [Google Scholar]
- [2].Ling S, Nheu L, Komesaroff PA. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini Rev Med Chem 2012; 12:175–83; PMID:22070689; http://dx.doi.org/ 10.2174/138955712798995057 [DOI] [PubMed] [Google Scholar]
- [3].Varki A. Selectin ligands. Proc Natl Acad Sci U S A 1994; 91:7390–7; PMID:7519775; http://dx.doi.org/ 10.1073/pnas.91.16.7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lutters BC, Leeuwenburgh MA, Appeldoorn CC, Molenaar TJ, Van Berkel TJ, Biessen EA. Blocking endothelial adhesion molecules: a potential therapeutic strategy to combat atherogenesis. Curr Opin Lipidol 2004; 15:545–52; PMID:15361790; http://dx.doi.org/ 10.1097/00041433-200410000-00008 [DOI] [PubMed] [Google Scholar]
- [5].Frostegard J, Wu R, Haegerstrand A, Patarroyo M, Lefvert AK, Nilsson J. Mononuclear leukocytes exposed to oxidized low density lipoprotein secrete a factor that stimulates endothelial cells to express adhesion molecules. Atherosclerosis 1993; 103:213–9; PMID:7507327; http://dx.doi.org/ 10.1016/0021-9150(93)90264-U [DOI] [PubMed] [Google Scholar]
- [6].Kim JA, Territo MC, Wayner E, Carlos TM, Parhami F, Smith CW, Haberland ME, Fogelman AM, Berliner JA. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler Thromb 1994; 14:427–33; PMID:8123647; http://dx.doi.org/ 10.1161/01.ATV.14.3.427 [DOI] [PubMed] [Google Scholar]
- [7].Jeng JR, Chang CH, Shieh SM, Chiu HC. Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochimica et Biophysica Acta 1993; 1178:221–7; PMID:7688576; http://dx.doi.org/ 10.1016/0167-4889(93)90013-F [DOI] [PubMed] [Google Scholar]
- [8].Chen W, Lu Y, Chen G, Huang S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anti-Cancer Agents Med Chem 2013; 13:979–87; PMID:23272908; http://dx.doi.org/ 10.2174/18715206113139990115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH, Chen SR, Le K, Wang YH, Liu PQ. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-kappaB and MAPK signaling pathways. Inflammation 2011; 34:111–8; PMID:20490642; http://dx.doi.org/ 10.1007/s10753-010-9214-3 [DOI] [PubMed] [Google Scholar]
- [10].Yoo KY, Park SY. Terpenoids as potential anti-Alzheimer's disease therapeutics. Molecules (Basel, Switzerland) 2012; 17:3524–38; PMID:22430119; http://dx.doi.org/ 10.3390/molecules17033524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ang KP, Tan HK, Selvaraja M, Kadir AA, Somchit MN, Akim AM, Zakaria ZA, Ahmad Z. Cryptotanshinone attenuates in vitro oxLDL-induced pre-lesional atherosclerotic events. Planta Med 2011; 77:1782–7; PMID:21614753; http://dx.doi.org/ 10.1055/s-0030-1271119 [DOI] [PubMed] [Google Scholar]
- [12].Ou HC, Song TY, Yeh YC, Huang CY, Yang SF, Chiu TH, Tsai KL, Chen KL, Wu YJ, Tsai CS. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol 2010; 108:1745–56; PMID:20203069; http://dx.doi.org/ 10.1152/japplphysiol.00879.2009 [DOI] [PubMed] [Google Scholar]
- [13].Zhao W, Ma G, Chen X. Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-kappaB pathway. Vasc Pharmacol 2014; 63:162–72; PMID:25135647; http://dx.doi.org/ 10.1016/j.vph.2014.06.008 [DOI] [PubMed] [Google Scholar]
- [14].Budnik A, Grewe M, Gyufko K, Krutmann J. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol 1996; 24:352–9; PMID:8641365. [PubMed] [Google Scholar]
- [15].Calabresi PA, Tranquill LR, Dambrosia JM, Stone LA, Maloni H, Bash CN, Frank JA, McFarland HF. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon beta-1b. Ann Neurol 1997; 41:669–74; PMID:9153530; http://dx.doi.org/ 10.1002/ana.410410517 [DOI] [PubMed] [Google Scholar]
- [16].Pigott R, Dillon LP, Hemingway IH, Gearing AJH. Soluble Forms of E-Selectin, Icam-1 and Vcam-1 Are Present in the Supernatants of Cytokine Activated Cultured Endothelial-Cells. Biochem Biophys Res Commun 1992; 187:584–9; PMID:1382417; http://dx.doi.org/ 10.1016/0006-291X(92)91234-H [DOI] [PubMed] [Google Scholar]
- [17].Ala A, Dhillon AP, Hodgson HJ. Role of cell adhesion molecules in leukocyte recruitment in the liver and gut. Int J Exp Pathol 2003; 84:1–16; PMID:12694483; http://dx.doi.org/ 10.1046/j.1365-2613.2003.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 2000; 101:2889–95; PMID:10869259; http://dx.doi.org/ 10.1161/01.CIR.101.25.2889 [DOI] [PubMed] [Google Scholar]
- [19].Kaperonis EA, Liapis CD, Kakisis JD, Dimitroulis D, Papavassiliou VG. Inflammation and atherosclerosis. Eur J Vasc Endovasc Surg 2006; 31:386–93; PMID:16359887; http://dx.doi.org/ 10.1016/j.ejvs.2005.11.001 [DOI] [PubMed] [Google Scholar]
