Abstract
High-grade serous ovarian carcinoma (HGSOC) is usually diagnosed at a late stage and is associated with poor prognosis. Understanding early stage disease biology is essential in developing clinical biomarkers to detect HGSOC earlier. While recent studies indicate that HGSOCs arise from fallopian tube secretory epithelial cells (FTSECs), a considerable body of evidence also suggests that HGSOC can also arise from ovarian surface epithelial cells (OSECs). PAX8 is overexpressed in HGSOCs and expressed in FTSECs, but there are conflicting reports about PAX8 expression in OSECs. The purpose of this study was to comprehensively characterize PAX8 expression in a large series of OSECs, and to investigate the role of PAX8 in early HGSOC development. PAX8 protein expression was analyzed in the OSECs of 27 normal ovaries and 7 primary OSEC cultures using immunohistochemistry and immunofluorescent cytochemistry. PAX8 mRNA expression was quantified in 66 primary OSEC cultures. Cellular transformation was evaluated in OSECs expressing a PAX8 construct. PAX8 was expressed by 44-71% of OSECs. Calretinin and E-cadherin were frequently co-expressed with PAX8. Expression of PAX8 in OSECs decreased cellular migration (P=0.028), but had no other effects on cellular transformation. In addition, PAX8 expression was significantly increased (P=0.003) in an in vitro stepwise model of neoplastic transformation. In conclusion, PAX8 is frequently expressed by OSECs and endogenous levels of PAX8 expression are non-transforming. These data indicate that in OSECs PAX8 expression may represent a normal state and that OSECs may represent an origin of HGSOCs.
Keywords: ovarian surface epithelium, high-grade serous ovarian carcinoma, PAX8, inclusion cyst, fallopian tube epithelial cells, CMYC
1. INTRODUCTION
Invasive ovarian carcinoma is a deadly disease marked by frequent late stage diagnosis and poor survival rates. It is also a heterogeneous disease encompassing several histological subtypes. High-grade serous ovarian carcinoma (HGSOC) is the most common subtype, accounting for approximately 60% of all ovarian carcinomas. Historically, HGSOCs were thought to arise from ovarian surface epithelial cells (OSECs), the mesothelial-type epithelium covering the ovary, and from the epithelial lining of cortical inclusion cysts (CICs), which derive from invaginations of the ovarian surface. Decades of research have indicated that HGSOCs may arise in these tissues [1-4]. However this hypothesis has been criticized because metaplastic ovarian epithelial cells are rarely found on the surface of the ovary, and because ovarian carcinomas exhibited markedly different histological features and marker expression when compared to normal OSECs. More recent evidence indicates that HGSOCs can originate from epithelial cells lining the fallopian tube fimbriae, through a precursor lesion termed serous tubal intraepithelial carcinoma (STIC). STICs are associated with 20-60% of sporadic HGSOCs [5-8], raising the possibility that the remaining 40-80% of HGSOCs may arise from an alternative cell of origin.
Several studies have shown that paired box 8 (PAX8) is highly expressed in most HGSOCs [9-14], suggesting that overexpression of this transcription factor plays a critical role in this tumor type. This is supported by functional evidence showing that PAX8 knockdown in ovarian carcinoma cell lines induces growth arrest, apoptosis, and decreases tumorigenesis [14-16]. The observation of PAX8 expression in fallopian tube secretory epithelial cells (FTSECs) and in many CICs but not OSECs could suggest that the epithelial lining of CICs is derived from the fallopian tube and that most, if not all HGSOCs likely originate from FTSECs. However, while it is widely considered that OSECs do not express PAX8, there are conflicting reports evaluating PAX8 expression in OSECs [9-12,17-19]. Evidence of PAX8 expression by OSECs has been reported in smaller sample sizes (n > 8 only in one study) [4,10,12,17,19], but this finding has been controversial [20] because it did not agree with the findings of others [9,11,18]. The purpose of the current study was to perform a comprehensive analysis of PAX8 protein and mRNA expression in both normal ovaries and primary OSECs in culture, and to evaluate the functional significance of PAX8 in early stage development of ovarian carcinoma using in vitro models of OSECs.
2. MATERIALS AND METHODS
2.1 Tissue Culture
Normal OSECs were isolated from histologically normal ovaries of women undergoing surgery for conditions not involving the ovaries (such as endometrial cancer or fibroids). Samples were collected with the approval of the University College London Hospital Ethics Committee and informed patient consent. OSECs were harvested by brushing the surface of the ovary with a cytobrush, and cultured in normal ovarian surface epithelial cell culture media (NOSE-CM) [21]. hTERT (human telomerase reverse transcriptase) -immortalized OSECs overexpressing CMYC ( (IOE19CMYC) have been previously described [22] and were cultured in NOSE-CM supplemented with 3 μg/ml blasticidin S hydrochloride (Sigma Aldrich, St. Louis, MO). HGSOC cell lines (n = 6) were cultured as follows: OVCA429 and PEO14: RMPI with 10% FBS (Seradigm, Providence, UT); OVMZ15: DMEM with 10% FBS, 1% nonessential amino acids, and sodium pyruvate; OVCA433: Eagle MEM with 10% FBS; COV318: DMEM with 10% FBS and 0.1M L-asparagine. HeyA8 cells were cultured in RMPI with 10% FBS. All cell lines used in this study were confirmed to be free of contaminating Mycoplasma infections by mycoplasma-specific PCR.
