Abstract
Complementation of the repair defect in hamster xrs mutants has been achieved by transfer of human chromosome 2 using the method of microcell-mediated chromosome transfer. The xrs mutants belong to ionizing radiation complementation group 5, are highly sensitive to ionizing radiation, and have an impaired ability to rejoin radiation-induced DNA double-strand breaks. Both phenotypes were corrected by chromosome 2, although the correction of radiation sensitivity was only partial. Complementation was achieved in two members of this complementation group, xrs6 and XR-V15B, derived independently from the CHO and V79 cell lines, respectively. The presence of human chromosome 2 in complemented clones was examined cytogenetically and by PCR analysis with primers directed at a human-specific long interspersed repetitive sequence or chromosome 2-specific genes. Complementation was observed in 25/27 hybrids, one of which contained only the q arm of chromosome 2. The two noncomplementing hybrids were missing segments of chromosome 2. The use of a back-selection system enabled the isolation of clones that had lost the human chromosome and these regained radiation sensitivity. Transfer of several other human chromosomes did not result in complementation of the repair defect in XR-V15B. These data show that the gene defective in xrs cells, XRCC5, which is involved in double-strand break rejoining, is located on human chromosome 2q.
Full text
PDF

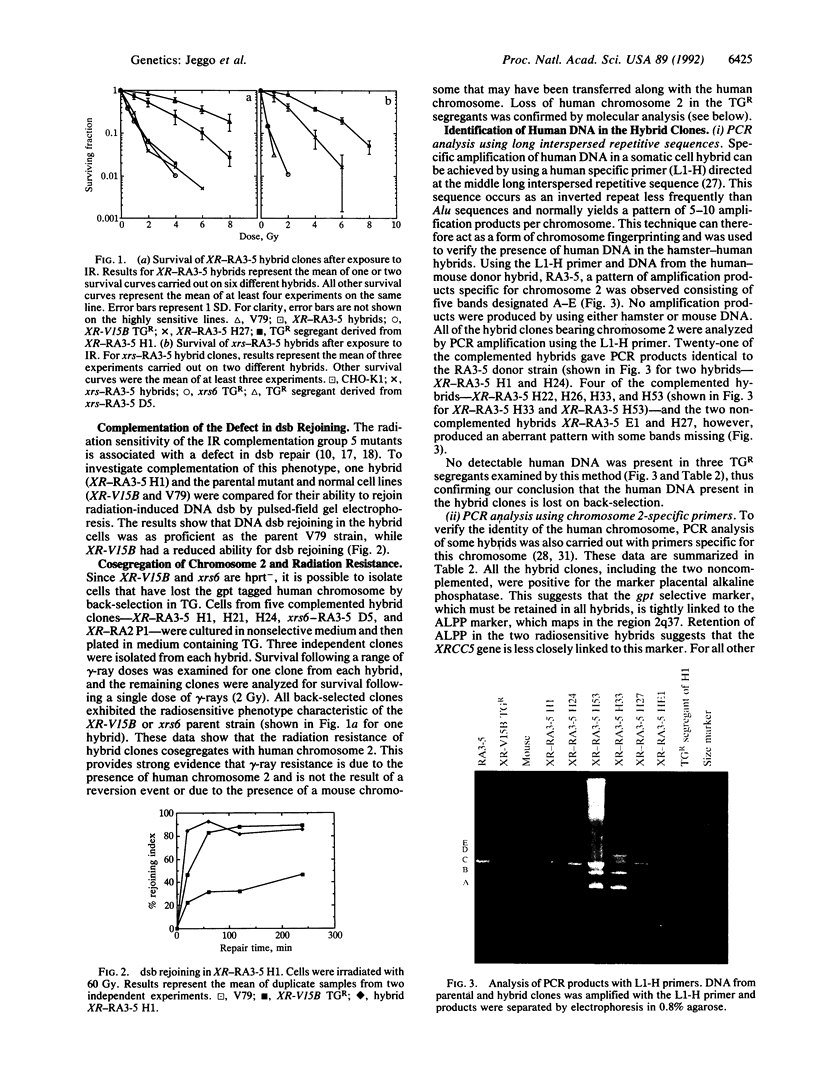

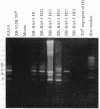
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott C., Povey S. Development of human chromosome-specific PCR primers for characterization of somatic cell hybrids. Genomics. 1991 Jan;9(1):73–77. doi: 10.1016/0888-7543(91)90222-z. [DOI] [PubMed] [Google Scholar]
- Athwal R. S., Smarsh M., Searle B. M., Deo S. S. Integration of a dominant selectable marker into human chromosomes and transfer of marked chromosomes to mouse cells by microcell fusion. Somat Cell Mol Genet. 1985 Mar;11(2):177–187. doi: 10.1007/BF01534706. [DOI] [PubMed] [Google Scholar]
- Evans H. H., Ricanati M., Horng M. F. Deficiency in DNA repair in mouse lymphoma strain L5178Y-S. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7562–7566. doi: 10.1073/pnas.84.21.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A., Weinstein R., Hu J., Stamato T. D. Cell cycle-dependent repair of double-strand DNA breaks in a gamma-ray-sensitive Chinese hamster cell. Somat Cell Mol Genet. 1985 Sep;11(5):485–491. doi: 10.1007/BF01534842. [DOI] [PubMed] [Google Scholar]
- Hariharan P. V., Hutchinson F. Neutral sucrose gradient sedimentation of very large DNA from Bacillus subtilis. II. Double-strand breaks formed by gamma ray irradiation of the cells. J Mol Biol. 1973 Apr 15;75(3):479–494. doi: 10.1016/0022-2836(73)90455-5. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. S. Induction of DNA double-strand breaks by X-rays in a radiosensitive strain of the yeast Saccharomyces cerevisiae. Mutat Res. 1975 Dec;30(3):327–334. [PubMed] [Google Scholar]
- Ishizaki K., Oshimura M., Sasaki M. S., Nakamura Y., Ikenaga M. Human chromosome 9 can complement UV sensitivity of xeroderma pigmentosum group A cells. Mutat Res. 1990 May;235(3):209–215. doi: 10.1016/0921-8777(90)90076-h. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A. Genetic analysis of X-ray-sensitive mutants of the CHO cell line. Mutat Res. 1985 Nov;146(3):265–270. doi: 10.1016/0167-8817(85)90067-7. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986 Aug;6(8):2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Tesmer J., Chen D. J. Genetic analysis of ionising radiation sensitive mutants of cultured mammalian cell lines. Mutat Res. 1991 Mar;254(2):125–133. doi: 10.1016/0921-8777(91)90003-8. [DOI] [PubMed] [Google Scholar]
