Abstract
It has been known that overexposure to Ni can induce nephrotoxicity. However, the mechanisms of underlying Ni nephrotoxicity are still elusive, and also Ni- and Ni compound-induced ER stress has been not reported in vivo at present. Our aim was to use broiler chickens as animal model to test whether the ER stress was induced and UPR was activated by NiCl2 in the kidney using histopathology, immunohistochemistry and qRT-PCR. Two hundred and eighty one-day-old broiler chickens were divided into 4 groups and fed on a control diet and the same basal diet supplemented with 300 mg/kg, 600mg/kg and 900mg/kg of NiCl2 for 42 days. We found that dietary NiCl2 in excess of 300 mg/kg induced ER stress, which was characterized by increasing protein and mRNA expression of ER stress markers, e.g., GRP78 and GRP94. Concurrently, all the three UPR pathways were activated by dietary NiCl2. Firstly, the PERK pathway was activated by increasing eIF2a and ATF4 mRNA expression. Secondly, the IRE1 pathway was activated duo to increase in IRE1 and XBP1 mRNA expression. And thirdly, the increase of ATF6 mRNA expression suggested that ATF6 pathway was activated. The findings clearly demonstrate that NiCl2 induces the ER stress through activating PERK, IRE1 and ATF6 UPR pathways, which is proved to be a kind of molecular mechanism of Ni- or/and Ni compound-induced nephrotoxicity.
Keywords: NiCl2, ER stress, UPR, PERK, IRE1, Immunology and Microbiology Section, Immune response, Immunity
INTRODUCTION
Ni is ubiquitously in our environment, and exists various forms of Ni compounds in soil, water, air and living organisms [1]. Ni is an ubiquitous transition metal that is industrially applied in many forms, which inevitably leads to a high degree of occupational and environmental exposure [2]. This widespread extraction and use increase Ni concentrations in biogeochemical cycles and enhance human exposure to Ni and Ni compounds through environmental contamination and occupational exposure. Ni and Ni compounds have long been recognized to cause adverse health effects including neurotoxicity, hepatotoxicity, nephrotoxicity, genetoxicity, reproductive toxicity and increase in risk of cancer [3-8].
There are several studies on oxidative stress, apoptosis and inflammation induced by Ni and Ni compounds in vivo and vitro have been reported [9-12]. In human, Ni can cause sensitivity, allergic skin reaction and cancer [13]. Oral NiCl2 can induce hepatic apoptosis and decrease liver weight and body weight in mice [4]. Amudha et al. [14] have suggested that NiCl2 (intraperitoneally) disrupts antioxidant system and induces kidney damage in rats. Our studies have also shown that dietary NiCl2 in 300 mg/kg and over can cause histopathological lesions, immunotoxicity, oxidative damage, apoptosis and cell cycle arrest in the kidney, thymus, spleen, small intestine, cecal tonsil and bursa of Fabricius of broiler chickens [15-31]. In the vitro studies, NiONPs induce human bronchial epithelial cell toxicity through increasing SIRT1-mediated apoptosis [32]. Pan et al. [33] have reported that NiCl2 induces apoptosis in human bronchial epithelial BEAS-2B cells. And Ni NPs reduce mitochondrial function and induces the leakage of LDH in dose- and time-dependent manner in human lung epithelial A549 cells [34]. NiONPs and NiSO4 can cause pulmonary inflammation through increasing IL-6 and IL-8 protein expression levels after 24 h treatment [35-37].
ER accurately ensures proteins to be folded and assembled before proteins are sent to other organelles [38]. Environmental and genetic factors that disrupt ER function cause an accumulation of misfolded and unfolded proteins in the ER lumen, which is termed ER stress [38]. ER stress leads to UPR which is a major hallmark of cytotoxicity [39]. To date, three UPR pathways have been documented: PERK, IRE1, and ATF6 [40]. Both PERK and IRE1 contain cytoplasmic kinase domains, which are well known to be activated by homodimerization and autophosphorylation in the presence of ER stressors [41]. In the case of ATF6, accumulation of unfolded proteins induces ATF6 transition from ER to the golgi, where it is cleaved by two transmembrane proteins, e.g., Site1 and Site2 proteases [42]. Cleaved ATF6 produces a cytoplasmic protein that acts as an active transcription factor. Short-term ER stress events lead to pro-survival transcriptional activities through UPR pathways. When cells undergo irreversible ER stress, UPR pathway eliminates damaged cells by apoptosis [39, 43, 44]. At present, it has been reported that As, Cd, Ag, Mn and Cu can induce ER stress [45-51]. However, there are no studies on Ni and Ni compounds-induced ER stress, except a report that nickel acetate can induce ER stress and increase CHOP protein expression in the NRK52E and the Hepa-1c1c7 [52]. Also, there are no reports on molecular mechanism of Ni and Ni compounds-induced ER stress in animals and human beings.
The objective of this study was to determine potential mechanisms of NiCl2-induced ER stress in kidneys of broiler chickens. We monitored the mRNA and protein expression of GRP78 and GRP94 which were the markers of ER stress. We also measured the mRNA expression of UPR pathways, e.g., PERK, IRE1 and ATF6 pathways.
RESULTS
Histopathological changes
The results were shown in the reference [25] and in Figure 1.
Figure 1. Histopathological changes in the kidney at 42 days of age (HE).