2.2 Immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks and hematoxylin and eosin (H&E) stained sections of normal ovaries (n = 27) and fallopian tubes (n = 7) were obtained from the Los Angeles County Hospital archive with approval of the University of Southern California Institutional Review Board. Samples came from women receiving gynecological surgery for non-cancer related conditions (such as fibroids). Ovarian sections were H&E stained and examined by a gynecological pathologist (PMF) to confirm presence of the surface epithelium. Formalin-fixed paraffin-embedded tissue sections (4 μm) were processed for immunohistochemistry. Endogenous peroxidase was blocked with 0.3% hydrogen peroxidase for 5 minutes. Antigen retrieval was carried out in high citrate buffer for 3 minutes in a steam-cooker. Sections were incubated overnight with PAX8 antibody (Biocare Medical, Concord, CA, 1:400 dilution) [23]. Serial sections of PAX8 expressing ovaries (n = 4) were incubated with calretinin antibody (Dako, Carpinteria, CA), and E-cadherin antibody (Novocastra, Wetzlar, Germany, ready to use). A subsequent reaction was performed with the biotin-free HRP enzyme labeled polymer of the Envision plus detection system (Dako, Carpinteria, CA). Diaminobenzidine was used as the chromogen, and hematoxylin was applied to counterstain. Positive controls were normal fallopian tubes for PAX8 staining, mesothelioma for calretinin, and normal fallopian tube for E-cadherin. As a negative control, normal goat serum was used instead of the primary antibody, resulting in a lack of detectable staining. PAX8 staining was nuclear and scored as negative, weak, moderate, or strong. Calretinin staining was cytoplasmic and scored as positive or negative. E-cadherin was membranous and scored as positive or negative. For all antibodies, weak, moderate and strong staining was scored as positive expression.
2.3 Immunofluorescent staining
Cells were grown to ~80% confluence on glass coverslips, fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton-X. Non-specific binding was blocked with 10% fetal bovine serum in DMEM. Primary antibodies (PAX8: Proteintech, Chicago, IL, 1:100 dilution; calretinin: EMD Millipore, Billerica, MA, 1:100 dilution) were incubated for 1 hour. Goat-anti rabbit Alexafluor 568 and rabbit-anti mouse Alexafluor 488 (Life Technologies, Carlsbad, CA) were used as secondary antibodies. Nuclei were stained with Hoechst 33342 (Thermo Scientific, Rockford, IL). HeyA8 cells were used as a positive PAX8 staining control, and negative controls lacked primary antibody and showed no detectable staining (data not shown). Coverslips were mounted on glass slides and imaged on a Zeiss Axio Imager Z1 fluorescent microscope. PAX8 staining was scored as negative, weak, moderate, or strong. Calretinin staining was scored as positive or negative. For all antibodies, weak, moderate and strong staining was scored as positive expression.
2.4 RNA expression analysis
Total RNA was harvested from all cells at ~80% confluence (Qiagen, Valencia, CA) and cDNA was generated (Life Technologies, Carlsbad, CA). Primary cells were used at early passages. cDNA was detected using real-time reverse transcription semi-quantitative PCR (RT-qPCR) and Taqman gene expression probes (Life Technologies, Carlsbad, CA). RT-qPCR was run on the ABI7900 (Applied Biosystems, Foster City, CA) standard program for 40 cycles. Expression levels were determined using the ΔΔCt method. Ct values were normalized to the average expression of β-Actin and GAPDH, and ΔΔCt values were calibrated to a single sample (OSEC250). Negative controls for cDNA synthesis and RT-qPCR lacked a cDNA template and showed no detectable gene expression for each probe. Unpaired Wilcoxon rank sum tests were used to test for significant differences in gene expression across cell line groups.
2.5 Generation of IOE19CMYC.PAX8 cells
The pEGFP-hPAX8a construct was generously provided by Peter Kopp [24]. IOE19CMYC cells were transfected using the BioT reagent (Bioland Scientific, Paramount, CA) according to the manufacturer’s protocol and positive cells were selected for and pooled using fluorescence associated cell sorting (FACSAria II, BD Biosciences, Franklin Lakes, NJ) for GFP expressing cells and maintained with 1 mg/ml G418 (Sigma Aldrich, St. Louis, MO) in culture, creating the stable IOE19CMYC.PAX8 cell line. The pEGFP-U6 construct was used in the same manner as a control (Addgene, Cambridge, MA) to generate stable IOE19CMYC.GFP cells. PAX8 expression was confirmed by RT-qPCR as described above. Stable construct expression was confirmed by PAX8 qPCR.