- [20].Ou HC, Chou FP, Lin TM, Yang CH, Sheu WH. Protective effects of honokiol against oxidized LDL-induced cytotoxicity and adhesion molecule expression in endothelial cells. Chem Biol Interact 2006; 161:1–13; PMID:16580656; http://dx.doi.org/ 10.1016/j.cbi.2006.02.006 [DOI] [PubMed] [Google Scholar]
- [21].Xu S, Liu Z, Huang Y, Le K, Tang F, Huang H, Ogura S, Little PJ, Shen X, Liu P. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-kappaB activation. Transl Res 2012; 160:114–24; PMID:22677363; http://dx.doi.org/ 10.1016/j.trsl.2012.01.008 [DOI] [PubMed] [Google Scholar]
- [22].Huang Z, Cai X, Li S, Zhou H, Chu M, Shan P, Huang W. Berberineattenuated monocyte adhesion to endothelial cells induced by oxidized lowdensity lipoprotein via inhibition of adhesion molecule expression. Mol Med Rep 2013; 7:461–5; PMID:23241897. [DOI] [PubMed] [Google Scholar]
- [23].Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 2014; 232:40–51; PMID:24401215; http://dx.doi.org/ 10.1016/j.atherosclerosis.2013.10.016 [DOI] [PubMed] [Google Scholar]
- [24].Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986; 320:454–6; PMID:3007998; http://dx.doi.org/ 10.1038/320454a0 [DOI] [PubMed] [Google Scholar]
- [25].Salvia EotIif, vivo mBoaamai, inflammatory responses. Phytotherapy ResearchKubes. Suzuki M P, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 1991; 88:4651–5; PMID:1675786; http://dx.doi.org/ 10.1073/pnas.88.11.4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med 2007; 17:48–54; PMID:17292046; http://dx.doi.org/ 10.1016/j.tcm.2006.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys 2004; 23:265–95; PMID:15638116. [PubMed] [Google Scholar]
- [28].Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 2000; 275:12633–8; PMID:10777555; http://dx.doi.org/ 10.1074/jbc.275.17.12633 [DOI] [PubMed] [Google Scholar]
- [29].Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V, Sawamura T. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem 2001; 276:13750–5; PMID:11278710. [DOI] [PubMed] [Google Scholar]
- [30].Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circulat Physiol 2005; 289:H813–22; PMID:15792994; http://dx.doi.org/ 10.1152/ajpheart.00092.2005 [DOI] [PubMed] [Google Scholar]
- [31].Heermeier K, Leicht W, Palmetshofer A, Ullrich M, Wanner C, Galle J. Oxidized LDL suppresses NF-kappaB and overcomes protection from apoptosis in activated endothelial cells. J Am Soc Nephrol 2001; 12:456–63; PMID:11181793. [DOI] [PubMed] [Google Scholar]
- [32].Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 1995; 9:899–909; PMID:7542214 [PubMed] [Google Scholar]
- [33].Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol Res 2000; 33:89–96; PMID:15693275; http://dx.doi.org/ 10.4067/S0716-97602000000200008 [DOI] [PubMed] [Google Scholar]
- [34].Kim CK, Cho DH, Lee KS, Lee DK, Park CW, Kim WG, Lee SJ, Ha KS, Goo Taeg O, Kwon YG, et al.. Ginseng Berry Extract Prevents Atherogenesis via Anti-Inflammatory Action by Upregulating Phase II Gene Expression. Evid Based Complement Alternat Med 2012; 2012:490301; PMID:23243449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Drabarek B, Dymkowska D, Szczepanowska J, Zablocki K. TNFalpha affects energy metabolism and stimulates biogenesis of mitochondria in EA.hy926 endothelial cells. Int J Biochem Cell Biol 2012; 44:1390–7; PMID:22687752; http://dx.doi.org/ 10.1016/j.biocel.2012.05.022 [DOI] [PubMed] [Google Scholar]
- [36].Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1:a000034; PMID:20066092; http://dx.doi.org/ 10.1101/cshperspect.a000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest 2013; 123:540–1; PMID:23485580; http://dx.doi.org/ 10.1172/JCI66843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vaziri ND, Ni ZM, Oveisi F, Liang KH, Pandian R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 2002; 39:135–41; PMID:11799092; http://dx.doi.org/ 10.1161/hy0102.100540 [DOI] [PubMed] [Google Scholar]
- [39].Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993; 362:801–9; PMID:8479518; http://dx.doi.org/ 10.1038/362801a0 [DOI] [PubMed] [Google Scholar]
- [40].Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol 1994; 24:1591–601; PMID:7963103; http://dx.doi.org/ 10.1016/0735-1097(94)90162-7 [DOI] [PubMed] [Google Scholar]


