Abstract
Background
Adrenocortical carcinoma (ACC) is a rare disease with a poor prognosis. Given the lack of data on readmission after resection of ACC, the objective of the current study was to define the incidence of readmission, as well as identify risk factors associated with readmission among patients with ACC who underwent surgical resection.
Methods
Two hundred nine patients who underwent resection of ACC between January 1993 and December 2014 at 1 of 13 major centers in the USA were identified. Demographic and clinicopathological data were collected and analyzed relative to readmission.
Results
Median patient age was 52 years, and 62 % of the patients were female. Median tumor size was 12 cm, and the majority of patients had an American Society of Anesthesiologists (ASA) class of 3–4 (n=85, 56 %). The overall incidence of readmission within 90 days from surgery was 18 % (n=38). Factors associated with readmission included high ASA class (odds ratio (OR), 4.88 (95 % confidence interval (CI), 1.75–13.61); P=0.002), metastatic disease on presentation (OR, 2.98 (95 % CI, 1.37–6.46); P=0.006), EBL (>700 mL: OR, 2.75 (95 % CI, 1.16–6.51); P=0.02), complication (OR, 1.91 (95 % CI, 1.20–3.05); P=0.007), and prolonged length of stay (LOS; ≥9 days: OR, 4.12 (95 % CI, 1.88–9.01); P<0.001). On multivariate logistic regression, a high ASA class (OR, 4.01 (95 % CI, 1.44–11.17); P=0.008) and metastatic disease on presentation (OR, 3.44 (95 % CI, 1.34–8.84); P=0.01) remained independently associated with higher odds of readmission.
Conclusion
Readmission following surgery for ACC was common as one in five patients experienced a readmission. Patients with a high ASA class and metastatic disease on presentation were over four and three times more likely to be readmitted after surgical treatment for ACC, respectively.
Keywords: Adrenocortical carcinoma, Surgery, Readmission
Introduction
Adrenocortical carcinoma (ACC) is a difficult disease to study due to its overall low incidence. In fact, there are only about 0.5–2 ACC cases per million adults in the USA each year.1 While rare, ACC can be associated with a poor prognosis with 5-year survival as low as 30 % among patients with advanced disease.2 Most information on ACC in the literature is derived from small, single-institution case series.2–9 In addition, most studies have focused exclusively on examining treatment strategies, as well as long-term outcome such as survival.10–13 More recently, other metrics of surgical quality have become an increasing topic of interest. In particular, unplanned hospital readmission has been emphasized as an area for quality improvement.14–16 Readmission can lead to adverse medical outcomes, lower patient satisfaction,17 as well as increased health care costs.18 The Centers for Medicare and Medicaid Services has targeted readmission as a quality metric and will financially penalize those hospitals with excess readmission in the future.
The incidence and risk factors associated with readmission have been reported by several investigators.19–22 In particular, using the American College of Surgeons National Surgical Quality Improvement Program, Lucas et al. reported on readmission after general, vascular, and thoracic surgery.20 In this study, the authors noted that length of stay (LOS) and American Society of Anesthesiologists (ASA) class could be used to predict readmission with moderate accuracy among a broad variety of patients. Other investigators have focused more on disease-specific populations and reported factors associated with readmission among patients with pancreatic, liver, cholangiocarcinoma, as well as gastric cancer.23–30 Data on readmission following surgery for ACC remains not defined. Information on ACC may be particularly interesting as readmission might be affected by patient presentation (e.g., age, comorbidities, etc.), tumor factors (e.g., size, functional vs. non-functional tumor, etc.), as well as perioperative considerations (e.g., operative approach, blood loss, complications, etc.). To our knowledge, no previous study has examined readmission after resection of ACC. Given this knowledge gap, the objective of the current study was to define the incidence of readmission after surgery for ACC. In particular, using a large, multicenter collaborative dataset, we sought to identify risk factors associated with a higher risk of readmission among patients with ACC who underwent surgical resection.
Methods
Study Population
All patients who underwent resection for ACC at 1 of 13 centers between 1993 and 2014 were identified. The medical centers included Johns Hopkins Hospital, Baltimore, MD; Emory University, Atlanta, GA; Stanford University, Palo Alto, CA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; The Ohio State University, Columbus, OH; Medical College of Wisconsin, Milwaukee, WI; New York University, New York, NY; University of California at San Diego, San Diego, CA; University of California at San Francisco, San Francisco, CA; University of Texas Southwestern Medical Center, Dallas, TX; and Vanderbilt University Medical Center, Nashville, TN). The institutional review board of the participating institutions approved the study. Only patients with complete data on hospital course and postdischarge status were included in the cohort (n=209).