2.6 CMYC expression and binding site analysis
A model of CMYC overexpression has been previously described [17]. PAX8 gene expression was measured using the Illumina HT12 Expression BeadChips. CMYC binding sites were identified using the Integrated Regulation and 100 Vertebrates Basewise Conservation (PhyloP) data tracks from the Encyclopedia of DNA Elements [25]. Expression and copy number variation co-occurrence was analyzed from The Cancer Genome Atlas Ovarian Serous Cystadenocarcinoma dataset using the cBioPortal (http://www.cbioportal.org/public-portal) [26].
2.7 Migration assay
Subconfluent cells were serum and growth factor starved for 24 hours. Cells were seeded into transwell inserts perforated with 8 micron pores. After 16-18 hours of exposure to the chemoattractant, 10% fetal bovine serum, membranes were fixed in methanol and stained with Hoechst 33342. Membranes were mounted onto slides and the number of migrated cells was counted using a fluorescent microscope. Statistical significance was determined using a paired two-tailed Student’s T-test and values were normalized to IOE19CMYC.GFP cells.
2.8 Invasion assay
The QCM ECMigration Cell Invasion Assay was used according to the manufacturer’s protocol (EMD Millipore, Billerica, MA). Cells were starved in the same manner as the migration assay, and the same chemoattractant was used. The membrane was covered by matrigel and a fluorescent dye was used to quantify the amount of cells that invaded into the bottom chamber after 24 hours of exposure to the chemoattractant. Fluorescence was measured using a Hidex Plate CHAMELEON plate reader (Turku, Finland) and MikroWin software (Mikrotech, Overath, Germany, V2000) and normalized to IOE19CMYC.GFP cells. Statistical significance was determined using a paired two-tailed Student’s T-test.
2.9 Anchorage independent growth assay
Cells were suspended in 0.3% agar diluted in culture media and allowed to solidify. After 4 weeks at 37°C and 5% CO2, cells were fixed and stained with p-iodonitrotetrazolium chloride (Sigma Aldrich, St. Louis, MO). The total number of colonies was counted under a light microscope. Colony forming efficiency was calculated as (number of colonies formed / total number of cells × 100) and normalized to IOE19CMYC.GFP cells. Statistical significance was determined using a paired two-tailed Student’s T-test.
3. RESULTS
3.1 PAX8 protein expression in normal ovaries
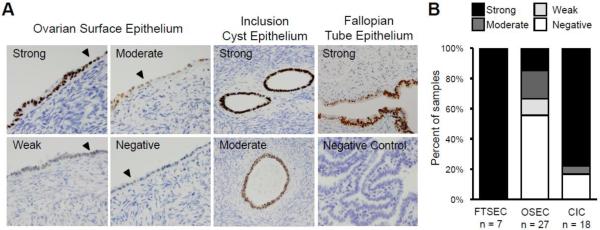
PAX8 expression was evaluated in 27 histologically normal ovaries with OSECs present on the surface, 18 of which also contained CICs. From the same patient cohort, seven fallopian tubes were analyzed as positive controls for PAX8 expression. PAX8 was expressed in the OSECs of 12 ovaries (44%), nine of which (33% of all ovaries) showed moderate to strong staining (similar to expression levels in FTSECs). OSECs in the remaining 15 ovaries (56%) showed no evidence of PAX8 staining. PAX8 was observed in the CICs of 15 ovaries (83%), 14 of which showed strong staining. Within the ovary, histiocytes stained strongly for PAX8, but there was no expression in ovarian stromal cells. In two cases, ovaries were from patients carrying deleterious BRCA1 mutations (the BRCA1 status of the remaining patients was unknown). In one of these cases there was no detectable PAX8 expression on the ovarian surface, and no CICs were present in the ovary. In the second case, OSECs showed weak PAX8 staining and strong staining was observed in the CIC epithelium. All seven fallopian tubes showed strong PAX8 staining in FTSECs, and no staining was observed in ciliated or stromal cells. Examples of PAX8 staining in OSECs, CICs and FTSECs are shown in Figure 1A, and are summarized in Figure 1B.
Figure 1.
PAX8 expression in normal ovarian surface epithelium. (A) PAX8 expression was evaluated in 27 normal ovaries using immunohistochemistry. PAX8 expression ranged from strong to negative in OSECs and was strong, moderate or negative in CIC epithelial cells. FTSECs stained positive for PAX8. Examples of normal OSEC morphology on the ovarian surface are indicated with a black arrowhead. Tissue sections are shown at 100x magnification, negative control shown at 40x. (B) Graphical illustration of the range and proportion of tissues with PAX8 expression in primary OSEC, CIC, and FTSEC tissue. OSEC = ovarian surface epithelial cell, CIC = cortical inclusion cyst, FTSEC = fallopian tube secretory epithelial cell.