- Kaur G. P., Athwal R. S. Complementation of a DNA repair defect in xeroderma pigmentosum cells by transfer of human chromosome 9. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8872–8876. doi: 10.1073/pnas.86.22.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Sedgwick S. G., Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984 Nov-Dec;132(5-6):189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Ledbetter S. A., Garcia-Heras J., Ledbetter D. H. "PCR-karyotype" of human chromosomes in somatic cell hybrids. Genomics. 1990 Dec;8(4):614–622. doi: 10.1016/0888-7543(90)90247-r. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Carrano A. V., Fertitta A., Perry B., Thompson L. H., Tucker J. D., Weber C. A. Refined mapping of the three DNA repair genes, ERCC1, ERCC2, and XRCC1, on human chromosome 19. Cytogenet Cell Genet. 1989;52(1-2):11–14. doi: 10.1159/000132829. [DOI] [PubMed] [Google Scholar]
- Picksley S. M., Attfield P. V., Lloyd R. G. Repair of DNA double-strand breaks in Escherichia coli K12 requires a functional recN product. Mol Gen Genet. 1984;195(1-2):267–274. doi: 10.1007/BF00332758. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Saxon P. J., Schultz R. A., Stanbridge E. J., Friedberg E. C. Human chromosome 15 confers partial complementation of phenotypes to xeroderma pigmentosum group F cells. Am J Hum Genet. 1989 Apr;44(4):474–485. [PMC free article] [PubMed] [Google Scholar]
- Siciliano M. J., Carrano A. V., Thompson L. H. Assignment of a human DNA-repair gene associated with sister-chromatid exchange to chromosome 19. Mutat Res. 1986 Aug;174(4):303–308. doi: 10.1016/0165-7992(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Smeets H., Bachinski L., Coerwinkel M., Schepens J., Hoeijmakers J., van Duin M., Grzeschik K. H., Weber C. A., de Jong P., Siciliano M. J. A long-range restriction map of the human chromosome 19q13 region: close physical linkage between CKMM and the ERCC1 and ERCC2 genes. Am J Hum Genet. 1990 Mar;46(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M., Anand R., Brown W. R., Fletcher D. S. A model for the separation of large DNA molecules by crossed field gel electrophoresis. Nucleic Acids Res. 1987 Aug 11;15(15):5925–5943. doi: 10.1093/nar/15.15.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J., Wilkinson R. E. The genetic basis of resistance to ionising radiation damage in cultured mammalian cells. Mutat Res. 1991 Mar;254(2):135–142. doi: 10.1016/0921-8777(91)90004-9. [DOI] [PubMed] [Google Scholar]
- Theune S., Fung J., Todd S., Sakaguchi A. Y., Naylor S. L. PCR primers for human chromosomes: reagents for the rapid analysis of somatic cell hybrids. Genomics. 1991 Mar;9(3):511–516. doi: 10.1016/0888-7543(91)90418-e. [DOI] [PubMed] [Google Scholar]
- Weeda G., Wiegant J., van der Ploeg M., Geurts van Kessel A. H., van der Eb A. J., Hoeijmakers J. H. Localization of the xeroderma pigmentosum group B-correcting gene ERCC3 to human chromosome 2q21. Genomics. 1991 Aug;10(4):1035–1040. doi: 10.1016/0888-7543(91)90195-k. [DOI] [PubMed] [Google Scholar]
- Weibezahn K. F., Lohrer H., Herrlich P. Double-strand break repair and G2 block in Chinese hamster ovary cells and their radiosensitive mutants. Mutat Res. 1985 May;145(3):177–183. doi: 10.1016/0167-8817(85)90025-2. [DOI] [PubMed] [Google Scholar]
- Whitaker S. J., McMillan T. J. Oxygen effect for DNA double-strand break induction determined by pulsed-field gel electrophoresis. Int J Radiat Biol. 1992 Jan;61(1):29–41. doi: 10.1080/09553009214550591. [DOI] [PubMed] [Google Scholar]
- Whitmore G. F., Varghese A. J., Gulyas S. Cell cycle responses of two X-ray sensitive mutants defective in DNA repair. Int J Radiat Biol. 1989 Nov;56(5):657–665. doi: 10.1080/09553008914551881. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Tran Q., van der Schans G. P., Simons J. W. Characterization of an X-ray-hypersensitive mutant of V79 Chinese hamster cells. Mutat Res. 1988 Nov;194(3):239–249. doi: 10.1016/0167-8817(88)90025-9. [DOI] [PubMed] [Google Scholar]