Baseline characteristics and demographic data were collected, including age, race, sex, ASA class, comorbidities, as well as body mass index (BMI). Clinical data such as tumor size, laterality of tumor (i.e., left, right), function of tumor (i.e., hormone-secreting, non-secreting), the presence or absence of capsular invasion, and final T, N, and M stage of disease were also collected. In addition, surgical approach (open abdominal or posterior, minimally invasive surgery (MIS), thoracoabdominal surgery), estimated blood loss (EBL), as well as overall length of stay (LOS), postoperative complications, and in-hospital mortality. Perioperative morbidity was classified according to the Clavien–Dindo classification system: grades 1 and 2 were categorized as minor complications, while grades 3 and 4 were categorized as major complications.31 Readmission was defined as rehospitalization within 90 days of discharge of the index hospitalization.25
Statistical Analysis
Categorical variables were reported as total frequencies and proportions; median and interquartile range (IQR) were used to describe continuous variables. Discrete variables were compared using the Chi-square and Fisher’s exact tests, where appropriate. The Wilcoxon test was used to compare continuous data variables, as well as to assess parametric and non-parametric data. For the univariate and multivariate models, logistic regression was utilized to evaluate the relationships between relevant baseline and/or clinicopathological characteristics and the odds of readmission. Variables significant on univariate analysis (P<0.05), as well as those deemed to be clinically important, were entered into the multivariate regression model. For multivariate logistic regression, variables with missing data were subjected to the multiple imputation method for missing values. Odds ratios (OR) were presented with 95 % confidence intervals (95 % CI) and P values, respectively. Collinearity was assessed using variance inflated factor (VIF). A sensitivity analysis was carried out to compare multivariate logistic regression models before and after multiple imputation, which showed similar results. For all statistical analysis, P<0.05 was considered statistically significant. All analyses were carried out using STATA®, version 13.1 (StataCorp, LP, College Station, Texas, USA).
Results
Patient Characteristics
A total of 209 patients who underwent resection of ACC met inclusion criteria and were included in the study group (Table 1). Median patient age was 52 years (IQR, 44–62), and most patients were female (n=130, 62.2 %). Most patients had at least one comorbidity, as the majority of patients had an ASA class of 3 or 4 (n=85, 55.9 %). In most patients, the ACC tumor was non-functioning (n=111, 55.5 %), while a subset had a tumor that was functional (glucocorticoid: n=50, 25.0 %; virilizing/feminizing: n=28, 14.0 %; mineralocorticoid hormone secreting: n=10, 5.0 %). Preoperatively, most ACC tumors were evaluated by computed tomography (CT; n=129, 62.3 %), while a subset of patients had both a CT and magnetic resonance imaging (MRI; n=61; 29.5 %); only a few patients (n=15, 7.3 %) had only an MRI. Median tumor size was 11.8 cm (IQR, 8.8–15.0), with tumors being roughly equally distributed on the right (n=93, 44.9 %) and left (n= 114, 55.1 %) side.
Table 1.