3.2 PAX8 mRNA expression in normal ovarian surface epithelial cells
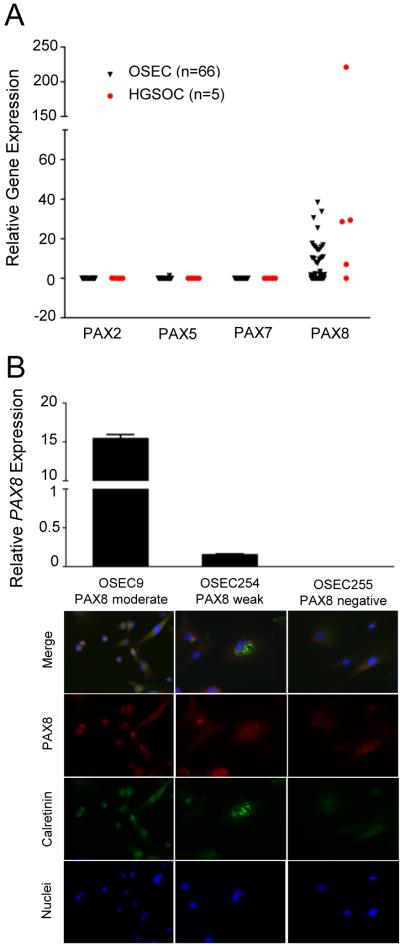
To investigate PAX8 expression in OSECs by an independent method, mRNA expression was measured in 66 early passage primary OSEC lines using semi-quantitative RT-qPCR. PAX8 mRNA expression was observed in 47/66 OSEC lines (71%); 18 OSEC lines (27%) showed moderate expression, and 29 OSEC lines (44%) showed detectable but lower PAX8 expression (Figure 2A). Negligible expression of other PAX family members (PAX2, PAX5 and PAX7) was found in these cell lines indicating the probe was specific to the PAX8 transcript (Figure 2A). Immunofluorescent staining in a proportion of OSEC lines (n = 7) indicated PAX8 mRNA and protein expression are correlated (Figure 2B).
Figure 2.
PAX8 protein and mRNA expression is correlated in OSECs. (A) Boxplot comparing relative PAX gene expression in primary OSEC cultures (black triangles), normalized to B-Actin and GAPDH and calibrated to a single OSEC line (OSEC250). HGSOC cell lines are shown as red circles. (B) OSEC cultures stained for PAX8 displayed PAX8 moderate expression (left panel and bar), weak expression (middle panel and bar), or negative expression (right panel and bar). Expression of PAX8 detected by immunofluorescence correlated with mRNA abundance. PAX8 moderate and weak mRNA expression was significantly different (P < 0.0001, one way ANOVA). 84% of OSEC9 and OSEC254 cells were PAX8 positive, and 48% of OSEC255 cells were weakly positive for PAX8. All cultures were calretinin positive by immunofluorescence using an anti-calretinin antibody. All images were taken at 200x magnification. OSEC = ovarian surface epithelial cell, HGSOC = high-grade serous ovarian carcinoma.
3.3 Correlations between PAX8 expression and OSEC morphology and marker expression
It has been postulated that PAX8 positive OSECs may be FTSECs ectopically located on the surface of the ovary through endosalpingiosis. To evaluate this, the relationship between cellular morphology and PAX8 expression in OSECs was examined. OSECs can be flattened and elongated, resembling classical mesothelial-type cells, or columnar and/or cuboidal, resembling differentiated epithelial monolayers. Of the 12 ovaries with PAX8 staining in OSECs, nine (75%) had a flattened morphology typical of OSECs, one (8%) had a cuboidal morphology, one (8%) exhibited mixed (flat and cuboidal) morphology, and the morphology of one specimen (8%) was ambiguous. In the 15 ovaries negative for PAX8 staining, 11 (73%) had flattened epithelium, two (13%) had cuboidal morphology, and two (13%) showed both flat and cuboidal cells on the same ovary (Table S1). There was no association between the cellular morphology of normal OSECs and PAX8 staining. In addition, we found no correlations between PAX8 staining and the presence of CICs (Table S2), or the following epidemiological risk factors: age, oral contraceptive use, parity, gravidity, or menopausal status (data not shown).