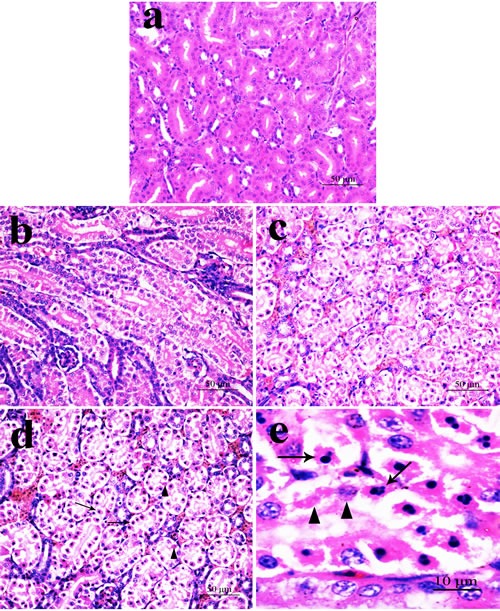
a. Control group. No changes are observed; b. 300 mg/kg group. Tubular cells show granular and vacuolar degeneration. Few necrotic tubular cells and apoptotic tubular cells are also observed. c. 600 mg/kg group. Tubular cells show marked granular and vacuolar degeneration. Also, some necrotic tubular cells and apoptotic tubular cells are observed. d. 900 mg/kg group. A large number of necrotic tubular cells (▲) and apoptotic tubular cells (↑) are observed. e. Necrotic tubular cells (▲) and apoptotic tubular cells (↑) are observed.
NiCl2 resulted in does- and time-dependent histopathological changes in the kidney, including tubular granular degeneration, vacuolar degeneration, necrosis and apoptosis. In the granular and vacuolar degenerated tubular cells, tiny particles and small or large vacuoles were appeared in the cytoplasm. Karyorrhexis, karyolysis and hypochromatosis were appeared in the necrotic cells. In the apoptotic cells, cytoplasm was intensely eosinophilic, and nuclei were shrunken, dense, ring-shaped and crescentic. Apoptotic bodies were also observed.
Effect of NiCl2 on ER stress markers in the kidney
We first examined whether NiCl2 could induce ER stress in the kidney. We examined the mRNA and protein expression of GRP78 and GRP94 which were the markers of ER stress.
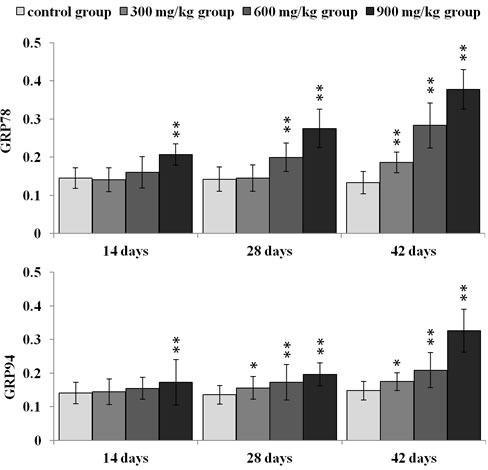
As shown in Figure 2, GRP78 mRNA expression was significantly higher (p < 0.05 or p < 0.01) in the three NiCl2-treated groups from 28 to 42 days of age, and in the 600 and 900 mg/kg group at 14 days of age than that in the control group. GRP94 mRNA expression was significantly increased (p < 0.05 or p < 0.01) in the three NiCl2-treated groups from 28 to 42 days of age and in the 900 mg/kg group at 14 days of age when compared with that in the control group.
Figure 2. Changes of GRP78 and GRP94 mRNA expression levels at 14, 28 and 42 days.
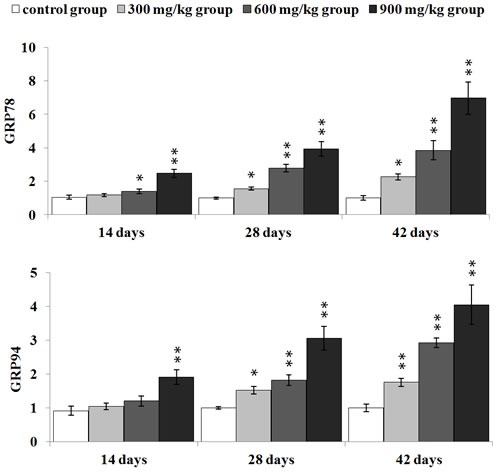
Data are presented with the mean ± standard deviation (n = 5) *P < 0.05, compared with the control group; ** P < 0.01, compared with the control group.
In Figures 3, 4, 5, GRP78 protein expression was significantly higher (p < 0.05 or p < 0.01) in the 900 mg/kg group at 14 days of age, in the 600 and 900 mg/kg group at 28 days of age and in the three NiCl2-treated groups at 42 days of age than that in the control group. GRP94 protein expression was significantly increased (p < 0.05 or p < 0.01) in the three NiCl2-treated groups from 28 to 42 days of age and in the 900 mg/kg group at 14 days of age in comparison with that in the control group.
Figure 3. Representative images of GRP78 protein expression by immunohistochemistry at 14, 28 and 42 days of age in the kidney.