Baseline characteristics of patients underwent surgical resection for ACC
| All (n=209) | Readmission (n=38) | No readmission (n=171) | P value | |
|---|---|---|---|---|
| Age (years, median (IQR)) | 52 (44–62) | 55 (49–64) | 51 (44–61) | 0.15 |
| Caucasian race | 161 (79.7) | 30 (78.9) | 131 (79.9) | 0.89 |
| Female gender | 130 (62.2) | 23 (61.0) | 107 (63.0) | 0.81 |
| ASA Class (n=152) | ||||
| 1 and 2 | 67 (44.1) | 5 (17.2) | 62 (50.4) | 0.001 |
| 3 and 4 | 85 (55.9) | 24 (82.8) | 61 (49.6) | |
| Comorbidities | ||||
| CAD | 18 (8.7) | 5 (13.9) | 13 (7.6) | 0.23 |
| CHF | 9 (4.4) | 4 (11.1) | 5 (2.9) | 0.03 |
| COPD | 13 (6.3) | 4 (10.8) | 9 (5.3) | 0.21 |
| Liver disease | 6 (2.9) | 1 (2.7) | 5 (2.9) | 0.93 |
| Chronic renal insufficiency | 12 (5.8) | 5 (13.5) | 7 (4.1) | 0.03 |
| DM | 37 (17.9) | 9 (24.3) | 28 (16.4) | 0.26 |
| BMI (kg/m2, median (IQR; n=161) | 27 (24–33) | 29 (23–37) | 27 (24–33) | 0.66 |
| Laterality | ||||
| Left | 114 (55.1) | 22 (57.9) | 92 (54.4) | 0.70 |
| Right | 93 (44.9) | 16 (42.1) | 77 (45.6) | |
| Tumor functionality | ||||
| Non-secreting | 111 (55.5) | 17 (48.6) | 94 (57.0) | 0.14 |
| Glucocorticoid | 50 (25.0) | 9 (25.7) | 41 (24.9) | |
| Mineralocorticoid | 10 (5.0) | 0 | 10 (6.1) | |
| Virilizing/feminizing | 28 (14.0) | 9 (25.7) | 19 (11.5) | |
| Capsular invasion | 91 (59.5) | 15 (56.6) | 76 (60.8) | 0.67 |
| T stage | ||||
| I | 9 (4.5) | 1 (2.8) | 8 (4.9) | 0.88 |
| II | 82 (40.8) | 16 (44.4) | 66 (40.0) | |
| III | 76 (37.8) | 12 (33.0) | 64 (38.8) | |
| IV | 33 (16.4) | 7 (19.4) | 26 (15.8) | |
| N stage | ||||
| N0 | 48 (23.5) | 9 (25.0) | 39 (23.2) | 0.16 |
| N1 | 24 (11.8) | 8 (22.2) | 16 (9.52) | |
| Nx | 131 (64.2) | 19 (52.8) | 112 (66.7) | |
| Metastasis at presentation | 42 (20.1) | 14 (36.8) | 28 (16.4) | 0.004 |
| Preop imaging | ||||
| CT | 129 (62.3) | 23 (62.2) | 106 (62.3) | 0.85 |
| MRI | 15 (7.3) | 2 (5.4) | 13 (7.7) | |
| Both | 61 (29.5) | 12 (32.4) | 49 (28.8) | |
| Tumor size (cm, median (IQR)) | 11.8 (8.8–15.0) | 12.2 (10.5–15.0) | 11.3 (8.5–15.0) | 0.31 |
| Surgical approach | ||||
| Open surgery | 136 (66.3) | 29 (80.6) | 107 (63.3) | 0.10 |
| MIS | 32 (15.6) | 2 (5.6) | 30 (17.8) | |
| Thoracoabdominal | 37 (18.1) | 5 (13.9) | 32 (18.9) | |
| OR time (min, median (IQR; n=135)) | 240 (159–330) | 298 (186–364) | 231 (155–318) | 0.051 |
| EBL (mL, median (IQR; n=158)) | 700 (200–1900) | 1000 (600–2300) | 600 (200–1500) | 0.008 |
| LOS (days, median (IQR; n=194)) | 6 (4–9) | 9 (6–13) | 6 (4–8) | <0.001 |
| Complication during hospitalization (n=180) | ||||
| Grades 1 and 2 | 44 (24.4) | 14 (40.0) | 30 (20.7) | 0.007 |
| Grades 3 and 4 | 27 (15.0) | 8 (22.8) | 19 (13.1) | |
| No complication | 109 (60.6) | 13 (37.1) | 96 (66.2) | |
| Death during hospitalization | 5 (2.4) | 0 | 5 (2.9) | 0.31 |
ASA American Society of Anesthesiology, CAD coronary artery disease, CHF chronic heart failure, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, BMI body mass index, OR operating room, EBL estimated blood loss, LOS length of stay
Variables with >5 % missing values indicate their total n next to their names
Most patients underwent surgery with an open abdominal or posterior approach (n=136, 66.3 %), while a smaller subset of patients had a minimally invasive approach (n=32, 15.6 %). The median operative time was 240 min (IQR, 159–330); median EBL was 700 mL (IQR, 200–1900) and 35.7 % patients received a blood transfusion with a median of 5 units transfused (IQR, 2–10). Postoperatively, 71 patients experienced a complication for an overall morbidity of 39.4 %. Most complications were minor (n=44, 24.4 %), while 27 (15.0 %) patients had a major complication. The median overall hospital LOS for the index hospitalization was 6 days (IQR, 4–9).