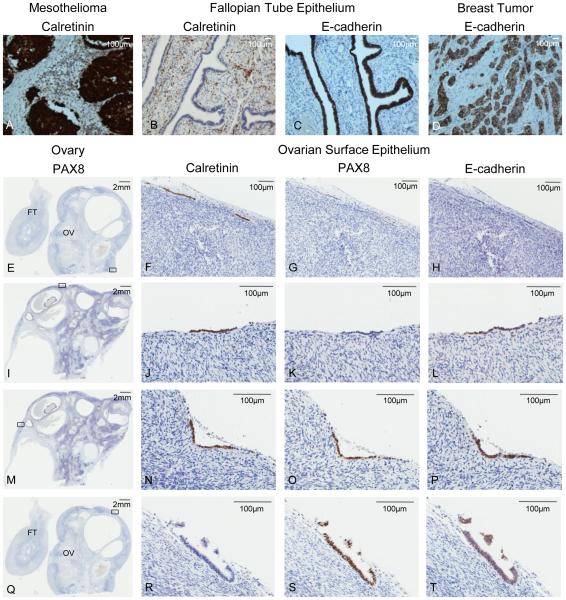
The expression of calretinin, a mesothelial cell marker expressed in OSECs but not FTSECs, and E-cadherin, which is expressed in FTSECs and, in some instances, OSECs [27], including deep clefts [28], and along the ovarian fimbria [17], was evaluated in OSEC samples that stained positive for PAX8 (n = 4). Diffuse co-expression of PAX8, calretinin, and E-cadherin was observed in three ovaries. In the fourth ovary there were two areas of focal PAX8 expression in OSECs that stained negative for calretinin, but positive for E-cadherin expression; three areas of OSECs that stained only for calretinin; and three areas of OSECs that co-expressed calretinin and E-cadherin. Areas of PAX8 and calretinin co-expression in one ovary and PAX8 and E-cadherin co-expression were observed in OSECs from three ovaries. Examples of PAX8, calretinin, and E-cadherin staining are shown in Figure 3. Early passage primary OSEC lines that were positive for PAX8 protein and mRNA expression also stained positive for calretinin (Fig. 2B).
Figure 3.
PAX8, calretinin, and E-cadherin expression in normal ovarian surface epithelium. (A-D) Controls are shown in the top panel. (A) mesothelioma with positive calretinin staining, (B) fallopian tube epithelium negative for calretinin and positive for E-cadherin expression (negative E-cadherin staining seen in stroma), (C) breast tumor specimen positive for E-cadherin expression. (E, I, M, Q) A section of normal ovary stained for PAX8. The black rectangle indicates the magnified region of serial sections in corresponding panels to the right. (F) Calretinin positive, (G) PAX8 negative, and (H) E-cadherin negative OSECs. (J) Calretinin positive, (K) PAX8 negative, and (L) E-cadherin positive OSECs. (N) Calretinin positive, (O) PAX8 positive, and (P) E-cadherin positive OSECs. (R) Calretinin negative, (S) PAX8 positive, and (T) E-cadherin positive OSECs. All tissue stained by DAB immunohistochemistry, and scale bars are shown in millimeters (mm) or micrometers (υm) at the top right of each image. FT = fallopian tube, OV = ovary, OSECs = ovarian surface epithelial cells.
3.4 The role of PAX8 in early stage transformation of OSECs
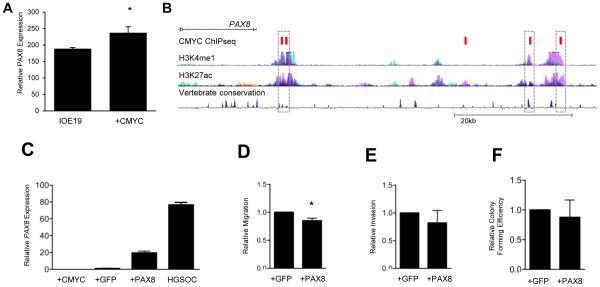
The role of PAX8 in the early stage transformation of OSECs was evaluated. Given its frequent overexpression in ovarian carcinomas we postulated that this overexpression promotes carcinogenesis. PAX8 expression was first evaluated in an established stepwise model of neoplastic transformation of OSECs created by overexpressing hTERT (to immortalize OSECs creating the IOE19 cell line) followed by overexpression of the CMYC oncogene (IOE19CMYC cells) [22]. Briefly, hTERT overexpression extends the lifespan of primary OSECs without inducing DNA copy number changes or evidence of transformation, while CMYC overexpression partially transforms these cells, as shown by their acquired ability to form colonies in anchorage independent growth assays. PAX8 mRNA expression was significantly increased in IOE19CMYC cells (P=0.003) compared to IOE19 cells (Figure 4A). PAX8 is not a known CMYC target gene [29]; but because CMYC is known to be frequently amplified and overexpressed in ovarian tumors [13,30], it suggests CMYC overexpression could drive upregulated PAX8 expression in ovarian carcinoma. Significant evidence was found that expression and copy number variation of CMYC and PAX8 occur together (odds ratio: 2.529, P=0.025, Fisher's Exact Test) in an analysis of primary ovarian tumors from The Cancer Genome Atlas dataset. Furthermore, data from the Encyclopedia of DNA Elements (ENCODE) project revealed two CMYC binding sites within active enhancer regions located ~4.2kb upstream of the PAX8 transcription start site. These binding sites were highly conserved in placental mammals (Figure 4B). To study the transformative ability of PAX8, a PAX8 cDNA construct was transfected into IOE19CMYC cells to create IOE19CMYC.PAX8 cells. PAX8 mRNA expression was increased significantly in IOE19CMYC.PAX8 cells compared to IOE19CMYC cells (P = 0.004), which mimicked endogenous expression but was lower than the levels typically observed in HGSOCs (Fig. 4C). Cellular transformation of cells was evaluated using several phenotypic assays. Compared to IOE19CMYC cells, IOE19CMYC.PAX8 cells were significantly less migratory (P = 0.028) (Fig. 4D), but PAX8 did not induce changes in invasion (Fig. 4E), anchorage independent growth (Fig. 4F), proliferation in three-dimensional culture, senescence, or cell cycle distribution (data not shown).