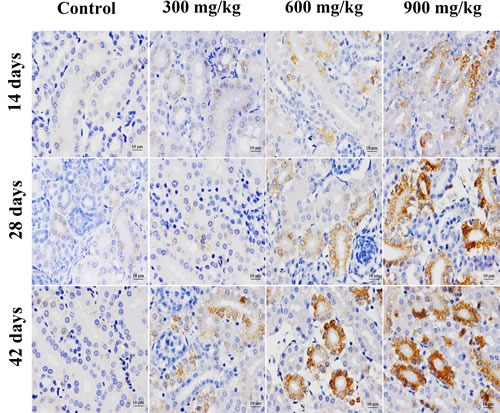
Figure 4. Representative images of GRP94 protein expression by immunohistochemistry at 14, 28 and 42 days of age in the kidney.
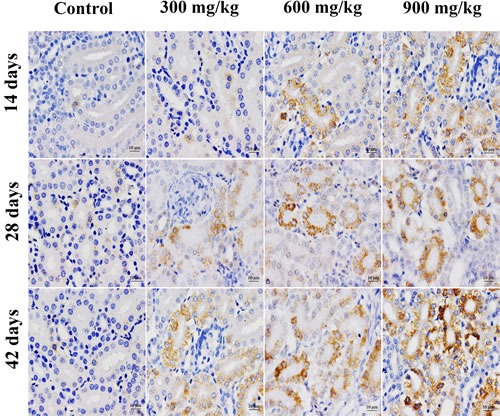
Figure 5. Changes of GRP78 and GRP94 protein expression levels at 14, 28 and 42 days.
Data are presented with the mean ± standard deviation (n = 5 × 5) *P < 0.05, compared with the control group; **P < 0.01, compared with the control group.
Effect of NiCl2 on the three UPR pathways in the kidney
To further confirm that UPR pathways were involved in NiCl2-induced ER stress, we examined all the three UPR pathways: PERK pathway, IRE1 pathway and ATF6 pathway.
NiCl2 activated the PERK pathway
In Figure 6, eIF2α mRNA expression was significantly higher (p < 0.05 or p < 0.01) in the 600 and 900 mg/kg groups from 28 to 42 days of age, and in the 900 mg/kg group at 14 days of age than that in the control group. ATF4 mRNA expression was significantly increased (p < 0.05 or p < 0.01) in the three NiCl2-treated groups from 28 to 42 days of age and in the 900 mg/kg group at 14 days of age when compared with that in the control group.
Figure 6. Changes of eIF2α and ATF4 mRNA expression levels at 14, 28 and 42 days.
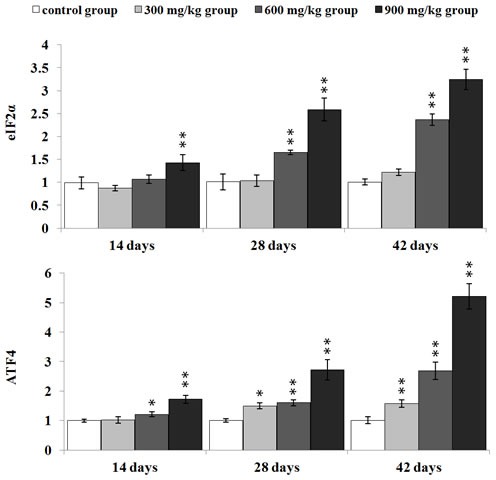
Data are presented with the mean ± standard deviation (n = 5) *P < 0.05, compared with the control group; **P < 0.01, compared with the control group.
NiCl2 activated the IRE1 pathway
IRE1 mRNA expression was significantly higher (p < 0.05 or p < 0.01) in the in the 600 and 900 mg/kg groups from 14 to 42 days of age and in the 300 mg/kg group at 42 days of age than that in the control group. XBP1 mRNA expression was significantly increased (p < 0.05 or p < 0.01) in the 600 and 900 mg/kg groups from 14 to 42 days of age in comparison with that in the control group, as shown in Figure 7.
Figure 7. Changes of IRE1 and XBP1 mRNA expression levels at 14, 28 and 42 days.
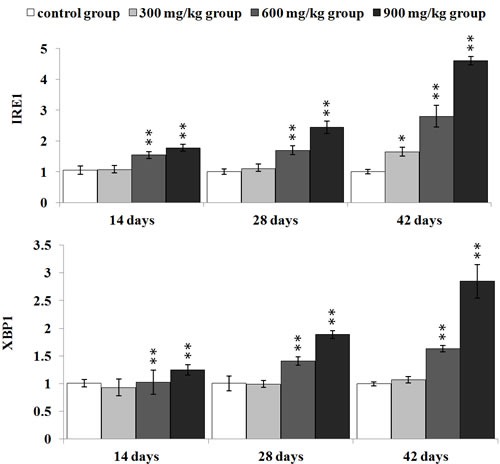
Data are presented with the mean ± standard deviation (n = 5) *P < 0.05, compared with the control group; **P < 0.01, compared with the control group.
NiCl2 activated the ATF6 pathway
As illustrated in Figure 8, ATF6 mRNA expression was significantly higher (p < 0.05 or p < 0.01) in the in the three NiCl2-treated groups from 28 to 42 days of age and in the 900 mg/kg group at 42 days of age than that in the control group.
Figure 8. Changes of ATF6 mRNA expression levels at 14, 28 and 42 days.