Readmission Analysis
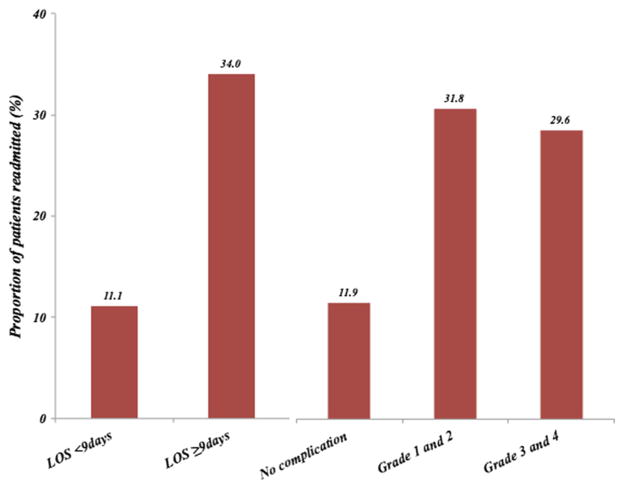
Within 90 days of discharge from the index hospitalization, 38 patients were readmitted for a 90-day all-cause readmission rate of 18.0 %. Perhaps as expected, there were differences in the baseline characteristics of patients who were and were not readmitted (Table 1). For example, most readmitted patients (n=24, 82.8 %) had a perioperative ASA class of 3–4 compared with non-readmitted patients (n=61, 49.6 %; P=0.001). The median operative time for patients who were readmitted was 298 min (IQR, 186–364) compared with a median operative time of 231 min (IQR, 155–318). In addition, patients who experienced a readmission were more likely to have had a complication during their index hospitalization (readmitted, 62.8 % vs. non-readmitted, 33.8 %; P=0.002). Of note, the incidence of minor (grades 1–2) vs. major (grades 3–4) complications was comparable among readmitted and non-readmitted patients (both P>0.05). Patients who experienced a readmission did have an initial longer index LOS (median, 9 days; IQR 6–13) compared with patients who were not readmitted (median, 6 days; IQR, 4–8). The percent of patients who experienced a readmission, stratified by LOS and complication grade, is shown in Fig. 1; of note, 34 % of patients with an extended LOS were readmitted within 90 days. Among patients who experienced a grades 1–2 or grades 3–4 complications during index hospitalization, 31.8 and 29.6 % were readmitted, respectively, while only 11.9 % of patients who did not have a complication were readmitted within 90 days (Fig. 1).
Fig. 1.
Proportion of patients readmitted, stratified by LOS and complication grades
On univariate analysis, patient-level and tumor-related factors such as sex, age, race, tumor size, T stage, or N stage were not associated with readmission (all P>0.05) (Table 2). On the other hand, patients with a higher pre-operative comorbidity index (ASA 3–4: OR, 4.88 (95 % CI, 1.75–13.61); P=0.002) and patients with more advanced disease (M1 disease: OR, 2.98 (95 % CI, 1.37–6.46); P=0.006) had higher odds of being readmitted. While various surgical factors such as operative time were not associated with risk of readmission (OR, 2.22 (95 % CI, 0.93–5.29); P=0.07), other factors such as high EBL increased the odds of being readmitted (EBL >700 mL: OR, 2.75 (95 % CI, 1.16–6.51); P=0.02). Patients who experienced complications also had higher odds of readmission (OR, 1.91 (95 % CI, 1.20–3.05): P=0.007) compared with patients who did not have a complication. Specifically, patients with either grades 1–2 complications (OR, 3.45 (95 % CI, 1.46–8.14); P=0.005) or grades 3–4 complications (OR, 3.11 (95 % CI, 1.13–8.53); P=0.03) had a higher odds of readmission vs. patients with no complications. In addition, prolonged LOS ≥9 days (OR, 4.12 (95 % CI, 1.88–9.01); P<0.001) during the index hospitalization was associated with readmission. On multivariate logistic regression, after controlling for competing risk factors, ASA classes 3–4 (OR, 4.01 (95 % CI, 1.44–11.17); P=0.008) and M1 disease (OR, 3.44 (95 % CI, 1.34–8.84); P=0.01) remained independently associated with higher odds for readmission.
Table 2.