Figure 4.
PAX8 expression does not induce neoplastic transformation in OSECs. (A) PAX8 expression is increased in normal, TERT immortalized OSECs following CMYC overexpression. (* P = 0.003) (B) Data from the ENCODE project shows five CMYC binding sites (demonstrated by ChIPseq, red boxes) located upstream of the PAX8 promoter, including four that lie within common enhancer regions (dashed boxes). (C-F) IOE19CMYC cells stably expressing GFP or a PAX8-GFP fusion protein were assayed for cellular transformation. (C) IOE19CMYC.PAX8 cells express higher levels of PAX8 than the parental cells (IOE19CMYC), IOE19CMYC.GFP control cells), and HGSOC cells (OVCA433)(P < 0.0001, unpaired T-test). (D) IOE19CMYC.PAX8 cells were less migratory than GFP control cells in an in vitro transwell migration assay. (* P = 0.028) (E) Cellular invasion was not significantly different in IOE19CMYC.GFP and IOE19CMYC.PAX8 cells in IOE19CMYC.GFP vitro. (F) Anchorage independent growth was not significantly different between and IOE19CMYC.PAX8 cells using a soft agar assay. OSECs = ovarian surface epithelial cells, IOE19 = immortalized ovarian surface epithelial cells.
4. DISCUSSION
Ovarian carcinoma is the most lethal gynecological malignancy in Western countries. HGSOC is characterized by frequent late-stage presentation, having no specific symptoms associated with early stage disease. Understanding the molecular mechanisms that underlie cancer initiation and identifying the precursor tissues of the specific subtypes of ovarian cancer may lead to the development of novel early-stage screening biomarkers for ovarian carcinoma, which would have a substantial impact on reducing disease mortality. The origin of the most common ovarian carcinoma subtype, HGSOC, remains a topic of debate. For many years it was widely considered that HGSOC arises from OSECs, either on the surface of the ovary or trapped within CICs where OSECs come into close contact with the mitogenic microenvironment of the ovarian stroma [31]. Recent histopathological data [5-7] and modeling studies [32] have elegantly demonstrated that HGSOCs can arise from FTSEC precursors; nonetheless, the relative proportion of HGSOCs that initiate from FTSECs is not yet clear.
While PAX8 is overexpressed in >80% of HGSOCs, its role in tumorigenesis is not well known. PAX8 is expressed by secretory cells of the fallopian tube and is widely considered absent in OSECs. In this study, PAX8 is expressed by 44-71% of OSECs on the ovaries of pre-and post-menopausal women, in contrast to published reports that state OSECs do not express PAX8 [9,11,18]. This study represents the largest analysis of PAX8 expression in normal OSECs published to date (27 normal ovarian tissues and 66 primary OSEC lines). In contrast to previous reports where only PAX8 protein expression was evaluated, PAX8 mRNA expression was measured to directly address common criticisms of immunohistochemical studies, largely that antibodies may lack specificity and that visual scoring of protein expression is subject to observer bias. In this study a PAX8-specific monoclonal antibody [23] was used, limiting the likelihood of non-specific staining. The proportion of OSECs showing PAX8 mRNA expression (71%) was higher than PAX8 protein expression in OSECs (44%). This may be sampling bias resulting from the smaller sample size in the PAX8 immunohistochemistry analyses, or may be a result of sampling error due to representative tissue sectioning biases. One section of each ovary was examined, and because these sections often contain only small populations of OSECs, it is possible that ovaries classified as PAX8 negative may express PAX8 in regions that were not examined.