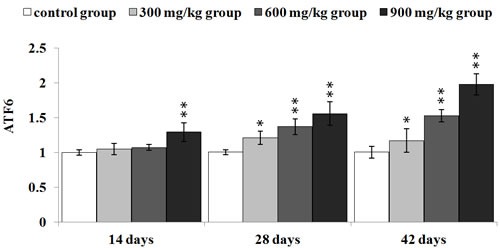
Data are presented with the mean ± standard deviation (n = 5) *P < 0.05, compared with the control group; **P < 0.01, compared with the control group.
DISCUSSION
This study explores whether the ER stress is induced and UPR pathways are activated by NiCl2 in the kidney of broiler chickens. We found consistent evidence that dietary NiCl2 in excess of 300 mg/kg caused ER stress, which was characterized by increasing protein and mRNA expression of ER stress markers, e.g., GRP78 and GRP94. When ER stress occurs, chaperone proteins, such as GRP78 and GRP94, are recruited to support the folding of new proteins [53]. Our findings are in agreement with the results of Hiramatsu et al. [52] who report that nickel acetate can induce ER stress and increase GRP78 protein expression in the rat renal proximal tubular cell line (NRK52E) and the mouse hepatoma cell line (Hepa-1c1c7). And, the heavy metals, such as CdCl2 and CoCl2 can induce ER stress in the rat renal proximal tubular cell line (NRK52E) and the mouse hepatoma cell line (Hepa-1c1c7) [52]. Tang et al. [48] have reported that As2O3 can trigger ER stress by increasing the protein and mRNA expression of only GRP78, not GRP94.
UPR is a defense mechanism against various cellular stress which causes accumulation of unfolded proteins in the ER [40]. We also monitored the three UPR pathways: PERK pathway, IRE1 pathway and ATF6 pathway. The chaperone proteins, such as GRP78 and GRP94, are major regulators of all three pathways. Under physiological conditions, the luminal domains of PERK, IRE1 and ATF6 proteins are bound to the ER resident chaperone GRP78 and GRP94, which keeps them inactive [40]. With the accumulation of unfolded proteins, GRP78 and GRP94 release enables PERK dimerization and activation to phosphorylate eIF2a, and then the phosphorylated eIF2a induces the translation of ATF4 mRNA [54]. In the present study, the results showed that dietary NiCl2 increased the eIF2a and ATF4 mRNA expression, implying that PERK pathway is one of mechanisms of NiCl2-induced ER stress. Wang et al. [55] have suggested that CdCl2 exposure significantly up-regulates the phosphorylated eIF2α protein and ATF4 mRNA expression in placenta. Also, the PERK pathway is involved in Nano-ZnO-induced ER stress in mice [56]. ATF4 promotes many adaptive responses that restore ER function and maintain cell survival [57]. And ATF4 can also promote apoptosis through regulating the CHOP and Noxa [58].
In the present study, NiCl2 also activated IRE1 pathway, which was characterized by increasing the IREI and XBP1 mRNA expression. GRP78 and GRP94 are released from IRE1 and permitted to dimerize, which activates XBP1 kinase and RNase activities to initiate XBP1 mRNA splice, which produces a potent transcriptional activator [59]. The XBP1 mRNA expression levels are increased in Nano-ZnO-treated mice [56]. As2O3 increases the protein and mRNA expression of XBP1 in Neuro-2a cells [45]. However, it has been reported that CdCl2 can't activate IRE1 signaling pathway in placenta [55].
Concurrently, the increase of ATF6 mRNA expression showed that NiCl2 activated the ATF6 pathway. ATF6 is transported from ER to the golgi compartment, where it is cleaved to a cytosolic fragment that migrates to the nucleus to further activate the transcription of UPR-responsive genes [60]. As2O3 can also increase ATF6 mRNA expression in FHL 124 cells [61]. Xu et al. [50] have suggested that MnCl2 activates both PERK and IRE1 pathway, not ATF6 pathway in brain.
Based on the results of our study and the above discussion, the mechanism of NiCl2-induced ER stress in the kidney is summarized in Figure 9.
Figure 9. Schematic diagram of NiCl2-caused ER stress.
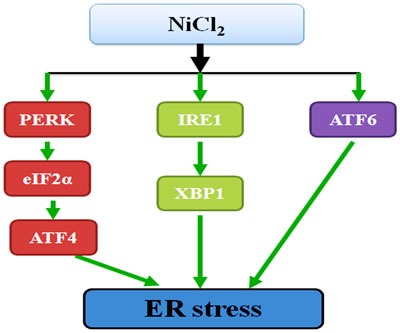
NiCl2 in excess of 300 mg/kg induces the ER stress. And PERK, IRE1 and ATF6 UPR pathways are all involved in NiCl2-induced ER stress.
In conclusion, our findings clearly demonstrate that dietary NiCl2 in excess of 300 mg/kg induces the ER stress through activating PERK, IRE1 and ATF6 UPR pathways, which is proved to be kind of molecular mechanism of Ni- or/and Ni compound-induced nephrotoxicity.
MATERIALS AND METHODS
Animals and treatment
Two hundred and eighty one-day-old healthy broilers were divided into four groups. There were seventy broilers in each group. Broilers were housed in cages with electrical heaters, and provided with water as well as under-mentioned experimental diets ad libitum for 42 days. The commercial broilers' growth period is about 42 days, and then they will be put into use for consumption. In this period they grow rapidly and a lot of diet will be consumed, and broilers will easily affected by diet containing metal pollutants (such as Ni). The aim of our study was to evaluate the effect of dietary NiCl2 on kidney in the broiler chicken of growth period.