Univariate and multivariate analyses of factors associated with readmission
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Prognostic factor | OR (95 % CI) | P value | OR (95 % CI) | P value |
| Age ≥65 years | 1.32 (0.57–3.07) | 0.51 | – | |
| Female sex | 0.92 (0.45–1.89) | 0.81 | – | |
| Caucasian race | 0.94 (0.40–2.25) | 0.90 | – | |
| ASA | ||||
| 1–2 | 1.00 (Reference) | 1.00 (Reference) | ||
| 3–4 | 4.88 (1.75–13.61) | 0.002 | 4.01 (1.44–11.17) | 0.008 |
| EBL >700 mL | 2.75 (1.16–6.51) | 0.02 | 1.48 (0.53–4.15) | 0.76 |
| OR time ≥4 h | 2.22 (0.93–5.29) | 0.07 | – | |
| Surgical approach | ||||
| MIS | 1.00 (Reference) | 1.00 (Reference) | ||
| Open | 3.67 (0.84–16.11) | 0.09 | 1.99 (0.38–10.47) | 0.42 |
| Tumor size ≥13 cm | 1.28 (0.61–2.69) | 0.51 | – | |
| N stage | ||||
| N0 | 1.00 (Reference) | |||
| N1 | 2.17 (0.71–6.61) | 0.18 | – | |
| T stage | ||||
| T1 | 1.00 (Reference) | |||
| T2 | 1.94 (0.23–16.64) | 0.55 | – | |
| T3 | 1.50 (0.17–13.11) | 0.71 | – | |
| T4 | 2.15 (0.23–20.23) | 0.50 | – | |
| Tumor functionality | ||||
| Non-secreting | 1.00 (Reference) | 1.00 (Reference) | ||
| Secreting | 1.42 (0.68–2.95) | 0.35 | 0.74 (0.29–1.84) | 0.51 |
| Metastasis on presentation | 2.98 (1.37–6.46) | 0.006 | 3.44 (1.34–8.84) | 0.01 |
| LOS | ||||
| <9 days | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥9 days | 4.12 (1.88–9.01) | <0.001 | 0.99 (0.97–1.07) | 0.98 |
| Complications | ||||
| No complication | 1.00 (Reference) | 1.00 (Reference) | ||
| Any complication | 1.91 (1.20–3.05) | 0.007 | 1.50 (0.77–2.91) | 0.24 |
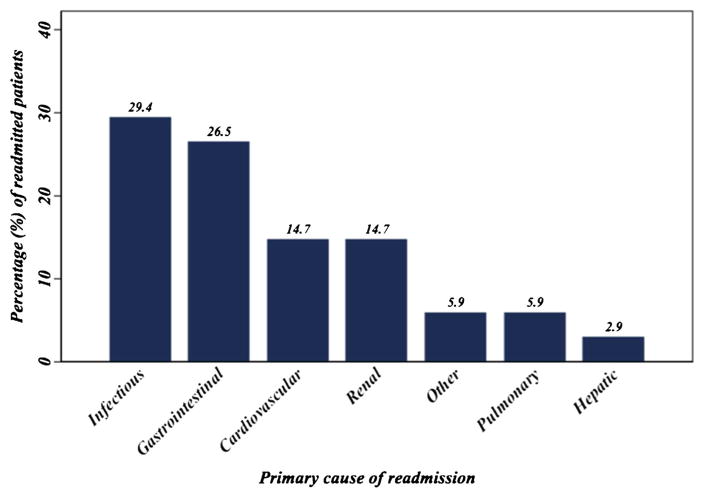
The most common causes of readmission were postoperative infections (n=10, 29.4 %); specifically, four (11.8 %) patients had a superficial wound infection, while four (11.8 %) had a deep organ site collection/abscess. Other causes of readmission included gastrointestinal (n=9, 26.5 %), pulmonary (n=2, 5.9 %), or cardiac (n=5, 14.7 %) issues (Fig. 2). The median LOS for the second hospitalization was 9.5 days (IQR, 7–12).
Fig. 2.
Primary cause of readmission among patients undergoing surgical resection for ACC
Discussion
Hospital readmission has become a standard measure for quality of care in healthcare.16 Unplanned readmission not only drives up health care costs but also can adversely impact patient satisfaction and safety.14,16,17 While several studies have reported on factors associated with readmission for patients undergoing a variety of disease-specific procedures, little data exist on readmission following surgery for ACC. In fact, information regarding outcomes related to ACC treatment is scarce due to the rarity of this cancer.6,10,32 Data on readmission following surgery for ACC is particularly uncommon, as no previous study has specifically examined this topic. As such, the current study is important because it was the first to define the incidence of readmission following surgery for ACC, as well as characterize factors associated with readmission. Specifically, the incidence of readmission rate was 18.0 %—indicating that nearly one in five patients undergoing surgery for ACC was readmitted within 90 days. Factors most associated with readmission among patients with ACC included a high ASA class and prolonged LOS.