It has been argued that PAX8 expressing cells on the surface of the ovary may be FTSECs that have ectopically relocated through endosalpingiosis both because OSECs are not expected to express PAX8, and because PAX8 positive cells on the ovarian surface can morphologically resemble FTSECs [18]. Some small regions showed focal co-expression of PAX8 and E-cadherin in the absence of calretinin expression, and it is possible that these cells may be fallopian in origin. However, our data do not support the hypothesis that all PAX8 positive cells on the ovary are FTSECs. First, endosalpingiosis only affects around 10% of women [33] whereas we found PAX8 expression in 44-71% of normal OSECs. Second, the morphology of OSECs expressing PAX8 did not resemble that of FTSECS, which are columnar. Instead, most OSECs expressing PAX8 had a flattened, cuboidal (mesothelial) morphology typical of OSECs, and no difference was found in morphology between OSECs of ovaries that expressed PAX8 and those that did not. However, most importantly, OSECs that expressed PAX8 co-expressed calretinin. Calretinin is a mesothelial cell marker expressed in OSECs but not FTSECs. PAX8 expression in OSECs was most frequently found in combination with calretinin and E-cadherin (which is expressed in both OSECs and FTSECs). Calretinin was also co-expressed with PAX8 in primary OSEC cultures. This indicates that PAX8 expressing cells on the ovarian surface are likely to be OSECs and not FTSECs.
Overexpression of PAX8 occurs frequently in HGSOC, and the correlation with CMYC expression led us to postulate that PAX8 expression is due to enhanced CMYC activity during tumorigenesis. While we expected PAX8 to behave as an oncogene, forced expression of PAX8 in partially transformed OSECs (at levels similar to primary OSECs) did not induce features of neoplastic transformation. The only significant phenotypic alteration observed was decreased migration in PAX8 expressing cells, in agreement with the literature [34]. This indicates that expression of PAX8 in OSECs may represent a non-pathological state, and is insufficient to induce transformation, even in cells partially transformed by CMYC overexpression. These data may indicate that PAX8 overexpression occurs later in HGSOC development, and that additional somatic alterations are likely to be required for PAX8 to promote carcinogenesis. PAX8 expression in OSECs may therefore be associated with normal physiological processes, such as ovulation, or may simply be a part of the phenotypic heterogeneity that characterizes this cell type.
While it is clear that a significant proportion of HGSOCs originate from FTSECs, including recent evidence from a genetically engineered murine model of ovarian carcinogenesis [32], the existing data do not explain the origin of all HGSOCs. One caveat of this study is that while it is shown that OSECs can express PAX8, further work is required to confirm that HGSOC can develop from OSEC precursors. Studies have shown that STICs are associated with 20-60% of HGSOCs [5-8], however, 15-30% of HGSOCs show no evidence of fallopian lesions and the remaining 40-60% of HGSOCs without accompanying STICs have a yet undetermined origin [6]. Our data add to existing histopathological data that suggest a proportion of these remaining HGSOCs may arise from OSECs, including findings of occult ovarian primary HGSOCs and dysplastic CICs [1-3]. Understanding the origins of HGSOC presents a major challenge to its clinical management, and the gaps in knowledge surrounding disease origins limit the development of early detection and prevention strategies. HGSOC is usually diagnosed at a late stage, and while therapeutic advances have marginally improved five-year survival rates over the last decade, the disease continues to be associated with a poor prognosis. Following the evidence that HGSOC can arise from FTSECs, many have proposed that salpingectomy should be considered or even routinely adopted when performing hysterectomies in post-menopausal women [6]. However, this research, together with other reports [4,35], continues to suggest that that the importance of OSECs in HGSOC should not be overlooked.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Lillian Young and the USC IHC core for immunohistochemistry services, and Lora Barsky and the USC Flow Cytometry Core Facility for flow cytometry services. We thank Dr. Peter Kopp and Aigerim Bizhanova for their kind efforts in generating and providing the pEGFP-hPAX8a construct. We also thank Eva Wozniak and Maria Notaridou for technical assistance in the OSEC collection and culture, and Rod Karevan for technical support in qPCR.