To observe the time-dependent dynamic change, we chose three time points (14, 28 and 42 days of age) for examining histopathological injury, alterations of markers protein expression and mRNA expression levels involved in ER stress.
In this study, a corn-soybean basal diet formulated by the National Research Council [62] was the control diet. NiCl2 (NiCl2·6H2O, Chengdu Kelong Chemical Co., Ltd., Chengdu, China) was mixed into the corn-soybean basal diet to produce the experimental diets containing 300, 600 and 900 mg/kg NiCl2, respectively.
The basis of doses (300, 600 and 900 mg/kg NiCl2) selection: Ling and Leach reported that dietary NiCl2 concentrations of 300 mg/kg and over resulted in significant reduction in growth rate. Mortality and anemia were observed in chicks receiving 1100 mg/kg nickel [63]. Weber and Reid found a significant growth reduction at 700 mg/kg NiSO4 and nickel acetate and over [64]. Chicks fed more than 250-300 mg/kg Ni in the diet exhibited depressed growth and reduced feed intake [65]. Bersenyi et al. [66] reported that supplementation of 500 mg/kg NiCl2 reduced weight gain (by 10%), feed intake (by 4%) and worse FCE (by 5%) in growing broiler cockerels. According to the above-mentioned research results and our preliminary experiment, we chose the doses of 300, 600 and 900mg/kg NiCl2 in this study for observing the does-dependent changes.
Our experiments involving the use of broilers and all experimental procedures were approved by Animal Care and Use Committee, Sichuan Agricultural University.
Histopathological examination
Method was appeared in the reference [25].
Detection of protein expression in the kidney by Immunohistochemistry
Five chickens in each group were humanely sacrificed for gross examination at 14, 28 and 42 days of age. Kidneys were collected and fixed in 4% paraformaldehyde, and then processed, trimmed, and embedded in paraffin wax.
The method used in this study was described by Wu et al. in the reference [17]. Tissue slices were dewaxed in xylene, rehydrated through a graded series of ethanol solutions, washed in distilled water and PBS and endogenous peroxidase activity was blocked by incubation with 3% H2O2 in methanol for 15 min. The slices were subjected to antigen retrieval procedure by microwaving in 0.01 M sodium citrate buffer pH 6.0. Additional washing in PBS was performed before 30 min of incubation at 37°C in 10% normal goat serum (Boster, Wuhang, China). The slices were incubated overnight at 4°C with anti-GRP78 (1:200) (Cat: 3177, Cell Signaling Technology, America); anti-GRP94 (1:100) (Cat: sc-11402, Santa Cruz, America). After washing in PBS, the slices were exposed to 1% biotinylated goat anti-mouse IgG secondary antibody (Boster, Wuhang, China) for 1 h at 37°C, and then incubated with strept avidin-biotin complex (SABC; Boster, Wuhang, China) for 30 min at 37°C. To visualize the immunoreaction, slices were immersed in DAB (Boster, Wuhang, China). The slices were monitored microscopically and stopped by immersion in distilled water, as soon as brown staining was visible. Slices were lightly counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene and mounted.
The expression of protein was counted using a computer-supported imaging system connected to a light microscope (OlympusAX70) with an objective magnification of 400×. The intensity of staining for each protein was quantified using Image-pro Plus 5.1 (USA). Each group was measured five slices and each slice was measured five visions and averaged.
Detection of mRNA expression in the kidney by qRT-PCR
Kidneys from five chickens in each group were taken at 14, 28, and 42 days of age and stored in liquid nitrogen. The kidneys were homogenized in liquid nitrogen with a mortar and pestle.
According to the method described in the reference [17], total RNA was extracted from the frozen kidney powders with RNAiso Plus (9108/9109, Takara, Japan) according to the manufacturer's protocol. Next, cDNA was synthesized with a Prim-Script™ RT reagent Kit (RR047A, Takara, Japan) according to the manufacture's protocol. The cDNA product was used as a template for qRT-PCR analysis. Sequences for target genes were obtained from the NCBI database. Oligonucleotide primers were designed by use of Primer 5 software and synthesized at Takara (Dalian, China), as shown in Table 2.
Table 2. Sequence of primers used in qRT-PCR.
| Gene symbol | Accession number | Primer | Primer sequence(5′-3′) | Product size | Tm (°C) |
|---|---|---|---|---|---|
| GRP78 | NM205491 | Forward | GAATCGGCTAACACCAGAGGA | 118bp | 59 |
| Reverse | CGCATAGCTCTCCAGCTCATT | ||||
| GRP94 | NM204289 | Forward | CTTCGCTTCCAGTCTTCCCATC | 149bp | 58 |
| Reverse | AGAAGGCGTTCAACAAATGGTG | ||||
| Eif2a | NM001031323 | Forward | GCTGCGAGTCAGTAATGGGTATAA | 103bp | 59 |
| Reverse | CTGCCAGGAAACTTGCCACA | ||||
| ATF4 | AB013138 | Forward | TTGATGCCCTGTTAGGTATGGAA | 139BP | 60 |
| Reverse | GGTATGAGTGGAGGTTCTTTGTTGT | ||||
| IRE1 | NM001285499 | Forward | TGAGGGCAATGAGAAATAAGAAGC | 127bp | 61 |
| Reverse | TGTAGGAGCAGGTGAGGGAAGC | ||||
| XBP1 | NM001006192 | Forward | GCGAGTCTACGGATGTGAAGGA | 140bp | 61 |
| Reverse | TGTGGAGGTTGTCAGGAATGGT | ||||
| ATF6 | XM422208 | Forward | GATTGTGGGCGTCACTTCTCG | 142bp | 57 |
| Reverse | TGGGATGCCAATGTTAGCCTG | ||||
| β-actin | L08165 | Forward | TGCTGTGTTCCCATCTATCG | 178bp | 62 |
| Reverse | TTGGTGACAATACCGTGTTCA |
Table 1. Abbreviations.