Most previous studies on ACC have focused exclusively on long-term outcomes such as survival. In these reports, several factors were identified as being associated with poor prognosis. In particular, increasing patient age, tumor size, and nodal status were each predictive of worse long-term outcome.10,32 Interestingly, in the current study, none of these patient or tumor-related factors were associated with readmission. Gratian et al. noted that outcomes for patients with ACC were associated with hospital case volume.10 While treatment at high-volume centers was associated with more aggressive surgical resection and chemotherapy use, overall survival was no different at high- vs. low-volume centers.10 More germane to the current paper, Gratian and colleagues similarly noted that 30-day readmission was the same at high- (4 %) vs. low-(3.9 %) volume centers. In the current study, only high-volume centers were included in the collaborative. However, the overall incidence of readmission was considerably higher (18 %) compared with the readmission rate reported by Gratian et al. The reasons for these disparate results are probably several fold. First, unlike the current study, Gratian and colleagues utilized a large, national administrative dataset, which may have been subject to underreporting. In addition, Gratian et al. only reported readmission within the first 30 days from surgery, while the current study reported events 90 days after discharge and therefore was more likely to capture all readmissions following surgery.
The two factors most strongly associated with readmission following surgery for ACC included ASA classification and LOS. Specifically, patients with a high ASA class had a fourfold increased odds of readmission, while a prolonged LOS was similarly associated with a higher risk of readmission. A high ASA class has been reported to be an important factor associated with readmission in several disease-specific specialties including orthopedics,33,34 thoracic-vascular surgery,20 and urology.35 In fact, similar to findings in the current study, Lucas et al. reported that ASA class and LOS were among the two strongest predictors of readmission.20 In fact, a simple integer-based score based on ASA class and LOS alone could predict risk of readmission with moderate accuracy (area under the receiver operator curve (0.702).20 Interestingly, we also noted that the presence of metastatic disease was associated with a higher likelihood of readmission. Others studies have similarly linked metastatic disease to an increased odds of readmission among patients with cancer.36,37
The current study had several limitations. Despite the participation of 13 major centers in the USA, the sample size was still relatively small (n~200). As such, some analyses were limited by sample size and may have been subject to a type II statistical error. In addition, due to the manner in which data on readmission were collected, information on the specific timing of readmission was not available. Moreover, while every attempt was made to account for patients readmitted to non-index hospitals (i.e., different hospital from where the surgery was performed), it is possible that some patients may have been readmitted to a different hospital without being detected. In turn, this would have led to an underreporting of readmission in the current study. Finally, analyses from the current study were based on data from large, experienced medical centers. As such, data herein presented on ACC and readmission may not be applicable to community hospitals that only operate on the occasional ACC patient.
In conclusion, data from the current study are among the first to evaluate specifically the incidence and risk of readmission among patients undergoing surgery for ACC. The results demonstrated that roughly one out of five patients were readmitted following resection of ACC. Factors most strongly associated with readmission included LOS, ASA class, and the presence of metastatic disease. Future research should aim to better understand and target areas of quality improvement to decrease unnecessary readmissions among patients with ACC.