Funding Disclosures: KL is funded by a K99/R00 award from the NCI (grant number 1K99CA184415). The project described was supported in part by award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, et al. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One. 2010;5:e10358. doi: 10.1371/journal.pone.0010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yates MS, Meyer LA, Deavers MT, Daniels MS, Keeler ER, Mok SC, et al. Microscopic and early stage ovarian cancers in BRCA1/2 mutation carriers: building a model for early BRCA-associated tumorigenesis. Cancer Prev Res. 2011;4:463–70. doi: 10.1158/1940-6207.CAPR-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–64. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [4].Auersperg N. Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecol Oncol. 2013;130:246–51. doi: 10.1016/j.ygyno.2013.03.021. [DOI] [PubMed] [Google Scholar]
- [5].Przybycin CG, Kurman RJ, Ronnett BM, Shihle M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- [6].Gilks C, Miller D. Opportunistic salpingectomy for women at low risk for development of ovarian carcinoma: The time has come. Gynecol Oncol. 2013;129:443–4. doi: 10.1016/j.ygyno.2013.04.021. [DOI] [PubMed] [Google Scholar]
- [7].Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- [8].Tang S, Onuma K, Deb P, Wang E, Lytwyn A, Sur M, et al. Frequency of Serous Tubal Intraepithelial Carcinoma in Various Gynecologic Malignancies: A Study of 300 Consecutive Cases. Int J Gynecol Pathol. 2012;2:103–10. doi: 10.1097/PGP.0b013e31822ea955. [DOI] [PubMed] [Google Scholar]
- [9].Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–7. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- [10].Ozcan A, Liles N, Coffey D, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic mullerian epithelial tumors: a comprehensive comparison. Am J Surg Pathol. 2011;35:1837–47. doi: 10.1097/PAS.0b013e31822d787c. [DOI] [PubMed] [Google Scholar]
- [11].Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:293–9. doi: 10.1097/PAI.0b013e3182025f66. [DOI] [PubMed] [Google Scholar]
- [12].Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–64. doi: 10.1038/modpathol.2011.3. [DOI] [PubMed] [Google Scholar]
- [13].TCGAR Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA. 2011;108:12373–7. doi: 10.1073/pnas.1109363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30:4824–34. doi: 10.1038/onc.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Di Palma T, Lucci V, de Cristofaro T, Fillippone MG, Zannini M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. BMC Cancer. 2014;14:292. doi: 10.1186/1471-2407-14-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Auersperg N. The origin of ovarian cancers: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, et al. Tubal origin of low-grade serous cancer. Modern Pathology. 2011;24:1488–99. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- [19].Banet N, Kurman RJ. Two types of ovarian cortical inclusion cysts: proposed origin and possible role in ovarian serous carcinogenesis. Int J Gynecol Pathol. 2015;34:3–8. doi: 10.1097/PGP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- [20].Tong GX, Hamele-Bena D. The differential expression of PAX2 and PAX8 in the ovarian surface epithelium and fallopian tubal epithelium is an important issue. Am J Surg Pathol. 2012;36:1099–100. doi: 10.1097/PAS.0b013e3182500c1b. author reply 100-2. [DOI] [PubMed] [Google Scholar]
- [21].Li NF, Wilbanks G, Balkwill F, Jacobs IJ, Dafou D, Gayther SA. A modified medium that significantly improves the growth of human normal ovarian surface epithelial (OSE) cells in vitro. Lab Invest. 2004;84:923–31. doi: 10.1038/labinvest.3700093. [DOI] [PubMed] [Google Scholar]
- [22].Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia. 2010;12:317–25. doi: 10.1593/neo.91948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tacha D, Qi W, Zhou D, Bremer R, Cheng L. PAX8 mouse monoclonal antibody [BC12] recognizes a restricted epitope and is highly sensitive in renal cell and ovarian cancers but does not cross-react with b cells and tumors of pancreatic origin. Appl Immunohistochem Mol Morphol. 2013;21:59–63. doi: 10.1097/PAI.0b013e318257cc1c. [DOI] [PubMed] [Google Scholar]
- [24].Congdon T, Nguyen LQ, Nogueira CR, Habiby RL, Medeiros-Neto G, Kopp P. A Novel Mutation (Q40P) in PAX8 Associated with Congenital Hypothyroidism and Thyroid Hypoplasia: Evidence for Phenotypic Variability in Mother and Child. J Clin Endocrinol Metab. 2001;86:3962–3967. doi: 10.1210/jcem.86.8.7765. [DOI] [PubMed] [Google Scholar]
- [25].Rosenbloom KR, Malladi VS, Dreszer TR, Learned K, Kirkup VM, Wong MC, et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41:D56–63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Okamoto S, Okamoto A, Nikaido T, Saito M, Takao M, Yanaihara N, et al. Mesenchymal to epithelial transition in the human ovarian surface epithelium focusing on inclusion cysts. Oncology Reports. 2014;21:1209–14. doi: 10.3892/or_00000343. [DOI] [PubMed] [Google Scholar]
- [28].Sundfeldt K, Piontkewitz Y, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, et al. E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Cancer. 1997;74:275–80. doi: 10.1002/(sici)1097-0215(19970620)74:3<275::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [29].Zeller KI, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tashiro H, Miyazaki K, Okamura H, Iwai A, Fukumoto M. c-myc over-expression in human primary ovarian tumours: Its relevance to tumour progression. International Journal of Cancer. 1992;50:828–833. doi: 10.1002/ijc.2910500528. [DOI] [PubMed] [Google Scholar]
- [31].Scully RE. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;59:208–18. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]
- [32].Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hesseling MH, De Wilde RL. Endosalpingiosis in laparoscopy. J Am Assoc Gynecol Laparosc. 2000;7:215–19. doi: 10.1016/s1074-3804(00)80043-2. [DOI] [PubMed] [Google Scholar]
- [34].Ruiz-Llorente S, de Pau ECS, Sastre-Perona A, Montero-Conde C, Gómez-López G, Fagin JA, et al. Genome-wide analysis of Pax8 binding provides new insights into thyroid functions. BMC Genomics. 2012;13:147. doi: 10.1186/1471-2164-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Auersperg N. The origin of ovarian cancers--hypotheses and controversies. Front Biosci (Schol Ed) 2013;5:709–19. doi: 10.2741/s401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.