| Ni | nickel |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| NiCl2 | nickel chloride |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| GRP78 | glucose-regulated protein 78 |
| GRP94 | glucose-regulated protein 94 |
| PERK | protein kinase RNA (PKR)-like ER kinase |
| eIF2a | elongation initiation factor 2a |
| ATF4 | activated transcription factor 4 |
| IRE1 | inositol-requiring enzyme 1 |
| XBP1 | X-boxbinding protein 1 |
| ATF6 | activated transcription factor 6 |
| NiONPs | NiO nanoparticles |
| Ni NPs | nickel nanoparticles |
| LDH | lactate dehydrogenase |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| As | arsenic |
| Cd | cadmium |
| Ag | silver |
| Mn | manganese |
| Cu | copper |
| CHOP | C/EBP homologous protein |
| NRK52E | rat renal proximal tubular cell line |
| Hepa-1c1c7 | mouse hepatoma cell line |
| CdCl2 | cadmium chloride |
| CoCl2 | cobalt chloride |
| As2O3 | arsenic trioxide |
| Nano-ZnO | Zinc oxide nanoparticles |
| MnCl2 | manganese chloride |
| FCE | feed conversion efficiency |
| PBS | phosphate buffer saline |
| DAB | 3,3′-diaminobenzidine |
| cDNA | complementary DNA |
| RNAiso | RNA isolate |
| NCBI | national center for biotechnology information |
All qRT-PCR were performed by use of the SYBR® Premix Ex TaqTM II system (DRR820A, Takara, Japan) with on a Model C1000 Thermal Cycler (Bio Rad, USA).
Chicken β-actin expression was used as an internal reference housekeeping gene. Gene expression values from control group subsamples at 14, 28, and 42 days of age were used to calibrate gene expression in subsamples from corresponding experimental subsamples. All data output from the qRT-PCR experiments were analyzed by use of the 2−ΔΔCT method [67].
Statistical analysis
The significance of difference among the four groups of broiler chicks was assessed with variance analysis, and results were presented as mean ± standard deviation (M ± SD). The variation was measured by use of one-way analysis of variance (ANOVA) test of SPSS 16.0 for windows. P < 0.05 was considered statistical significance.
Acknowledgments
The study was supported by the program for Changjiang scholars and innovative research team in university (IRT 0848) and the Shuangzhi project of Sichuan Agricultural University (03570327; 03571198).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Chau Y, Kulikovsky-Cordeiro O. Occurrence of nickel in the Canadian environment. Environ Rev. 1995;3:95–120. [Google Scholar]
- 2.Henderson RG, Durando J, Oller AR, Merkel DJ, Marone PA, Bates HK. Acute oral toxicity of nickel compounds. Regul Toxicol Pharm. 2012;62:425–432. doi: 10.1016/j.yrtph.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Xu S, He M, Zhong M, Li L, Lu Y, Zhang Y, Zhang L, Yu Z, Zhou Z. The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett. 2015;590:52–57. doi: 10.1016/j.neulet.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Gathwan KH, Al-Karkhi IHT, Jaffar Al-Mulla EA. Hepatic toxicity of nickel chloride in mice. Res Chem Intermed. 2012;39:2537–2542. [Google Scholar]
- 5.Scutariu MD, Ciupilan C. Nickel and magnesium effects in the rat kidney, treated with acid retinoic. Comparative study. Rev Med Chir Soc Med Nat Iasi. 2007;111:744–747. [PubMed] [Google Scholar]
- 6.Chen CY, Lin TK, Chang YC, Wang YF, Shyu HW, Lin KH, Chou MC. Nickel(II)-induced oxidative stress, apoptosis, G2/M arrest, and genotoxicity in normal rat kidney cells. J Toxicol Environ Health A. 2010;73:529–539. doi: 10.1080/15287390903421250. [DOI] [PubMed] [Google Scholar]
- 7.Forgacs Z, Massanyi P, Lukac N, Somosy Z. Reproductive toxicology of nickel - review. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2012;47:1249–1260. doi: 10.1080/10934529.2012.672114. [DOI] [PubMed] [Google Scholar]
- 8.Goodman JE, Prueitt RL, Dodge DG, Thakali S. Carcinogenicity assessment of water-soluble nickel compounds. Crit Rev Toxicol. 2009;39:365–417. doi: 10.1080/10408440902762777. [DOI] [PubMed] [Google Scholar]
- 9.Zheng GH, Liu CM, Sun JM, Feng ZJ, Cheng C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol. 2014;147:105–111. doi: 10.1016/j.aquatox.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Ma C, Song M, Zhang Y, Yan M, Zhang M, Bi H. Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicol Rep. 2014;1:114–121. doi: 10.1016/j.toxrep.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubrak OI, Husak VV, Rovenko BM, Poigner H, Kriews M, Abele D, Lushchak VI. Antioxidant system efficiently protects goldfish gills from Ni(2+)-induced oxidative stress. Chemosphere. 2013;90:971–976. doi: 10.1016/j.chemosphere.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Chuang HC, Hsueh TW, Chang CC, Hwang JS, Chuang KJ, Yan YH, Cheng TJ. Nickel-regulated heart rate variability: the roles of oxidative stress and inflammation. Toxicol Appl Pharmacol. 2013;266:298–306. doi: 10.1016/j.taap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157–173. doi: 10.1515/reveh.2007.22.2.157. [DOI] [PubMed] [Google Scholar]