Footnotes
Funding/support None
References
- 1.Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, Dousset B, Bertagna X, Bertherat J. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. The Journal of Clinical Endocrinology and Metabolism. 2006;91(7):2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 2.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World Journal of Surgery. 2001;25(7):891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 3.Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer. 1989;64(3):765–769. doi: 10.1002/1097-0142(19890801)64:3<765::aid-cncr2820640333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Kasperlik-Zaluska AA, Migdalska BM, Zgliczynski S, Makowska AM. Adrenocortical carcinoma. A clinical study and treatment results of 52 patients. Cancer. 1995;75(10):2587–2591. doi: 10.1002/1097-0142(19950515)75:10<2587::aid-cncr2820751028>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Didolkar MS, Bescher RA, Elias EG, Moore RH. Natural history of adrenal cortical carcinoma: a clinicopathologic study of 42 patients. Cancer. 1981;47(9):2153–2161. doi: 10.1002/1097-0142(19810501)47:9<2153::aid-cncr2820470908>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Wanis KN, Kanthan R. Diagnostic and prognostic features in adrenocortical carcinoma: a single institution case series and review of the literature. World Journal of Surgical Oncology. 2015;13(1):117. doi: 10.1186/s12957-015-0527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152(6):1150–1157. doi: 10.1016/j.surg.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Donatini G, Caiazzo R, Do Cao C, Aubert S, Zerrweck C, El-Kathib Z, Gauthier T, Leteurtre E, Wemeau JL, Vantyghem MC, Carnaille B, Pattou F. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Annals of Surgical Oncology. 2014;21(1):284–291. doi: 10.1245/s10434-013-3164-6. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AB, Habra MA, Grubbs EG, Bednarski BK, Ying AK, Perrier ND, Lee JE, Aloia TA. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surgical Endoscopy. 2013;27(11):4026–4032. doi: 10.1007/s00464-013-3034-0. [DOI] [PubMed] [Google Scholar]
- 10.Gratian L, Pura J, Dinan M, Reed S, Scheri R, Roman S, Sosa JA. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Annals of Surgical Oncology. 2014;21(11):3509–3514. doi: 10.1245/s10434-014-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihai R. Diagnosis, treatment and outcome of adrenocortical cancer. The British Journal of Surgery. 2015;102(4):291–306. doi: 10.1002/bjs.9743. [DOI] [PubMed] [Google Scholar]
- 12.Przytulska J, Rogala N, Bednarek-Tupikowska G. Current and emerging therapies for adrenocortical carcinoma—review. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University. 2015;24(2):185–193. doi: 10.17219/acem/30645. [DOI] [PubMed] [Google Scholar]
- 13.Saade N, Sadler C, Goldfarb M. Impact of regional lymph node dissection on disease specific survival in adrenal cortical carcinoma. Hormone and Metabolic Research = Hormon - und Stoffwechselforschung=Hormones et metabolisme. 2015 doi: 10.1055/s-0035-1549877. [DOI] [PubMed] [Google Scholar]
- 14.Dimick JB, Ghaferi AA. Hospital readmission as a quality measure in surgery. JAMA. 2015;313(5):512–513. doi: 10.1001/jama.2014.14179. [DOI] [PubMed] [Google Scholar]
- 15.Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483–495. doi: 10.1001/jama.2014.18614. [DOI] [PubMed] [Google Scholar]
- 16.Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. The New England Journal of Medicine. 2013;369(12):1134–1142. doi: 10.1056/NEJMsa1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai TC, Orav EJ, Jha AK. Patient satisfaction and quality of surgical care in US hospitals. Annals of Surgery. 2015;261(1):2–8. doi: 10.1097/SLA.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. Journal of Clinical Anesthesia. 2002;14(5):349–353. doi: 10.1016/s0952-8180(02)00371-9. [DOI] [PubMed] [Google Scholar]
- 19.Autorino R, Zargar H, Butler S, Laydner H, Kaouk JH. Incidence and risk factors for 30-day readmission in patients undergoing nephrectomy procedures: a contemporary analysis of 5276 cases from the national surgical quality improvement program database. Urology. 2015;85(4):843–849. doi: 10.1016/j.urology.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Lucas DJ, Haider A, Haut E, Dodson R, Wolfgang CL, Ahuja N, Sweeney J, Pawlik TM. Assessing readmission after general, vascular, and thoracic surgery using ACS-NSQIP. Annals of Surgery. 2013;258(3):430–439. doi: 10.1097/SLA.0b013e3182a18fcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen MG, LaPar DJ, Daniel SK, Turrentine FE, Hanks JB, Smith PW. Risk factors for 30-day hospital readmission after thyroidectomy and parathyroidectomy in the United States: An analysis of National Surgical Quality Improvement Program outcomes. Surgery. 2014;156(6):1423–1430. doi: 10.1016/j.surg.2014.08.074. discussion 1430–1421. [DOI] [PubMed] [Google Scholar]
- 22.Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. The Journal of Arthroplasty. 2013;28(9):1499–1504. doi: 10.1016/j.arth.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang CL, Wang SL, Huang DD, Pang WY, Lou N, Chen BC, Chen XL, Yu Z, Shen X. Risk factors for hospital readmission after radical gastrectomy for gastric cancer: a prospective study. PloS ONE. 2015;10(4):e0125572. doi: 10.1371/journal.pone.0125572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad R, Schmidt BH, Rattner DW, Mullen JT. Factors influencing readmission after curative gastrectomy for gastric cancer. Journal of the American College of Surgeons. 2014;218(6):1215–1222. doi: 10.1016/j.jamcollsurg.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Spolverato G, Maqsood H, Vitale A, Alexandrescu S, Marques HP, Aldrighetti L, Gamblin TC, Pulitano C, Bauer TW, Shen F, Poultsides G, Maithel S, Marsh JW, Pawlik TM. Readmission after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract. 2015 doi: 10.1007/s11605-015-2826-z. [DOI] [PubMed] [Google Scholar]
- 26.Schneider EB, Hyder O, Wolfgang CL, Hirose K, Choti MA, Makary MA, Herman JM, Cameron JL, Pawlik TM. Patient readmission and mortality after surgery for hepato-pancreatobiliary malignancies. Journal of the American College of Surgeons. 2012;215(5):607–615. doi: 10.1016/j.jamcollsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimbrough CW, Agle SC, Scoggins CR, Martin RC, Marvin MR, Davis EG, McMasters KM, Jones CM. Factors predictive of readmission after hepatic resection for hepatocellular carcinoma. Surgery. 2014;156(4):1039–1046. doi: 10.1016/j.surg.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 28.Jeong O, Kyu Park Y, Ran Jung M, Yeop Ryu S. Analysis of 30-day postdischarge morbidity and readmission after radical gastrectomy for gastric carcinoma: a single-center study of 2107 patients with prospective data. Medicine. 2015;94(11):e259. doi: 10.1097/MD.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyder O, Dodson RM, Nathan H, Schneider EB, Weiss MJ, Cameron JL, Choti MA, Makary MA, Hirose K, Wolfgang CL, Herman JM, Pawlik TM. Influence of patient, physician, and hospital factors on 30 - day readmission following pancreatoduodenectomy in the United States. JAMA Surgery. 2013;148(12):1095–1102. doi: 10.1001/jamasurg.2013.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, Patel SH, Bentram DJ, Weber SM, Cho CS, Winslow ER, Scoggins CR, Martin RC, Kim HJ, Baker JJ, Merchant NB, Parikh AA, Kooby DA. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Annals of Surgery. 2012;256(3):529–537. doi: 10.1097/SLA.0b013e318265ef0b. [DOI] [PubMed] [Google Scholar]
- 31.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Aziz TE, Rajeev P, Sadler G, Weaver A, Mihai R. Risk of adrenocortical carcinoma in adrenal tumours greater than 8 cm. World Journal of Surgery. 2015;39(5):1268–1273. doi: 10.1007/s00268-014-2912-5. [DOI] [PubMed] [Google Scholar]
- 33.Sathiyakumar V, Molina CS, Thakore RV, Obremskey WT, Sethi MK. ASA score as a predictor of 30-day perioperative readmission in patients with orthopaedic trauma injuries: an NSQIP analysis. Journal of Orthopaedic Trauma. 2015;29(3):e127–132. doi: 10.1097/BOT.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 34.Schaeffer JF, Scott DJ, Godin JA, Attarian DE, Wellman SS, Mather RC., 3rd The Association of ASA Class on Total Knee and Total Hip Arthroplasty Readmission Rates in an Academic Hospital. The Journal of Arthroplasty. 2015;30(5):723–727. doi: 10.1016/j.arth.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Leow JJ, Gandaglia G, Sood A, Ruhotina N, Klett DE, Sammon JD, Schmid M, Sun M, Chang SL, Kibel AS, Trinh QD. Readmissions after major urologic cancer surgery. The Canadian Journal of Urology. 2014;21(6):7537–7546. [PubMed] [Google Scholar]
- 36.Granda-Cameron C, Behta M, Hovinga M, Rundio A, Mintzer D. Risk factors associated with unplanned hospital readmissions in adults with cancer. Oncology Nursing Forum. 2015;42(3):E257–268. doi: 10.1188/15.ONF.E257-E268. [DOI] [PubMed] [Google Scholar]
- 37.Schairer WW, Carrer A, Sing DC, Chou D, Mummaneni PV, Hu SS, Berven SH, Burch S, Tay B, Deviren V, Ames C. Hospital readmission rates after surgical treatment of primary and metastatic tumors of the spine. Spine. 2014;39(21):1801–1808. doi: 10.1097/BRS.0000000000000517. [DOI] [PubMed] [Google Scholar]