- 14.Amudha K, Pari L. Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chem Biol Interact. 2011;193:57–64. doi: 10.1016/j.cbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Tang K, Guo H, Deng J, Cui H, Peng X, Fang J, Zuo Z, Wang X, Wu B, Li J, Yin S. Inhibitive Effects of Nickel Chloride (NiCl2) on Thymocytes. Biol Trace Elem Res. 2015;164:242–252. doi: 10.1007/s12011-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Toxicological Effects of Nickel Chloride on IgA+ B Cells and sIgA, IgA, IgG, IgM in the Intestinal Mucosal Immunity in Broilers. Int J Environ Res Public Health. 2014;11:8175–8192. doi: 10.3390/ijerph110808175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem Toxicol. 2014;63:18–29. doi: 10.1016/j.fct.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Analysis of the Toll-like receptor 2-2 (TLR2-2) and TLR4 mRNA expression in the intestinal mucosal immunity of broilers fed on diets supplemented with nickel chloride. Int J Environ Res Public Health. 2014;11:657–670. doi: 10.3390/ijerph110100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang K, Li J, Yin S, Guo H, Deng J, Cui H. Effects of Nickel Chloride on Histopathological Lesions and Oxidative Damage in the Thymus. Health. 2014;6:2875. [Google Scholar]
- 20.Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B. Effect of dietary nickel chloride on splenic immune function in broilers. Biol Trace Elem Res. 2014;159:183–191. doi: 10.1007/s12011-014-0003-y. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B. Downregulation of TLR4 and 7 mRNA expression levels in broiler's spleen caused by diets supplemented with nickel chloride. Biol Trace Elem Res. 2014;158:353–358. doi: 10.1007/s12011-014-9938-2. [DOI] [PubMed] [Google Scholar]
- 22.Guo H, Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Deng J, Yin S, Li J, Tang K. NiCl2-down-regulated antioxidant enzyme mRNA expression causes oxidative damage in the broiler(‘)s kidney. Biol Trace Elem Res. 2014;162:288–295. doi: 10.1007/s12011-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Cui H, Peng X, Fang J, Zuo Z, Huang J, Luo Q, Deng Y, Wang H, Liu J. Changes of the serum cytokine contents in broilers fed on diets supplemented with nickel chloride. Biol Trace Elem Res. 2013;151:234–239. doi: 10.1007/s12011-012-9554-y. [DOI] [PubMed] [Google Scholar]
- 24.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Dietary nickel chloride induces oxidative intestinal damage in broilers. Int J Environ Res Public Health. 2013;10:2109–2119. doi: 10.3390/ijerph10062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Deng H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Chen K. Nickel chloride (NiCl2)-caused inflammatory responses via activation of NF-kappaB pathway and reduction of anti-inflammatory mediator expression in the kidney. Oncotarget. 2015;6:28607–28620. doi: 10.18632/oncotarget.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Chen K, Deng J. Modulation of the PI3K/Akt pathway and Bcl-2 family proteins involved in chicken's tubular apoptosis induced by nickel chloride (NiCl2) Int J Mol Sci. 2015;16:22989–23011. doi: 10.3390/ijms160922989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Chen K, Deng J. Dietary NiCl2 causes G2/M cell cycle arrest in the broiler's kidney. Oncotarget. 2015;6:35964–35977. doi: 10.18632/oncotarget.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin S, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Guo H. Toxic effect of NiCl2 on development of the bursa of Fabricius in broiler chickens. Oncotarget. 2016;7:125–139. doi: 10.18632/oncotarget.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu B, Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Pathway underlying small intestine apoptosis by dietary nickel chloride in broiler chickens. Chem-Biol Interact. 2016;243:91–106. doi: 10.1016/j.cbi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Yin S, Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Tang K, Li J. Nickel chloride (NiCl2) induces histopathological lesions via oxidative damage in the broiler's bursa of Fabricius. Biol Trace Elem Res. 2015 doi: 10.1007/s12011-015-0528-8. [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Toxicological effects of nickel chloride on the cytokine mRNA expression and protein levels in intestinal mucosal immunity of broilers. Environ Toxicol. 2015;30:1309–1321. doi: 10.1002/tox.22001. [DOI] [PubMed] [Google Scholar]
- 32.Duan WX, He MD, Mao L, Qian FH, Li YM, Pi HF, Liu C, Chen CH, Lu YH, Cao ZW, Zhang L, Yu ZP, Zhou Z. NiO nanoparticles induce apoptosis through repressing SIRT1 in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2015;286:80–91. doi: 10.1016/j.taap.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Pan JJ, Chang QS, Wang X, Son YO, Liu J, Zhang Z, Bi YY, Shi X. Activation of Akt/GSK3beta and Akt/Bcl-2 signaling pathways in nickel-transformed BEAS-2B cells. Int J Oncol. 2011;39:1285–1294. doi: 10.3892/ijo.2011.1157. [DOI] [PubMed] [Google Scholar]
- 34.Ahamed M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol In Vitro. 2011;25:930–936. doi: 10.1016/j.tiv.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Capasso L, Camatini M, Gualtieri M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol Lett. 2014;226:28–34. doi: 10.1016/j.toxlet.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Brant KA, Fabisiak JP. Nickel and the microbial toxin, MALP-2, stimulate proangiogenic mediators from human lung fibroblasts via a HIF-1alpha and COX-2-mediated pathway. Toxicol Sci. 2009;107:227–237. doi: 10.1093/toxsci/kfn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattiwale SH, Saha S, Yendigeri SM, Jargar JG, Dhundasi SA, Das KK. Protective effect of L-ascorbic acid on nickel induced pulmonary nitrosative stress in male albino rats. Biometals. 2013;26:329–336. doi: 10.1007/s10534-013-9617-3. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 39.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 41.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 42.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 43.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833:3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 45.Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ, Chen YW. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett. 2014;224:130–140. doi: 10.1016/j.toxlet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardarin A, Chedin S, Lagniel G, Aude JC, Godat E, Catty P, Labarre J. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol Microbiol. 2010;76:1034–1048. doi: 10.1111/j.1365-2958.2010.07166.x. [DOI] [PubMed] [Google Scholar]
- 48.Tang CH, Chiu YC, Huang CF, Chen YW, Chen PC. Arsenic induces cell apoptosis in cultured osteoblasts through endoplasmic reticulum stress. Toxicol Appl Pharmacol. 2009;241:173–181. doi: 10.1016/j.taap.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Simard JC, Vallieres F, de Liz R, Lavastre V, Girard D. Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome. J Biol Chem. 2015;290:5926–5939. doi: 10.1074/jbc.M114.610899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu B, Shan M, Wang F, Deng Y, Liu W, Feng S, Yang TY, Xu ZF. Endoplasmic reticulum stress signaling involvement in manganese-induced nerve cell damage in organotypic brain slice cultures. Toxicol Lett. 2013;222:239–246. doi: 10.1016/j.toxlet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Hancock CN, Stockwin LH, Han B, Divelbiss RD, Jun JH, Malhotra SV, Hollingshead MG, Newton DL. A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo. Free Radic Biol Med. 2011;50:110–121. doi: 10.1016/j.freeradbiomed.2010.10.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiramatsu N, Kasai A, Du S, Takeda M, Hayakawa K, Okamura M, Yao J, Kitamura M. Rapid, transient induction of ER stress in the liver and kidney after acute exposure to heavy metal: evidence from transgenic sensor mice. FEBS Lett. 2007;581:2055–2059. doi: 10.1016/j.febslet.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 53.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Wang H, Xu ZM, Ji YL, Chen YH, Zhang ZH, Zhang C, Meng XH, Zhao M, Xu DX. Cadmium-induced teratogenicity: association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol Appl Pharmacol. 2012;259:236–247. doi: 10.1016/j.taap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Shao H, Liu W, Gu W, Shu X, Mo Y, Chen X, Zhang Q, Jiang M. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234:40–49. doi: 10.1016/j.toxlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gautam S, Kirschnek S, Wiesmeier M, Vier J, Hacker G. Roscovitine-induced apoptosis in neutrophils and neutrophil progenitors is regulated by the Bcl-2-family members Bim, Puma, Noxa and Mcl-1. Plos One. 2013;8:e79352. doi: 10.1371/journal.pone.0079352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 60.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Duncan G, Wang L, Liu P, Cui H, Reddan JR, Yang BF, Wormstone IM. Arsenic trioxide initiates ER stress responses, perturbs calcium signalling and promotes apoptosis in human lens epithelial cells. Exp Eye Res. 2007;85:825–835. doi: 10.1016/j.exer.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 62.NRC . Nutrient Requirements of Poultry. 9. National Academy Press; Washington, DC, USA: 1994. [Google Scholar]
- 63.Ling J, Leach R. Studies on nickel metabolism: interaction with other mineral elements. Poultry Sci. 1979;58:591–596. doi: 10.3382/ps.0580591. [DOI] [PubMed] [Google Scholar]
- 64.Weber CW, Reid BL. Nickel toxicity in growing chicks. J Nutr. 1968;95:612–616. doi: 10.1093/jn/95.4.612. [DOI] [PubMed] [Google Scholar]
- 65.Szilagyi M., Szentmihalyi S., Anke M. Changes in Some of the Biochemical Parameters in Ni and Mo Deficient Animals (Goat, Sheep, Pig, Chicken, Rat) Proceeding (Hungary) 1983;1:257–260. Available online: http://agris.fao.org/agris-search/search.do?recordID=HU8200908. [Google Scholar]
- 66.Bersényi A, Fekete SG, Szilágyi M, Berta E, Zöldág L, Glávits R. Effects of nickel supply on the fattening performance and several biochemical parameters of broiler chickens and rabbits. Acta Vet Hungarica. 2004;52:185–197. doi: 10.1556/AVet.52.2004.2.7. [DOI] [PubMed] [Google Scholar]
- 67.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]


